Abstract
Background
The present study investigated the potential wound healing activity comparisons between ethanol leaf & seed extracts of Costus speciosus topical formulation using mice Excision wound models, compared to Neomycin sulphate ointment as a standard drug. To assess the efficacy of Costus speciosus ointment as a potential alternative to commercially available ointments, a study was conducted using a cohort of 16 healthy male mice, aged between 3 and 4 months. The ointment formulation was prepared utilizing ethanol extracts from both the leaves and seeds, presented in a dosage form for application. This investigation seeks to provide insights into the comparative effectiveness of Costus speciosus ointments in relation to conventional market preparations, with a focus on its potential applicability as an alternative therapeutic option. The parameter measured was wound contraction and epithelization period.
Result
The mice groups treated with seed & leave extracts ointment showed better wound size changes than the negative and standard groups. However, the leaf extract ointment promotes the formation of granulation in tissue, allowing the reepithelization phase to occur more rapidly than seed extract. From the 1st day to the results of the 9th day of the extract indicate that there is a significant increase (P < 0.05) in the percentage of wound contraction in the group. After 21 days the wound was healed fully. These results suggest that ointment of ethanol leaf extract of Costus speciosus could be an answer to facilitate wound healing compared to seed extract, to approve the traditional claims of the plant in wound healing activity.
Conclusion
The study concludes that ethanol leaf and seed extracts of Costus speciosus exhibit potential wound healing activity in mice excision wound models. The topical formulation of these extracts significantly enhances wound closure, reduces wound size, and increases tensile strength compared to the control group. The efficacy of the extracts is comparable to the standard drug, Neomycin sulphate ointment. Therefore, the use of Costus speciosus extracts in topical formulations can be considered a promising alternative for the treatment of wounds. However, further studies are needed to determine the safety and efficacy of these extracts in humans.
Similar content being viewed by others
Introduction
Wounds are the outcome of skin damage that disrupts the outer soft tissue. Healing is a process that is fundamentally a connective tissue response; the initial stage of this process involves the acute inflammatory phase, followed by the synthesis of collagen and other extracellular macromolecules, which are later remodeled to form a scar [1].
Wound healing is an effective mechanism that substitutes missing and impaired tissues and cellular structures. This is attained in four specific phases, hemostasis, inflammation, propagation, and remodeling, that maintain the functional integrity of the injured muscles [2].
The in-vivo model for wound healing uses donated skin from reduction surgeries, sustaining it under controlled conditions long enough to study the molecular mechanisms involved and evaluate therapies intended to speed recovery. The skin goes through overlapping phases of inflammation, proliferation (or migration), and remodeling during wound healing. Although the process of acute wound healing isn’t studied in humans in-vivo for ethical reasons, it can be closely mimicked in-vivo. For designing an excellent wound-healing system, some key factors should be considered. For example, it should be biocompatible, provide a moist environment to the wound, maintain good absorption capacity of wound exudate and fluids, and allow the finest gas diffusion, thus stimulating healing and preventing the intrusion of germs at the wound bed. In all the above factors, biocompatibility plays a crucial role [3].
Costus speciosus is a valuable medicinal plant that’s commonly employed to treat a variety of health issues. This plant contains numerous active components and has been discovered to offer a range of health benefits, including fighting off harmful molecules, battling cancer, reducing inflammation, managing diabetes, regulating lipid levels, safeguarding the liver, supporting hormone production, enhancing the body’s ability to adapt, and combating various microbes [4].
Some key components identified from different sections of the Costus speciosus include the following: Diosgenin has been recognized as the primary element isolated from the Costus speciosus. The stem hosts the highest amount of diosgenin at 0.65%, while the leaves contain 0.37% and the flowers have 1.21%. Additional isolated constituents comprise Tigogenin, dioscin, gracillin, β-sitosterol glucoside. Moreover, the seeds contain around 6% of a pale-yellow fatty oil with a sweet aroma. The physical and chemical properties of the oil are as follows:
-
Specific gravity − 0.9125.
-
Refractive index − 1.4672.
-
Acid value − 23.84.
-
Saponification value − 179.84.
-
Iodine value − 76.4 [5].
Phytochemical screening of C. speciosus detected the presence of alkaloids, glycosides, steroids, phenolic, flavonoids, polyphenols, tannins, and βcarotene. Diosgenin, βsitosterol, furostanol saponinscostusosides, βDglucoside, prosapogenins, dioscin, gracillin, dihydrophytylplastoquinone, and αtocopherolquinone [4]. Among them; Flavonoids, alkaloids, tannins, and polyphenols exert antiviral properties, anti-inflammatory properties, anti-bacterial, antiproliferative, and wound healing properties [6,7,8].
Currently, available methods of wound management, including irrigation, debridement, antibiotics, proteolytic enzymes, and tissue grafts, are associated with significant drawbacks such as invasiveness, and are expensive [9].
The main goals of the research in wound healing are to evaluate the influence of various actions in wound management programs on healing and to screen drugs that encourage the healing process efficiently. Different plant products have been used to treat wounds over the years. Phytoconstituents derived from plants need to be identified and screened for antimicrobial activity to manage injuries [10].
The natural plant elements generated from them account for a sizable share of the worldwide medical business. Throughout human civilization, herbal treatments and medications have played an important role in illness treatment. Despite a significant body of research on their restorative properties, there are no standard protocols for quality control of plant materials in terms of categorization (phytochemical, pharmacological, and remedial activity). The standardizing of medicinal plants ensures uniformity and therapeutic efficacy. Herbal remedies are examined for their identification (portrayal), quality, and the quality of the extracts contained, since this is essential to assess their medicinal efficacy, i.e., to understand their pharmacological activity in order to demonstrate authenticity [11].
The plant extracts are more efficacious, free from undesirable side effects than the pure active principle, and due to the totality of constituents rather than a single molecule. However, most synthetic drugs currently used to treat wounds are expensive and pose problems such as allergy and drug resistance. The investigation of potent wound healing agents is one of the most promising areas in biomedical sciences [12].
To understand such plants in detail and for them to assume their proper role in contributing to affordable healthcare, a robust scientific assessment is required. Successive solvent extraction techniques, chromatographic separations, and spectroscopic methods have been used to determine the chemical constituents of plants and study their bioactivity. The present study investigated the wound-healing activity of the ointment formulation of the ethanolic extract of Costus speciosus leaves and seeds and their comparison.
Risk factors and conditions associated with wound healing
Healing a wound is a delicate process, prone to disruption and failure for a multitude of reasons. Impairment of the wound-healing function can occur when any of its stages, or the timing and order in which they appear, are altered by external stimuli. In addition to raising the risk of severe complications and mortality, these variables also slow wound healing [13]. There are indeed local and systemic wound healing risk factors. An individual’s propensity to recuperate naturally is affected by systemic elements, which in turn impact the individual’s general health. Age, stress, degenerative diseases (including diabetes mellitus and hepatic and renal failure), adiposity, alcoholism, medication, and immunocompromising conditions are also contributors (such as cancer and AIDS) [14]. It’s essential to establish the local elements that have an impact on the wound’s distinctive features. Foreign bodies, poor circulation, lack of oxygen, and infection are potential complications of an such injury. There is a linkage between such parameters, with systemic factors frequently influencing wound healing via environmental variables [15].
Methods and resources
Plant collection
At Baro Aoulia, Kumira, near Chittagong, in December of the following year, a specimen of Costus speciosus was collected. The leaves and seeds were then rinsed thoroughly with water and placed in a drying shed. Dr. Shaikh Bokhtiyar Uddin confirmed this plant. The leaf weight was roughly 2 kg, and the seeds were 700 gm. It was placed on a tray following thorough leaf cleaning and placed in an area with good airflow and shade from the sun. The leaves took roughly a month to dry out. Brownish was the hue of the withered leaves. The seeds took 14 days to dry out. The dried leaves and seeds were ground into a fine powder in a blender. Then leaf was 800gm and seed were 400gm after converting into course powder. The powder was collected and sent to the lab for additional processing using a glass vial [16].
Plant drug extraction
This was then used for extraction by soaking in a solvent. The solvent, in this case, is 95% ethanol. Ethanol was used to split the leaves and seed powder into two large glass jars, and the jars were then filled with the leaves. A mixture of 95% ethanol and leaves & seed powder were kept in separate 1-liter containers for 14 days at room temperature (25 °C), sometimes containers were shaken and stirred, then sieved and filtered using cotton plugs and Whatman No. 1 filter paper. The resulting solution was filtered again using a piece of filter paper. Filtration yielded 800 ml since ethanol evaporates when exposed to the air. A rotating evaporator at 50 °C was used to remove solvent from the filtrate, and a specific extract was obtained from the process. To achieve a firm residue, this had to be driest. Therefore, it was possible to begin laboratory testing on Costus speciosus’ leaf and seed extract.
Percentage yield of the crude extract
The weight of crude extract was measured, and the percentage yield was calculated per weight of the sample. The yield (%) of the dried extract was determined by:
Preparation of formulation and standard use
The simple ointment was prepared from the ethanolic extract of Costus speciosus using a white soft and hard paraffin mixture, cetostearyl alcohol, and beeswax obtained from the Department of Pharmacy, IIUC. A simple ointment base was used as the control group and was applied twice per day. Both extract ointments were used twice per day to treat different animals. Nebanol ointment (Neomycin sulphate) obtained from Square Pharmaceuticals Ltd. was used as a standard drug for comparing the wound healing potential of extract in animal models and both sample and standard were applied twice per day on experimental animals.
Formulation of topical ointment of Costus speciosus leaves & seeds
Effective concentration, optimal dosage for mice, and procedure for preparation of extract ointment followed by Sawant, S.E., and M.D. Tajane [17] with slight modification. Simple ointment base B.P. in the concentration of 0.5% (Tables 1 and 2; Fig. 1) was applied using excision wound models in mice.
Stability of formulated Costus speciosus’ ointment
Animals
For the animal experiments, all efforts were made to minimize the suffering of the animals. Swiss Albino mice of either sex (aged 6–7 weeks old and weighing 25–35 g) were obtained from the appropriate source. The animals were familiarized with the laboratory conditions for 14 days at room temperature (25 °C ± 2 °C) with a 12-h light/dark cycle with food pellets and ample water supply. The institutional animal ethical committee approved this study under an approved reference number [18]. All sections of this report adhere to the Animal Research: Reporting of In Vivo Experiments guidelines for reporting animal research.
Animals and experimental groups
Healthy young Swiss albino mice were randomly divided into four groups. Before commencing the experiment, each animal was assigned a unique identification marking with a marker-like head, tail, body, and unmarked.
-
Group I: The Control group was treated with ordinary ointment base.
-
Group II: Test group treated with Costus speciosus leaf ointment.
-
Group III: Test group treated with Costus speciosus seeds ointment.
-
Group IV: Reference Standard Market Preparation Nebanol (Neomycin sulphate).
We conducted an irritancy test where we applied the ointment to a person’s skin and carefully observed its effects. Additionally, we performed a stability study to assess the physical stability of the herbal ointment over four weeks under different temperature conditions, including 2 ºC, 25ºC, and 37ºC (Table 3). The results showed that the ointment remained physically stable at all of these temperatures for the entire four-week duration. The physicochemical properties of C. speciosus ointment was also determined which is described in Table 4.
Acute dermal toxicity study
The acute dermal toxicity test of the crude extract of Costus speciosus was carried out per OECD draft guideline number 404. Three mice with standard skin surfaces were randomly selected and maintained in a cage individually and acclimatized to the working environment for a week before the test’s commencement. Around 10% of the body surface area fur was shaved from the dorsal area of the trunk 24 h prior to the study. The extract formulation was uniformly applied over the shaved area for 24 h. Mice were housed individually during the exposure period. The residual test substance was removed at the end of the exposure period, and the mice were observed daily for 14 days to identify any adverse skin reactions [19].
Excision wound model
The Mice were anesthetized by administering ketamine (0.5 ml/kg b.w. i.p.). A total thickness of the straight excision wound area (approx. 130 mm) and 1 mm depth was made on the shaved backs of the mice 30 min after the administration of ketamine injection. The wounding day was considered day 0 (Fig. 2). The wounds were treated with topical application of the ointments as described above till the wounds were completely healed. The wounds were monitored, and the area of the wound was measured on the 3rd, 6th, 9th, 12th, 15th, 18th & 21st post-wounding days, and the mean of % wound closure is reported in Table 3. The epithelization period was calculated as the number of days required for the falling of the dead tissue remnants without any residual raw wound [20, 21].
Where N = number of days 3rd, 6th, 9th, 12th, and 15th day.
Wound contraction measurement
Wound contraction was assessed by measuring the narrowing of the size of the wound using a ruler. In order to quantify the size of the wound, the area of the wound was measured planimetrically every other day until the complete healing of the wound was observed.
Epithelization time measurement
The epithelization time (Fig. 2) was calculated as the Number of days required for falling off the dead tissue remnants without any residual raw wound [22].
Statistical analysis
Results obtained from the animal models have been expressed as mean ± SEM and were compared with the corresponding control group (simple ointment B.P.) by applying ANOVA analysis and Dunnett’s test & Tukey test were applied to compare values, as appropriate, using SPSS software. Values were considered significant at p < 0.05, 0.01, and 0.001 [23, 24].
Result and discussion
Percentage yield of the crude extract
The percentage yield of the crude leaf and seed extract of Costus speciosus was determined by using the formula described in method section.
Wound area
A better healing pattern with complete wound closure was observed in treated mice within 21 days, while it took about 25–30 days in control mice with given concentrations of ointments of ethanol extract (Table 5; Figs. 2 and 3). There was a significant reduction in wound area from day three onwards in treated mice, and also, on later days, the closure rate was much faster than when compared with control mice. Table 5 shows the effect of ointment and extract of Costus speciosus leaves and seeds on the Excision wound model in mice (Fig. 4).
Chromatic study
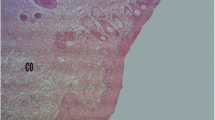
Wound photography of the same group of mice was illustrated in Fig. 5. The chosen days (0/3/6/9/12/15/18/21) corresponded to the wound induction day, inflammatory phase, granulation tissue formation, and epithelialization, respectively.
In this way, the recovery process is quickened. The wound contraction and epithelization time were the variables measured in this research. Nevertheless, the healing process is influenced by several factors, including the capabilities of the tissue to regenerate, the damage’s intensity, and the source material’s health state. Values of wound contraction of treatments are presented in Table 4. The extract ointment showed a noteworthy effect on narrowing the wound area (130 mm2) compared to the standard drug Neomycin ointment. The time required for epithelialization of the excision wound of the extract is described in Table 4.
Epithelization period
Significant shortened epithelization period was manifested by the extract ointment compared to standard drugs and placebo. The medicated concentration shortened the period of epithelization into 6 days compared negative group. Wound contraction and epithelization occur in the proliferation phase. However, these two stages are not related, although the contraction may facilitate the re-epithelization process. The shortening and thickening of the wound resulted in the reduction of wound size and the amount of extracellular matrix needed to recover the damaged tissues [25]. This resulted in the acceleration of wound closure.
Discussion
Wound healing is a complex, multifactor sequence of events involving several cellular and biochemical processes. These processes aim to regenerate and reconstruct the skin’s disrupted anatomical continuity and functional status. The healing process, a natural body reaction to injury, initiates immediately after wounding and occurs in four stages. The first phase is coagulation which controls excessive loss of blood from the damaged vessels. The second stage of the healing process is inflammation and debridement of the wound, followed by re-epithelization, which includes proliferation, migration, and differentiation of the epidermis squamous epithelial cells. In the third or final stage of the healing process, collagen deposition and remodeling occur within the dermis [26,27,28,29]. The basic principle of optimal wound healing is to minimize tissue damage and provide adequate tissue perfusion and oxygenation, proper nutrition, and a moist wound-healing environment to restore the anatomical continuity and function of the affected part. The result of the wound excision model indicates that in the first 3 consequent days, there was no significant increase in wound contraction in all the groups, compared to the control group. The results of the 8th day indicate a significant increase (P < 0.05) in the percentage of wound contraction in the group treated with standard drug & ethanolic extract, this reveals that the extract has the ability to induce cellular proliferation.
When it comes to the progression of repairing wounds, herbal medicines are frequently used. The bioactive chemical constituents isolated from plant materials (leaves and seeds) have been scientifically demonstrated to aid wound healing potential. In this recent study, an investigation was conducted on the efficacy of Costus speciosus leaves and seed extract in augmenting the wound healing process within wound excision models. Notably, the chosen solvent for the extraction process was 90% ethanol, renowned for its universal solvency that adeptly attracts both polar and nonpolar molecules concurrently. The selection of an oily medicinal formulation was deliberate, given its inherent qualities of being gentle, facile to apply, and versatile for employment in both therapeutic and aesthetic contexts.
The rationale behind opting for an oily basis, specifically an ointment, lies in its capacity to serve as a conducive medium for enhancing the absorption of active constituents into the targeted tissue. The oily nature of the base ensures a sustained interaction of the ointment’s active components with the target tissue over an extended duration. This characteristic proves instrumental in facilitating and optimizing the therapeutic effects of the formulated substance. From the above remarks, it can be concluded that all the parameters of the selected extract ointment are done carefully. The extract studies showed a significant amount of selected active healing constituents. The wound contraction studies revealed that wound contraction increases by increasing the Costus speciosus extract concentration. The study also reveals that ointment formulation’s better activity may be due to the synergistic action of the plant’s constituents present in the prepared formulation. Thus, the prepared topical ointment is versatile in healing wound contraction.
The wound-healing activities of C. speciosus extracts are hypothesized to be mediated by the synergistic effects of the various bioactive phytoconstituents. Phytochemical investigations have revealed the presence of various secondary metabolites in Costus speciosus, including steroids, flavonoids, alkaloids, diosgenin, tigogenin, terpenes and saponins. Extracts prepared from the leaves and seeds of Costus speciosus exhibit antioxidant, antimicrobial, and anti-inflammatory properties in preliminary in vitro studies which were proven [30]. Further phytochemical standardization and elucidation of molecular mechanisms through in vivo models are warranted to substantiate the ethnopharmacological use of this species for cutaneous wound repair.
Comparing the leaf extract of Kalanchoe pinnata with the results of standard medication, on day 12 of treatment, the extract of the plant demonstrated significant wound healing, greater re-epithelialization, and denser collagen fibers, and on the 15th day of treatment, wounds were completely healed [31], moreover, on the 16th day of treatment, the hydromethanolic Bersama abyssinica leaves extract’ ointment caused significant wound contraction [32]. Vernonia auriculifera Hiern leaves developed as ointment preparations with concentrations of reveal significant wound healing activity when extracted with 80% methanol and solvent fractions [33]. On the 16th day, the group treated with Elaeis guineensis leaf extract showed complete wound healing [34]. On the 20th day of treatment, the Manilkara zapota leaves extract ointments with the two concentrations 5% and 10% demonstrated significantly enhanced in-vitro and in-vivo wound reconstruction activities [35]. Whereas our tested C. speciosus extracts, both leaf and seeds extract took 21 days to heal the wounds completely and 18 days to significantly contract the wound in the animal model.
Based on assessments of C. speciosus, the plant’s antifungal properties were introduced to light by the abundance of sapogenins and saponins [36, 37]. Plenty of biologically active chemical compounds, notably dehydrodihydrocostus lactone, reynosin, arbusculin A, santamarine, and stigmasterol, which were extracted from the chloroform extract of C. speciosus, have been investigated on blood mononuclear cells which have been isolated. These substances have been found to have anti-inflammatory properties by triggering a decline in the levels of prostaglandins TNF-α and different interleukins, including interleukins 1 and 6 [38]. Positive antibacterial activity is possessed by C. speciosus. Terpenoids, or flavonoids, which have strong antifungal properties, antibacterial as well as anti-insect properties, could potentially be contributing factors to this [39].
The study had a limitation, as it didn’t use techniques like chromatographic separations or spectroscopy to pinpoint the exact chemical components in the extracts and their effects on biological systems. This limitation arose mainly because of the limitation of facilities which made it difficult to perform advanced chemical analyses. Consequently, we couldn’t gain a complete understanding of the specific compounds in the extracts and their potential impacts on biological systems. This constraint may have limited the study’s findings, leaving some aspects of the extracts’ composition and potential bioactivity uninvestigated.
Conclusion
This study highlights Costus speciosus’s significant wound-healing efficacy, emphasizing its potential to expedite recovery. Both leaves and seeds contribute to an ointment with accelerated healing compared to a control group. Combining Costus speciosus with other treatments enhances its therapeutic impact. The ethanol extract, used as an ointment, proves effective in wound treatment, showcasing its promise in advancing wound management methodologies due to its rich phenol constituents. These findings underscore the promising potential of Costus speciosus in innovating and advancing methodologies for the management and treatment of wounds.
In summary, the findings of this study posit the leaves and seeds of Costus speciosus as potential sources of wound-healing properties, with the derived ointment emerging as a promising avenue for fostering tissue repair. The prospect of utilizing extracts from Costus speciosus is particularly compelling, offering a potential means to synergistically enhance therapeutic benefits. The notion of combining these extracts suggests a promising avenue for achieving a synergistic effect, thereby potentially magnifying the overall therapeutic impact on tissue repair. Chromatographic separations or spectroscopic analysis can be done as a future approach to identify potential lead molecules for wound healing purposes from leaves and seeds of Costus speciosus.
Data availability
All the datasets manifested here are generated during the tests and analyzed by the authors themselves.
References
Nandanwar R, Gurjar H, Sahu VK, Saraf H. Studies on wound healing activity of gel formulation containing cow ghee and Aloe vera. Int J Pharma Sci Res. 2010;1:50–4.
Shah SA, Sohail M, Khan S, Minhas MU, De Matas M, Sikstone V, Hussain Z, Abbasi M, Kousar M. Biopolymer-based biomaterials for accelerated diabetic wound healing: a critical review. Int J Biol Macromol. 2019;139:975–93.
Ambekar RS, Kandasubramanian B. Advancements in nanofibers for wound dressing: a review. Eur Polymer J. 2019;117:304–36.
El-Far A, Shaheen H, Alsenosy A, El-Sayed Y, Al Jaouni S, Mousa S. Costus speciosus: traditional uses, phytochemistry, and therapeutic potentials. Pharmacogn Rev. 2018;12(23).
Tyagi B, Gupta M. Ploidy status and diosgenin content in Costus speciosus. (Koen) Sm Cytologia. 1987;52(1):41–6.
de Albuquerque RDDG, Perini JA, Machado DE, Angeli-Gamba T, dos Santos Esteves R, Santos MG, et al. Wound Healing Activity Chem Stand Eugenia Pruniformis Cambess. 2016;12(48):288.
Sdayria J, Rjeibi I, Feriani A, Ncib S, Bouguerra W, Hfaiedh N et al. Chemical composition and antioxidant, analgesic, and anti-inflammatory effects of methanolic extract of Euphorbia retusa in mice. 2018;2018.
Criollo-Mendoza MS, Contreras-Angulo LA, Leyva-López N, Gutiérrez-Grijalva EP, Jiménez-Ortega LA, Heredia JB. Wound Healing Prop Nat Products: Mech Action. 2023;28(2):598.
Muluye AB, Desta AG, Abate SK, Dano GT. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar J. 2019;18(1):1–8.
Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evidence-based complementary and alternative medicine. 2011;2011.
Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Future J Pharm Sci. 2021;7(1):1–13.
Majumder P, Taxo-cognostic. Phyto-Physicochemical & Biological screening of a plant: Taxo-cognostic, phytochemical, physicochemical parameters & Biological screening of Zyziphus Oenoplia (L.) Mill plant root. LAP LAMBERT Academic Publishing; 2012.
Gonzalez ACO, Costa TF, Andrade ZA, Medrado ARAP. Wound healing-A literature review. An Bras Dermatol. 2016;91:614–20.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29.
Anderson K, Hamm RL. Factors that impair wound healing. J Am Coll Clin Wound Spec. 2012;4(4):84–91.
Banu N, Alam N, Nazmul Islam M, Islam S, Sakib SA, Hanif NB, et al. Insightful valorization of the biological activities of Pani Heloch leaves through experimental and computer-aided mechanisms. Molecules. 2020;25(21):5153.
Sawant SE, Tajane MD. Formulation and evaluation of herbal ointment containing neem and turmeric extract. J Sci Innovative Res. 2016;5(4):149–51.
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10.
Charmeau-Genevois C, Sarang S, Perea M, Eadsforth C, Austin T, Thomas P. A simplified index to quantify the irritation/corrosion potential of chemicals–part I: skin. Regul Toxicol Pharmacol. 2021;123:104922.
Nayak B, Anderson M, Pereira LP. Evaluation of wound-healing potential of Catharanthus roseus leaf extract in rats. Fitoterapia. 2007;78(7–8):540–4.
Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124(2):311–5.
Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. J Ethnopharmacol. 2007;114(2):103–13.
Mukherjee PK, Verpoorte R, Suresh B. Evaluation of in-vivo wound healing activity of Hypericum patulum (family: Hypericaceae) leaf extract on different wound model in rats. J Ethnopharmacol. 2000;70(3):315–21.
Alam N, Banu N, Aziz M, Ibn A, Barua N, Ruman U, et al. Chemical profiling, pharmacological insights and in Silico studies of methanol seed extract of Sterculia foetida. Plants. 2021;10(6):1135.
Paju N, Yamlean PV, Kojong N. Uji Efektivitas salep ekstrak daun binahong (Anredera cordifolia (ten.) Steenis) pada kelinci (Oryctolagus cuniculus) Yang Terinfeksi Bakteri Staphylococcus aureus. Pharmacon. 2013;2(1).
Leibovich S, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78(1):71.
Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46.
Phillips GD, Whitehead RA, Knighton DR. Initiation and pattern of angiogenesis in wound healing in the rat. Am J Anat. 1991;192(3):257–62.
Ahsan A, Miana G, Naureen H, RehmanA MU, Anum K, Malik I, Extraction. Phytochemical Screening and Wound Healing Activity of Herbal Formulation of Saussurea lappa: Wound Healing and Anti-bacterial Activity of Saussurea lappa. Proceedings of the Pakistan Academy of Sciences: B Life and Environmental Sciences. 2019;56(3):83-96-83–96.
Saraf A. Phytochemical and antimicrobial studies of medicinal plant Costus speciosus (Koen). J Chem. 2010;7:405–S13.
Coutinho MAS, Casanova LM, Nascimento LBS, Leal D, Palmero C, Toma HK et al. Wound healing cream formulated with Kalanchoe pinnata major flavonoid is as effective as the aqueous leaf extract cream in a rat model of excisional wound. 2021;35(24):6034–9.
Taddese SM, Gurji TB, Abdulwuhab M, Aragaw TJJE-BC, Medicine A. Wound healing activities of hydromethanolic crude extract and solvent fractions of Bersama abyssinica Leaves in mice. 2021;2021.
Lambebo MK, Kifle ZD, Gurji TB, Yesuf JSJJEP. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera Hiern (Asteraceae) in mice. 2021:677– 92.
Sasidharan S, Logeswaran S, Latha LYJI. Wound healing activity of Elaeis guineensis leaf extract ointment. 2011;13(1):336–47.
Alsareii SA, Alzerwi NA, Alasmari MY, Alamri AM, Mahnashi MH, Shaikh IA et al. Manilkara zapota L. extract topical ointment application to skin wounds in rats speeds up the healing process. 2023;14.
Islam R, Ullah A, Mukim M, Ashraf M, Haque R. Pharmacological investigation of the Wound Healing ActivitY of Costus Specious Plant2015.
Sohrab S, Mishra P, Mishra SKJBNRC. Phytochemical competence and pharmacological perspectives of an endangered boon—Costus speciosus. (Koen) Sm: Compr Rev. 2021;45(1):1–27.
Al-Attas AA, El-Shaer NS, Mohamed GA, Ibrahim SR. Esmat AJJoe. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. 2015;176:365– 74.
Malabadi RBJJopr. Antibacterial activity in the rhizome extract of Costus speciosus. (Koen). 2005;18(1):83–5.
Acknowledgements
We are grateful & thankful to the Department of Pharmacy, International Islamic University Chittagong, Bangladesh, for the laboratory and other vital logistic support for this research. We are also grateful to Shaikh Bokhtiyar Uddin for his cooperation in identifying this plant. Special thanks to Saiful Islam Arman, Najmul Alam, and Abdul Karim for providing guidance and arranging the necessary facilities for a hassle-free research project.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
SAZ planned and executed the whole test & MU helped him throughout the procedure, MM made a graph and did statistical data & proofreading, AK did plagiarism check & wrote the manuscript according to the journal, SSI & JN critically reviewed and revised the manuscript, SIA & SSI helped with supervision and guidelines.
Corresponding authors
Ethics declarations
Ethical approval
From a bioethical point of view, the experimental protocol used in this study complies with the recommendations for rendering the ethical morals laid down in the 1964 Declaration of Helsinki. In addition, this work has been validated by the institutional research ethics committee of the Faculty of Science & Engineering and the Department of Pharmacy of IIUC.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zishan, S.A., Uddin, M.M., Mohammad, M. et al. Costus speciosus leaf and seed extracts for wound healing: a comparative evaluation using mice excision wound models. Clin Phytosci 10, 5 (2024). https://doi.org/10.1186/s40816-024-00368-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-024-00368-9