Abstract
Background
Momordica. charantia is popularly used as a medicinal herb in ethnomedicine for the management of cardiovascular diseases. In this study, we evaluated the beneficial effects of the ethanolic extract of M. charantia (Linn.) in experimentally induced cardiovascular disorders using cholesterol-fed Wistar rat.
Methods
Seventy-two experimental rats were randomly assigned into nine 9 groups of 8 rats each and treated as follows: Rats in group A (control) were given distilled water only; Rats in group B were given 30 mg/kg of cholesterol dissolved in coco-nut oil (cholesterol solution); Rats in group C were given cholesterol solution and 100 mg/kg Atorvastatin; Rats in group D were given 250 mg/kg of M. charantia and cholesterol solution; Rats in group E were given 500 mg/kg of M. charantia and cholesterol solution; Rats in group F were given 250 mg/kg. M. charantia; Rats in group G were given 500 mg/kg M. charantia; Rats in group H were given 1 ml of coconut oil; Rats in group I were given 100 mg/kg of Atorvastatin.
Results
Mean LDL-cholesterol was significantly (P < 0.05) lower in groups F, E and H as compared with the control groups. Histological analysis of the heart and aortic branch of the experimental rats show that cholesterol administration induced myocardial degeneration, vascular ulceration and stenosis in the aorta and heavy perivascular infiltrates of inflammatory cells. However, these deleterious effects were ameliorated upon treatment with Momordica charantia and Atorvastatin as compared with the control groups.
Conclusion
Our findings indicate the possible cardiovascular benefits of M. charantia.
Similar content being viewed by others
Introduction
Cardiovascular diseases result from the death of cardiac tissues due to prolonged and severe ischemia [1]. The occurrence also correlates with genetic and behavioral dispositions to atherosclerosis [2]. Significant increase in morbidity and mortality due to cardiovascular diseases are often associated with aortic and carotid atherosclerotic disease and stroke [2]. Conventionally, cardiovascular diseases such as myocardial infarction and atherosclerosis are treated or managed with the administration of orthodox drugs or by surgical intervention. These conventional procedures are often associated with unpleasant side-effects and complications. In a view to mitigating these side effects, the search for alternative and safer remedy have become the focus of many researchers. Medicinal plants have been acclaimed to be safe as an alternative form of therapy. They have been harnessed as phyto-remedy since the dawn of civilization and some herbs have emerged as a mainstay of man’s pharmacotherapy.
The World Health Organization in 2013 [3] estimated that 80% of inhabitants of developing countries rely on plant-based drugs because they are natural and supposedly innocuous with little or no side effects. In ethnomedicine, M. charantia has been acclaimed to have a broad spectrum of activities in the management and treatment of cardiovascular disorders, diabetes, inflammation, etc [4] Commonly called bitter gourd or Balsam pear, M. charantia belongs to the Cucurbitaceae family and grows in the tropical area. It is native to tropical and subtropical Africa. It is popularly consumed as vegetable and has been acclaimed to possess high medicinal values. M. charantia has been reported to contain active phytochemicals which are responsible for its therapeutic activities. An example is the protection against atherosclerosis by regulating blood cholesterol. It has been proposed that the crude extract of Momordica charantia, as well as Momordicatin (which is obtained from the fruits of Momordica charantia) may be considered as potential sources of new chemotherapeutics [4]. However, there is dearth of scientific information about the determination of the safe dose and possible cardiovascular benefits of this plant. In this study, we investigated the possible cardiovascular benefits of M. charantia using standard scientific methods.
Methods
Chemicals and reagents
HDL-Cholesterol Kit, LDL-Cholesterol Kit, Triglyceride (TG) kit, cholesterol powder, coconut oil, 50% ethanol, phosphate buffer (pH 7.4), 6 M H2SO4, 0.01 M KMnO4, EDTA, formalin, alcohol, xylene, paraffin wax, haematoxylin and eosin dyes, 1% acid alcohol, phosphotungstic acid haematoxylin (PTAH) stain. All chemicals and reagents are of analytical grades from Sigma-Aldrich, Germany. The standard drug used was Atorvastatin (Lipitor) from Pfizer Pharmaceuticals, Great Britain.
Plant materials and preparation of ethanol extract
Fresh leaves and stem of M. charantia were collected from Ovbiogie community in Ovia North-East Local Government Area of Edo State, Nigeria. They were identified and authenticated in the Department of Plant Biology and Biotechnology, Faculty of Life Sciences, University of Benin, Nigeria. The plant was assigned Herbarium Number: UBHc0294 and a sample of the plant material was deposited in the Herbarium. The fresh leaf and stem were air dried and ground into fine powder using an electric blender (pyeUnicam, Cambridge, England). The powdered plant material was subjected to extraction using 50% ethanol and intermittent maceration. Dried ethanol extracts were obtained after removing the solvent by evaporation under reduced pressure using Rotary evaporator. The extract was stored in an air-tight container and kept in the refrigerator at 4 °C for further use.
Percentage yield of the extracts was determined using the formula.
Phytochemical analysis of extract
The extract was subjected to preliminary phytochemical screening for tentative identification of carbohydrates, cardiac glycosides, saponins, steroidal nucleus, alkaloids, tannins, flavonoids using standard established methods.
Animals, experimental design and procedure
Adult Wistar rats weighing between 160 g and 180 g were used for this study. The animals were bred in the animal facility of the Department of Anatomy, School of Basic Medical Sciences, University of Benin. Food and water were provided ad libitum. The experimental procedures and care of the animals were in accordance with the institution’s (School of Basic Medical Sciences, University of Benin, Nigeria) animal and ethics guidelines. A total of 72 healthy Wistar rats of either sexes weighing between 160 g and 180 g were randomly assigned into nine groups (A to I) of eight rats each, as follows:
-
Group A: (Control) received 1 ml distilled water orally daily for a period of sixty (60) days.
-
Group B: Received freshly prepared cholesterol powder dissolved in coconut oil at a dose of 30 mg/kg body weight orally for a period of sixty (60) days.
-
Group C: (Standard) received the standard drug Atorvastatin (Lipitor) at a dose of 100 mg/kg body weight orally for a period of sixty (60) days and freshly prepared cholesterol at a dose of 30 mg/kg body weight.
-
Group D: Received 250 mg/kg orally, ethanol extract of M. charantia (LDMC) for a period of sixty (60) days and freshly prepared cholesterol at a dose of 30 mg/kg body weight.
-
Group E: Received 500 mg/kg orally, ethanol extract of M. charantia (HDMC) for a period of sixty (60) days and freshly prepared cholesterol at a dose of 30 mg/kg body weight.
-
Group F: Received 250 mg/kg orally, ethanol extract of M. charantia (LDMC) only for a period of 60 days.
-
Group G: Received 500 mg/kg orally, ethanol extract of M. charantia (HDMC) only for a period of 60 days.
-
Group H: Received 1 ml of coconut oil only for 60 days.
-
Group I: Received 100 mg/kg of Atorvastatin (Lipitor) only for 60 days.
Acute toxicity study of extract
Acute oral toxicity (AOT) of M. charantia was determined using 42 Wistar rats by the method described by Lorke in 1983 [5]. The animals were fasted overnight prior to the commencement of the experiment. They were divided into seven groups of six animals each and were administered with single dose of the extract orally. The different groups received different doses of the extract (1, 2, 4, 8 and 12 g/kg body weight). The animals were observed for mortality up to 48 h (acute toxicity) and for another 14 days for signs of sub-acute toxicities.
Induction of cholesterol-induced myocardial infarction and atherosclerosis
Cholesterol powder was made into solution in pure coconut oil (vehicle) and administered to the treated groups (B – E) at a dose of 30 mg/kg body weight for 60 days to induce myocardial infarction on the heart and atherosclerosis in the aorta.
Sample collection
After 60 days of administration, the rats were weighed, anesthetized and cut open (sacrificed). Blood samples were collected by cardiac and aortic puncture from the heart and aorta of each rat using sterile syringes. The samples were kept in plane sample bottles. The heart and aorta were excised from the rats and promptly transferred into 10% formal saline for fixation.
Determination of serum lipid profile
The blood samples were centrifuged using Hettich Centrifuge (Rototix 32A, Germany) at 4000rmp for 10 min. The sera were separated and used for determination of total cholesterol (TC), triglycerides (TG), low density lipoproteins (LDL) and high-density lipoproteins (HDL) using standard diagnostic test kits (Randox laboratories, UK) on Automated Clinical System (VIS-7220G, Biotech Engineering Management Company Limited, UK) following the manufacturer’s instructions.
Histological procedure
The fixed tissues were completely dehydrated by passing them through ascending grades of alcohol solutions (70% for 2 h, 90% for 18 h and 100% for 4 h). The tissues were immersed in xylene for 5 h for complete removal of the alcohol solution. The tissues were infiltrated in an oven using molten paraffin wax at a temperature range of 56 °C to 60 °C. The molten paraffin wax was poured into an embedding mound and the infiltrated tissues were placed in it. The tissues were oriented as to allow the cutting of longitudinal and transverse sections. The molten paraffin wax was allowed to cool and form the tissue block. Prior to sectioning, the tissue blocks were trimmed and mounted on a wooden block holder. The tissues were clipped to the microtome (Leica RM 2235, UK) and sectioned at 4 μm thickness. Sections came out as ribbons and were placed in 20% alcohol for spreading. The ribbons were cut and allowed to float in a water bath at a temperature of 30 °C. The sectioned tissues were placed in xylene for 5 min to allow the removal of paraffin wax. Hydration was carried out by passing the tissues through descending grades of alcohol solutions (100%, 90% and 70%) and water for 5 min each. The tissues were stained in haematoxylin for 10 min and washed in water for 2 min. They were briefly differentiated in 1% acid alcohol and subsequently washed in water. The tissues were further counterstained in eosin for 5 min and rinsed in 90% alcohol solution [6]. Stained slides were viewed using an optical photomicroscope (Leica MC170 HD, Leica Bio systems, Germany) at × 100 and × 400 magnifications. Special stains used are Elastic stain (ES) technique for elastic fibers [7]; Phosphotungstic acid haematoxylin (PTAH) for smooth muscle fibres [8].
Statistical analysis
Statistical analysis was by One Way Analysis of Variance (ANOVA) using IBM-SPSS statistics version 23 followed by Duncan’s comparison test for significance (P < 0.05).
Results
The effects of ethanol extract of Momordica charantia on the body and organ weight, lipid profile and the histological sections of the heart and aortic branch of the experimental rats are as described below;
In Table 1, the phytochemical screening of Momordica charantia shows the presence of some phytochemicals such as alkaloids, flavonoids, saponins, steriods, terponoids, tanins and anthroquines and cardiac glycosides. The results also indicate comparatively, the highest concentration of alkaloids in the plant extract.
Comparison of body weight and the organ weight
There were no significant (P > 0.05) differences between the body weight and the organ (heart) weight of the experimental animals across the different groups (Table 2, Figs. 1, 2 and 3).
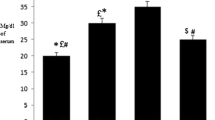
Lipid profile of the experimental animals. Our results of the lipid profile of the experimental animals (fig) show that the mean total cholesterol, triglyceride and high-density lipoproteins (HDL) were not significantly different (P > 0.05) in all the test groups as compared to the control group. However, the mean low-density lipoproteins was significantly (P < 0.05) lower in the groups given LDMC, cholesterol plus HDMC and coconut oil only as compared with the control group
Histological sections of the heart of the experimental rats (H&E X 100). Figure 2 shows; 1. Control rat heart composed of bundles of myocardiac fibres (A), coronary artery (B) and interstitial space (C). 2. Rat given low dose extract only showing, moderate interstitial congestion (A) and mild vascular dilatation (B). 3. Rat given high dose extract only showing, normal myocardiac fibres (A) and mild vascular dilatation (B). 4. Rat given Atorvastatin only showing, normal myocardiac fibres (A) and normal vascular architecture (B). 5. Rat given cholesterol diet only showing, focal myocardiac degeneration (A), vascular ulceration, stenosis and heavy perivascular infiltrates of inflammatory cells (B). 6. Rat given cholesterol and low dose extract showing, normal myocardiac fibres (A) and asymmetric media hypertrophy (B). 7. Rat given cholesterol and high dose extract showing, normal myocardiac fibres (A) and mild vascular dilatation and congestion (B). 8. Rat given cholesterol and Atorvastatin showing, normal myocardiac fibres (A) and normal vascular architecture (B). 9. Rat given coconut oil only showing, normal myocardiac fibres (A) and normal vascular architecture (B)
Histological sections of the aortic branch of the experimental rats (H&E X 100). Figure 3 shows; 1. Control rat aortic branch composed of tunica, intima (A), media (B) and adventitia (C). 2. Rat given low dose extract only showing, normal vascular wall (A). 3. Rat given high dose extract only showing, normal vascular wall (A) and mild dilatation (B). 4. Rat given Atorvastatin only showing, normal vascular wall (A). 5. Rat aortic branch given high cholesterol diet only showing, moderate vascular stenosis (A) and ulceration (B). 6. Rat given high cholesterol diet and low dose extract showing, normal vascular wall (A). 7. Rat given high cholesterol diet and high dose extract showing, normal vascular wall (A). 8. Rat given cholesterol diet and Atorvastatin showing, normal vascular wall (A). 9. Rat given coconut oil only showing, normal vascular wall (A)
Discussion
Myocardial infarction and atherosclerosis are major manifestations of cardiovascular diseases with high rate of morbidity and mortality globally. Nature has made available certain therapeutic agents of plant origin capable of protecting or improving cardiovascular health. This may provide an avenue to reduce cholesterol without recourse to synthetic drugs and their undesirable side-effects. Due to the long historical utilization of ethnomedicinal plants by humans, useful data on the acute and sub-acute toxicity evaluations of medicinal plants or extracts derived from them should be accessed. This is necessary to ensure the certainty of their safety to humans and also set the criteria for choosing a safe dose in humans [9]. Preliminary analysis of some phytochemical constituent of the extract of M. charantia revealed the presence of high amounts of alkaloids, flavonoids and cardiac glycosides. Other phytochemicals of biochemical importance and cardiovascular relevance found include saponins, steroids, terpenoids tannins and anthraquinones. As observed in our study, we infer that the presence of saponins, tannins and other hypolipidemic phytocomponents such as flavonoids in the extract could account for its bioactivity.
Our preliminary toxicity (acute and chronic) study of M. charantia (although not published) revealed that; the oral administration of the plant extract at different doses (up to 12,000 mg/kg body weight) for 14 days did not cause any signs of toxicity, morbidity or mortality in the experimental rats. Toxicological evaluation involving sub-acute, acute and chronic studies are often employed for the evaluation of the undesirable side effects of exposure to medicinal plants over a period of time [10]. These investigations provide useful information on target organ toxicity and can be used to determine appropriate dose regimens for longer term investigations in humans [11]. It has been reported that significant changes in body weight gain and internal organ weights are direct and sensitive indices of toxicity after exposure to a toxic substance [12,13,14]. We did not observe any significant changes in the behavior of the test animals, as well as their food consumption habits. The body weight changes as well as relative organ weights of the experimental rats (both male and female) did not differ significantly from those of the control rats. It may therefore be appropriate to infer that M. charantia is relatively safe with respect to its use in ethnomedicine. M. charantia is widely used in ethnomedicine; however, studies investigating its biological properties and mechanisms of action are still insufficient. Different therapeutic indications of the plant in ethnomedicine are found in literature, especially its pharmacological activities in the control of diabetes, viral infections and cancer [15]. M. charantia is a medicinal herb which possesses various bioactive compounds with health benefits [16].
Li et al. in 1994 and Verschuren et al. in 1995 [17, 18] reported a linear correlation between dietary cholesterol intake and mortality from coronary heart disease (CHD). It has also been shown that the administration of dietary cholesterol speeds up the formation of aortic plaques in the apolipoprotein E null mutant mouse [19]. Results of our present study, which focused on the cardiovascular benefits of the plant (M. charantia), shows that the mean total cholesterol, triglyceride and high-density lipoproteins (HDL) of the experimental rats were not significantly affected in all the test groups as compared to the control. It can therefore be inferred that the administration of the extracts and the standard drug (atorvastatin) had similar effect with respect to the reduction of the lipids in the test animals. Previous studies have shown that in mice fed high fat diet (HFD), administration of freeze-dried M. charantia juice was found to reduce adiposity and it was reported that extracts from this plant may reduce visceral obesity [20, 21]. Our findings also show that the mean low-density lipoproteins were significantly lower in the groups given LDMC, cholesterol plus HDMC and coconut oil only as compared with the control group. Low-density lipoprotein is the most important risk factor for development of atherosclerosis and cardiovascular diseases in humans and animals [22,23,24]. Our results portend the cardiovascular benefit of the extract as reduction in low-density lipoproteins correlates with decreased risk of cardiovascular diseases. Willeck et al. in 2015 [22] reported observable increases in total cholesterol, triglyceride and LDL-cholesterol, with corresponding decrease in HDL- cholesterol in cholesterol-induced hyperlipidemic rats as compared to the control rats. Pre-treatment with M. charantia resulted in reduced LDL-cholesterol with concomitant increase in HDL-cholesterol. Decrease in LDL-cholesterol and increase in HDL-cholesterol in M. charantia–treated group may be due to inhibition of hepatic cholesterol biosynthesis, increased fecal bile acid secretion, stimulation of receptor-mediated catabolism of LDL-cholesterol, or increase in uptake of LDL-cholesterol from the blood by the liver [25]. These activities may be due to the presence of active phytochemicals with lipid lowering properties in M. charantia. This may also be true since chronic administration of the extract did not significantly alter the total food intake of the treated animals. This suggests that the anti-hyperlipidaemic effects of the extract were not mediated through inhibition of appetite.
Histological examination has been described as the golden standard for evaluating treatments related to pathological changes in tissues and organs [26]. Histological analysis of the heart and aortic branch of the experimental rats show that cholesterol administration induced myocardial degeneration, vascular ulceration and stenosis in the aorta and heavy perivascular infiltrates of inflammatory cells. However, there was amelioration of these deleterious effects to a very appreciable degree or a near normal condition relatively, upon treatment with Atorvastatin and M. charantia, as compared with the control groups. These, among others, also suggest a similar activity between the extract and the standard drug. Our results also support the report that dietary cholesterol contributes significantly to elevation of plasma cholesterol. This has been demonstrated to increase serum and aortic tissue cholesterol and as such predisposes to aortic atherosclerosis or cardiovascular diseases [27, 28].
Conclusion
Our study shows that the extract of M. charantia exhibited hypolipidemic properties, as well as restored and maintained the functional and structural integrity of the tissues of the heart and aorta. It ameliorated the degenerative changes in the myocardium and aortic tissues. The lowering of LDL-cholesterol level and improvement in the histological features of the aorta portend the cardio-protective effect or cardiovascular benefits of M. charantia. However, more detailed and relevant studies are required to give scientific justification to these benefits.
Availability of data and materials
Not applicable.
References
Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease, professional edition e-book. In: Elsevier health sciences. 10th ed. New York: Saunders; 2016.
Braunwald E, Zipes D, Libby P. Heart disease. 6th ed: WB Saunders; 2001. p. 1301–8.
World Health Organization. Regulatory situation of herbal medicines: a worldwide review. Geneva: World Health Organisation; 2013.
Gupta S, Raychaudhuri B, Banerjee S, Das B, Mukhopadhaya S, Datta SC. Momordicatin purified from fruits of Momordica charantia is effective to act as a potent antileishmania agent. Parasitol Int. 2010;59(2):192–7. https://doi.org/10.1016/j.parint.2010.01.004.
Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54(4):275–87. https://doi.org/10.1007/BF01234480.
Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. London: Churchill Livingstone; 2008.
Bancroft JD, Stevens A. Bancroft’s theory and practice of histological Technques. 7th ed. London: Churchill Livingstone; 1977.
Mallory FB. A contribution to staining methods: A differential stain for connective- tissue fibrillae and reticulum.II. Chloride of iron haematoxylin for nuclei and fibrin III. Phosphotungstic acid haematoxylin for neuroglia fibres. J Exp Med. 1900;5(1):15.
Ukwuani AN, Abubakar MG, Hassan SW, Agaie BM. Toxicological studies of hydromethanol leaves extract of Grewia crenata. Int J Pharm Sci Drug Res. 2012;4(4):245–9.
National Research Council. Toxicity testing for assessing environmental agents. Interim Report. Washington DC: National Academic Press; 2006.
Yuet PK, Darah I, Chen Y, Sreeramanan S, Sasidharan S. Acute and sub-chronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMedical Res Int. 2013. https://doi.org/10.1155/2013/182064.
Raza M, Al-Shabanah OA, El-Hadiya TM, Al-Majed AA. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm. 2002;70(2):135–45. https://doi.org/10.3797/scipharm.aut-02-16.
Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of d-methylphenidate and d, l-methylphenidate in Sprague–Dawley rats. Toxicology. 2002;179(3):183–96. https://doi.org/10.1016/S0300-483X(02)00338-4.
Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N. Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J Ethnopharmacol. 2003;89(1):115–21. https://doi.org/10.1016/S0378-8741(03)00273-3.
Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004;93(1):123–32. https://doi.org/10.1016/j.jep.2004.03.035.
Ruda-Kucerova J, Kotolova H, Koupy D. Effectiveness of phytotherapy in supportive treatment of type 2 diabetes mellitus III. Momordica (Momordica charantia). Ceska Slov Farm. 2015;64(4):126–32.
Li N, Tuomilehto J, Dowse G, Virtala E, Zimmet P. Prevalence of coronary heart disease indicated by electrocardiogram abnormalities and risk factors in developing countries. J Clin Epidemiol. 1994;47(6):599–611. https://doi.org/10.1016/0895-4356(94)90208-9.
Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures: twenty-five—year follow-up of the seven countries study. J Am Med Assoc. 1995;274(2):131–6. https://doi.org/10.1001/jama.1995.03530020049031.
Yuan YV, Kitts DD, Godin DV. Influence of dietary cholesterol and fat source on atherosclerosis in the Japanese quail (Coturnix japonica). Br J Nutr. 1997;78(6):993–1014. https://doi.org/10.1079/BJN19970214.
Alam MA, Uddin R, Subhan N, Rahman MM, Jain P, Reza HM. Beneficial role of bitter melon supplementation in obesity and related complications in metabolic syndrome. J Lipids. 2015;2015:1–18. https://doi.org/10.1155/2015/496169.
Mahmoud MF, El-Ashry FZ, El Maraghy NN, Fahmy A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm Biol. 2017;55(1):758–65. https://doi.org/10.1080/13880209.2016.1275026.
Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, et al. Effects of high fat feeding and diabetes on regression of atherosclerosis induced by low-density lipoprotein receptor gene therapy in LDL receptor-deficient mice. PLoS One. 2015;10(6):e0128996. https://doi.org/10.1371/journal.pone.0128996.
Silverman SAM, Janssen DL. Diagnostic imaging. Reptile Med Surg. 2006;2:471–89.
Brunkwall J, Mattsson E, Bergqvist D. Diet-induced atherosclerosis in rabbits alters vascular prostacyclin release. Eicosanoids. 1992;5(3–4):197–202.
Khanna AK, Chander R, Kapoor NK. Terminalia arjuna: an ayurvedic cardiotonic, regulates lipid metabolism in hyperlipaemic rats. Phytother Res. 1996;10(8):663–5. https://doi.org/10.1002/(SICI)1099-1573(199612)10:8<663::AID-PTR935>3.0.CO;2-W.
OECD. Repeated Dose 28-day oral toxicity study in Rodents, OECD guideline for testing of chemicals, vol. 407; 1995. p. 1–8.
Lofdand HB, Clarkson TB, Goodman HO. Interactions among dietary fat, protein, and cholesterol in atherosclerosis-susceptible pigeons: effects on serum cholesterol and aortic atherosclerosis. Circ Res. 1961;9(4):919–24. https://doi.org/10.1161/01.RES.9.4.919.
Adaramoye OA, Nwaneri VO, Anyanwu KC, Farombi EO, Emerole GO. Possible anti-atherogenic effect of kolaviron (a Garcinia kola seed extract) in hypercholesterolaemic rats. Clin Exp Pharmacol Physiol. 2005;32(1–2):40–6. https://doi.org/10.1111/j.1440-1681.2005.04146.x.
Acknowledgements
The Authors acknowledge the Department of Anatomy, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, (Edo State, Nigeria) for providing the necessary laboratory facilities.
Funding
This research did not receive any funding from any institution or organisation.
Author information
Authors and Affiliations
Contributions
SOI and IGE conceived, designed and performed the experiments; SOI, IGE and KO performed the analysis and interpretation of the data, as well as preparation of the draft of the manuscript. All authors have reviewed and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental procedures and care of the animals were in accordance with the institution’s (School of Basic Medical Sciences, University of Benin, Nigeria) animal and ethics guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare there are no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Innih, S.O., Eze, I.G. & Omage, K. Cardiovascular benefits of Momordica charantia in cholesterol-fed Wistar rats. Clin Phytosci 7, 65 (2021). https://doi.org/10.1186/s40816-021-00303-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-021-00303-2