Abstract
Background
At present, even the first-line medication epinephrine still shows no evidence of a favourable neurological outcome in patients with sudden cardiac arrest (SCA). The high mortality of patients with postcardiac arrest syndrome (PCAS) can be attributed to brain injury, myocardial dysfunction, systemic ischaemia/reperfusion response, and persistent precipitating pathology. Targeted temperature management, the only clinically proven method in the treatment of PCAS, is still associated with a series of problems that have not been completely resolved.
Acupuncture is a crucial therapy in traditional Chinese medicine. On the basis of the results of previous studies, we hypothesize that electroacupuncture (EA) might provide therapeutic benefits in the treatment of PCAS. This study will explore the feasibility of EA on SCA patients.
Methods
This is a prospective pilot, randomized controlled clinical trial. Eligible patients with PCAS after in-hospital cardiac arrest (IHCA) admitted to our department will be randomly allocated to the control group or the EA group. Both groups will receive standard therapy according to American Heart Association guidelines for cardiopulmonary resuscitation. However, the EA group will also receive acupuncture at the Baihui acupoint (GV20) and Zusanli acupoint (ST36) with EA stimulation for 30 min using a dense-dispersed wave at frequencies of 20 and 100 Hz, a current intensity of less than 10 mA, and a pulse width of 0.5 ms. EA treatment will be administered for up to 14 days (until either discharge or death). The primary endpoint is survival with a favourable neurological outcome. The secondary endpoints are neurological scores, cardiac function parameters, and other clinical parameters, including Sequential Organ Failure Assessment (SOFA) scores and Acute Physiology and Chronic Health Evaluation (APACHE) II scores, on days 0 to 28.
Discussion
This study will provide crucial clinical evidence on the efficacy of EA in PCAS when used as an adjunctive treatment with AHA standard therapy.
Trial registration
chictr.org.cn: ChiCTR2000040040. Registered on 19 November 2020. Retrospectively registered. http://www.chictr.org.cn/.
Similar content being viewed by others
Background
Sudden cardiac arrest (SCA) is a significant cause of death worldwide, and more than 550,000 deaths due to SCA occur annually in the USA and Europe [1]. A recent study showed that epinephrine, the first-line medication for patients with SCA, was associated with more severe neurological impairment in survivors than placebo in adults with out-of-hospital cardiac arrest (OHCA) [2]. In addition, SCA patients will have sepsis-like changes, which may be caused by ischaemia, hypoxia, and reperfusion injury, after the return of spontaneous circulation (ROSC), resulting in multiple organ dysfunction syndromes (MODSs) and a poor prognosis,. This series of reactions is called postcardiac arrest syndrome (PCAS) [3]. According to studies performed in the USA, Europe, and Asia, whether in adults, children, or infants, the mortality rate of PCAS patients is 65–80% [4,5,6,7,8]. The high mortality rate can be attributed to a unique pathophysiological process. The special features of this unique pathophysiological process of PCAS are often superimposed on the disease or injury that caused the cardiac arrest and underlying comorbidities [3]. There are four critical components of PCAS: brain injury [9, 10], myocardial dysfunction [11, 12], systemic ischaemia/reperfusion response [13], and persistent precipitating pathology [14]. Therefore, therapies focusing on a single organ may further damage other damaged organ systems. Targeted temperature management (TTM) is currently the only clinically proven method to improve SCA patients’ survival and neurological outcomes after the ROSC [15, 16]; however, TTM is still associated with a series of problems that have not been completely resolved [17].
Acupuncture is a crucial therapy in traditional Chinese medicine (TCM). Acupuncture involves the insertion of fine needles at “acupoints”, followed by stimulation via manual or mechanical techniques [18]. It has been reported that acupuncture may have a bidirectional regulating effect and an anti-systemic inflammatory response effect [19, 20]. The acupuncture effect of balancing the autonomic nervous system has been demonstrated to inhibit neuronal apoptosis and reduce oxidative stress [21,22,23]. These effects are in line with the critical components of PCAS mentioned above.
Nevertheless, the effects of acupuncture on PCAS have never been investigated comprehensively. Therefore, the present pilot, randomized, controlled study was designed to explore the feasibility of electroacupuncture (EA) on PCAS patients and furthermore evaluate the impact in the main trial.
The specific objectives of this pilot study were to test the trial procedures, explore the feasibility of the program, and provide data for recruitment, follow-up rates, and sample size calculation for the main trial.
Method
Aim
The study is a prospective randomized controlled pilot trial designed to evaluate whether EA can improve survival with a favorable neurological outcome of PCAS after in-hospital cardiac arrest (IHCA).
Design
This study is a prospective randomized controlled pilot clinical trial that will be carried out in the Emergency Department of Guangdong Provincial Hospital of Chinese Medicine and Intensive Care Unit of Fangcun Branch Hospital of Guangdong Provincial Hospital of Chinese Medicine. This trial is embedded with a study of survival with a favourable neurological outcome of PCAS following IHCA.
The trial received ethics approval from the Guangdong Provincial Hospital of Chinese Medicine Institutional Review Board (approval number ZF2020-051-01). If any changes are made to this protocol, a draft of the new version must be submitted for approval by the Institutional Review Board of Guangdong Provincial Hospital of Chinese Medicine. This clinical trial will conform to the national laws and the principles of the Declaration of Helsinki. The eligible participants’ authorized representatives will sign informed consent papers before the participants are enrolled.
The protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Additional file 1).
Participant selection criteria
Patients aged 18 to 85 years who suffer SCA in the hospital caused by respiratory failure or hypovolemic shock and with a ROSC sustained for ≥ 20 min and met the following criteria at the same time are eligible to enrol in this study: Glasgow coma score ≤ 8 after ROSC, and before sedation (if any); the legal representative signed the informed consent; advanced cardiovascular life support is conducted. Those who meet the exclusion criteria (listed in Table 1) will not be enrolled in this study.
Participants can withdraw from the trial for any reason at any time. Participants for whom, upon regaining consciousness (if any), the legal representative or patient requests study withdrawal may be removed from the study:
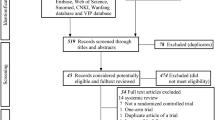
The flow chart of the study is presented in Fig. 1.
Study flow chart. CA, cardiac arrest; ROSC, return of spontaneous circulation; BLS, basic life support; CPR, cardiopulmonary resuscitation; ACLS, advanced cardiovascular life support; ICU, intensive care unit; EA, electroacupuncture; CPC, cerebral performance category; GCS, Glasgow Coma Scale; CT, computed tomography; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation
Randomization
Eligible patients enrolled in this trial will be randomly assigned to the EA or control groups in a 1:1 ratio. Randomization assignment will be carried out by a researcher (Fang Lai) not involved in the treatment and assessment. This randomization will be run with a block randomization size of four by SPSS 17.0. The randomization block size will be concealed to guarantee rigorous methodology, and the allocation number will be sequentially assigned and stored. The unique code assigned to each newly eligible participant will be adequately preserved throughout this study. This procedure will ensure that each eligible patient could be assigned to either of the two groups with equal probability and that group assignment will not be influenced by the researchers. Decai Zhu and Baijian Chen will enrol and assign participants to interventions according to the preprepared allocation sequence. Considering that an included patient could withdraw from the study, their randomization number would be invalidated, so we will ultimately take the first 52–60 patients. Finally, the number of patients in these two groups may be different because of the actual procedures performed.
Blinding and masking
Since this study involves acupuncture, it is challenging to achieve double blindness. However, to avoid bias in this process, the following specific measures are proposed:
-
1.
Participants will be recruited in strict accordance with the principle of randomization.
-
2.
The physicians participating in the outcome evaluation will be nonresearchers, making the trial efficacy evaluation blinded.
-
3.
The laboratory technicians and the biostatisticians will be blinded to the assigned treatments.
Sample size determination
Since there is no previous clinical study on EA to improve the survival of patients with PCAS and given this trial's pilot nature, we will follow the recommendation of 20 participants or more to achieve sufficient precision based on the sample size calculation of a previous study [24]. We plan to recruit 50 participants since a 20% dropout rate is expected.
Interventions and procedures
Eligible patients for whom consent is provided by the legal representative or the patient themselves will receive EA. In addition, participants will receive standard therapy according to American Heart Association guidelines for CPR recommendations [14, 25,26,27], while any other adjunct treatment will be prohibited. Investigators will be trained before the start of the study. Fang Lai, who will not be involved in participants’ enrolment or data analysis, will monitor the outcomes of the interventions at least once per month during the trial.
Standard therapy
Standard therapy will be based on current recommendations [14, 25,26,27], including basic life support (BLS), advanced cardiovascular life support (ACLS), and postcardiac arrest care. BLS includes immediate recognition of SCA, EMS activation, early CPR, and rapid defibrillation. ACLS consists of airway control and ventilation, antiarrhythmic drugs and vasopressors during and immediately after SCA, and extracorporeal CPR. Postcardiac arrest care is considered coronary angiography, which will be performed in an emergency setting. Comatose adult patients with an ROSC after SCA will receive TTM; fever will be actively prevented in these comatose patients after TTM, and other standard critical care interventions will be administered.
EA treatment
Disposable stainless steel acupuncture needles will be inserted at the Baihui acupoint (GV20, located at the intersection of the sagittal midline and the line linking the ears) and unilaterally on the side of the left leg at the Zusanli acupoint (ST36, situated on the front to one side of the leg, 3 cun below the acupoint ST35 and a cross-finger (middle finger) away from the leading edge of the tibia) to a depth of approximately 0.5 cm [28]. Furthermore, an EA instrument (Suzhou Medical Supplies Factory Co., Ltd., Jiangsu, China) will be connected to perform EA stimulation for 30 min using a dense-dispersed wave at frequencies of 20 and 100 Hz. The current intensity will be less than 10 mA (adjusted to induce a slight muscle twitch), and the pulse width will be 0.5 ms. One of the two electrodes of the EA stimulator will be connected to the needle at GV20, and the other electrode will be connected to the needle at ST36. This EA intervention will begin immediately after the ROSC for 20 min and will continue for up to 14 days, discharge, or death, whichever comes first. The EA will be performed by Decai Zhu, Baijian Chen, or Wei Huang, who will conduct the research and have previously received equal training.
Principles of the formula
GV20 is located at the top of the apex, which is the intersection of three yang meridians of the hand, three yang meridians of the foot, and the governor vessel. At the same time, the head is the meeting of yang and the ancestor of meridian vessels. The functional brain injury after PCAS is located in the head, and the pathogenesis is yin-yang disharmony, the reversal of qi and blood reversal. Therefore, the choice of acupuncture at GV20, where vessels and qi converge, plays an essential role in regulating the body’s yin-yang balance [29, 30].
ST36 is the acupoint of the stomach meridian (ST), the sea point in the five transport points. The stomach is the sea of water and food and the source of qi and blood engendering transformation. It has an exterior and interior relationship with the spleen meridian (SP), and the spleen and stomach are the roots of acquisition. “Suwen” indicated that “the treatment of wilting disease takes yang brightness meridian alone.” Acupuncture at ST36 can stimulate the qi of the viscera and bowels, encourage the movement of qi and blood, and dredge the block of the meridian vessel [31].
EA at GV20 and ST36 can restore yang to prevent collapse and open the orifices. According to long-term experience, acupuncture used in our trials is considered safe for patients who suffer from SCA. However, according to modern clinical research principles, it has been criticized for a lack of adequate assessment. Therefore, we designed this observational trial to evaluate the efficacy of EA.
Outcome measures
The primary feasibility outcomes are as follows:
-
Evaluate the acceptability and compliance of EA in PCAS as an additional intervention of usual care.
-
Explore the feasibility of recruiting, randomizing, and retaining participants.
-
Evaluate outcome measures’ appropriateness.
-
Collect data for effect size calculation in future sample size calculating.
-
Develop an appropriate protocol for further study.
The length of time for recruitment of 50 eligible patients, recruitment rate and dropout rate will be measured. Successful recruitment is defined as at least half (50%) of eligible patients enrolled, with a dropout rate of no more than 20%.
The secondary patient-centred outcomes include the following:
-
We define a favourable neurological outcome as a cerebral performance category (CPC) score of 1 or 2 on the 28th day after the ROSC. We define an ROSC as a spontaneous pulse and blood pressure, an abrupt sustained increase in end-tidal CO2 partial pressure (PETCO2) (typically ≥ 40 mmHg), or spontaneous arterial pressure waves with intra-arterial monitoring.
-
Neurological scores: Glasgow Coma Scale (GCS) score, CPC score, modified Rankin Scale (mRS) score, and cranial computed tomography (CT) findings;
-
Evaluation of cardiac function: troponin and lactic acid levels and echocardiography parameters.
-
Sequential Organ Failure Assessment (SOFA) scores
-
Acute Physiology and Chronic Health Evaluation (APACHE) II scores
-
Length of stay in the ICU, length of hospital stay, hospital mortality/discharge, and hospital costs
-
Adverse events
Data acquisition and biological parameter assessment
The recruited patients in this study will receive a participant ID, which will be labelled in the chart containing the personal information of the participants. Only investigators involved in this study will have the right to access the participant IDs on an as-needed basis.
The following data will be collected from all participants:
-
Clinical, laboratory, and imaging-study findings on day 0, day 3, day 7, day 14, and day 28 (if available)
-
Emergency medical service (EMS) record, ICU mortality, ICU length of stay, hospital mortality, hospital length of stay, 28-day all-cause mortality, and inpatient cost
-
Details on the decision-making process
If patients are discharged early, investigators will call them back to the hospital as outpatients for free data acquisition and examinations.
The clinical parameters will include age, sex, cause of SCA, comorbidities, APACHE II scores, and SOFA scores. The EMS record contains the time of the call for EMS, the time of arrival, the initial cardiac rhythm, and management information. The data above will be collected on the day of enrolment and 28 days after registration. The schedule of the trial is shown in Fig. 2.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) timeline of measurements. Laboratory examinations include tests for inflammation parameters (C-reactive protein and procalcitonin), complete blood counts, general urine analyses, liver function tests (ALT, AST, ALP, TBIL, and GGT), renal function tests (BUN, Scr), and tests to measure troponin and lactic acid
Routine laboratory examinations (complete blood counts (CBCs), C-reactive protein (CRP) and procalcitonin (PCT) tests, liver function tests (ALT, AST, ALP, TBIL, and GGT), and renal function tests (BUN and Scr)) will be performed by the clinical laboratory of Guangdong Provincial Hospital of Chinese Medicine.
Investigators will be trained to collect trial data according to the standard protocol. Data will ultimately be input into the Clinical Trial Management Public Platform, ResMan, designed for clinical trials. Double data entry will be performed by two researchers independently and will be checked by the third data clerk separately. All collected forms will be kept in the scientific research department of Guangdong Provincial Hospital of Chinese Medicine. Investigators will be trained to collect trial data according to the standard protocol.
Any adverse event that occurs in participants, regardless of whether it is related to the EA, will be recorded from recruitment to the 28th day. Severe adverse events and unexpected adverse events will be reported to the Ethics Committee within 2 days. EA will be suspended, and symptomatic treatment will be offered when needed. The principal investigator will decide whether the participants should discontinue the trial.
Both data and safety will be monitored by the Institutional Review Board and the scientific research department of Guangdong Provincial Hospital of Chinese Medicine, independent of the sponsor, with no competing interests.
Statistical analysis
The data will be analysed statistically according to the method of a previous study [32]. Enrolled participants who cannot continue the study or follow up after treatment will remain in an “intention to treat” analysis. All statistical analyses will be performed with SPSS (version 17.0, Chicago, USA) for Windows by researchers not implementing the intervention. The analysis of this trial will be mainly descriptive focusing on estimation rather than hypothesis testing. All baseline outcomes will be summarized for each group using the appropriate descriptive statistics. No formal comparison of groups will take place. A full statistical analysis plan will be developed prior to the final analysis of the trial.
Discussion
In addition to high mortality, SCA remains a cause of disability in survivors [17]. Hypothermia has been applied for the treatment of SCA from the 1950s to the present. Hypothermia has been shown to improve neurological outcomes and survival in patients with severe ischaemia–reperfusion brain injury after the ROSC [6, 33,34,35]. Animal studies have shown that the mechanism underlying the effect of mild hypothermia on PCAS is complicated and that it does not merely involve a decline in brain metabolism [36, 37]. Furthermore, TTM is currently the only clinically proven way to improve neurological outcomes and survival after the ROSC. However, TTM is still associated with a series of problems that have not been completely resolved.
Retrospective studies have shown that for IHCA, the earlier TTM is implemented, the higher the proportion of favourable neurological outcomes, and this trend is also seen in the survival rate [38, 39]. Because of the low quality of evidence, the data showing that the effects of prehospital induction of cooling are superior to those of in-hospital initiation of cooling are not convincing. Moreover, prehospital cooling might result in higher rearrest rates after the ROSC [40, 41]. The delay of mild hypothermia in retrospective studies may be related to problems such as a lack of simplified procedures and experience in PCAS, hemodynamic instability, recurrence, and coronary angiography. Therefore, whether early initiation of mild hypothermia can result in a favourable neurological outcome and induce hypothermia remains controversial.
Acupuncture is an integral part of TCM. Many documents show that it was the primary means of treating emergencies such as SCA in ancient times. Whether acupuncture can be used as a new technique in CPR to improve survival with a favourable outcome is worthy of in-depth study.
Studies have shown that after EA, oligodendrocyte regeneration increases [42, 43], motor and memory function improves [44, 45], and cognitive impairment is ameliorated [46]. The role of EA is related to the upregulated expression of neurotrophin-4/5-tyrosine receptor kinase B (NT4/5-TrkB) signalling [42], cAMP response element-binding protein/brain-derived neurotrophic factor (CREB/BDNF) signalling [44, 45, 47, 48], stromal cell-derived factor-1α (SDF-1α) [49], B cell lymphoma-2 (Bcl-2) and B cell lymphoma-2 related X protein (Bax) [46, 50]. Animal studies have shown that acupuncture at GV20 and ST36 can significantly reduce blood–brain barrier permeability and brain oedema, and the effect of acupuncture may inhibit the expression of phosphorylated caveolin-1 in endothelial cells [51]; downregulate reactive oxygen species (ROS) and NADPH oxidase type 4 (NOX4) [52]; and upregulate matrix metalloproteinase-2 (MMP2), aquaporin-4 (AQP4) and aquaporin-9 (AQP9) [53]. It was found in a middle cerebral artery occlusion (MCAO) rat model study that EA could inhibit neuronal apoptosis, impede the activation of microglia and inhibit inflammatory responses [54]. Acupuncture may involve specific effects on the protease A (PKA)/CREB pathway [23] and erythropoietin (EPO)-mediated Janus family tyrosine kinase 2 (JAK2) signal transduction and transcription activator 3 (STAT3) cell pathway [22], reduce S100B-mediated neurotoxicity [55] and finally achieve anti-apoptosis. In addition, anti-ischaemic apoptosis may be obtained through the downregulation of the oxidative stress response, which is associated with a decrease in tumour necrosis factor α (TNF-α), interleukin 6 (IL-6), neuron-specific enolase (NSE), malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) in the serum and hippocampus [56]. Furthermore, EA reduced the infarct area and increased the ejection fraction (EF) by inhibiting the expression of NLR family pyrin domain containing 3 (NLRP3) and AMP-activated protein kinase (AMPK)-dependent autophagy in an animal study of an acute myocardial infarction rat model [57, 58].
Our decision to use an EA in PCAS is based on the results of our retrospective study published in Chinese, in which we found that EA attenuates the multiorgan failure of patients with PCAS [59], and those of a systematic review indicating that acupuncture ameliorates the injuries induced by experimental sepsis [60]. Whether EA is associated with mortality or a favourable neurological outcome after PACS is unclear. However, this trial is the first registered interventional and experimental study evaluating the potential benefits of EA on the neurological outcome of PCAS. The results of this trial might establish the importance of EA as a promising complementary strategy for the treatment of PCAS.
This study is the first study of EA for the treatment of SCA. The initial design was to consider SCA due to all causes; however, the ethics committee suggested that a pilot study in a focused area was needed to clarify the feasibility and safety of EA. The next step will be to expand the research. Therefore, there are several limitations to this trial. First, this is a pilot study with a small sample size and no blinding of the recruited participants. Second, the causes of SCA vary, and EA may be useful only for some causes of SCA, which could ultimately lead to bias; therefore, matched control and subgroup analyses are needed. Third, the treatment strategy received by ICU patients is highly complicated; therefore, the results of this trial might not be adequately explained solely by the therapeutic effect of EA. Fourth, as stated throughout this paper, this was a pilot study and no sample size calculation was performed; this was not powered for hypothesis testing of clinical outcomes. The clinical results will be treated as preliminary and interpreted with caution.
Trial status
We are currently recruiting participants.
Availability of data and materials
The ethical, consent, and funding documents of this study are available for review by the Editorial Office. Ruifeng Zeng and Banghan Ding are responsible for the data and will have the final dataset. Trial results will be reported to the Guangzhou Science and Technology Department for public publication. Additionally, the investigators plan to publish the results in journals. Participants can reach out for information on the study progress and related data.
Abbreviations
- SCA:
-
Sudden cardiac arrest
- ROSC:
-
Return of spontaneous circulation
- MODS:
-
Multiple organ dysfunction syndrome
- PCAS:
-
Postcardiac arrest syndrome
- TTM:
-
Targeted temperature management
- TCM:
-
Traditional Chinese medicine
- CPR:
-
Cardiopulmonary resuscitation
- ACLS:
-
Advanced cardiovascular life support
- OHCA:
-
Out-of-hospital cardiac arrest
- IHCA:
-
In-hospital cardiac arrest
- ICU:
-
Intensive care unit
- CCF:
-
Chest compression fraction
- EA:
-
Electroacupuncture
- BLS:
-
Basic life support
- CPC:
-
Cerebral performance category
- GCS:
-
Glasgow Coma Scale
- mRS:
-
Modified Rankin scale
- CT:
-
Computed tomography
- SOFA:
-
Sequential Organ Failure Assessment
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- EMS:
-
Emergency medical service
- CBC:
-
Complete blood count
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- NT4/5-TrkB:
-
Neurotrophin-4/5-tyrosine receptor kinase B
- CREB:
-
cAMP response element binding protein
- BDNF:
-
Brain-derived neurotrophic factor
- SDF-1α:
-
Stromal cell-derived factor-1α
- Bcl-2:
-
B cell lymphoma-2
- Bax:
-
B cell lymphoma-2-related X protein
- ROS:
-
Reactive oxygen species
- NOX4:
-
NADPH oxidase type 4
- MMP2:
-
Matrix metalloproteinase-2
- AQP:
-
Aquaporin
- MCAO:
-
Middle cerebral artery occlusion
- PKA:
-
Protease A
- EPO:
-
Erythropoietin
- JAK2:
-
Janus family tyrosine kinase 2
- STAT3:
-
Signal transduction and transcription activator 3
- TNF-α:
-
Tumor necrosis factor α
- IL-6:
-
Interleukin 6
- NSE:
-
Neuron-specific enolase
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- EF:
-
Ejection fraction
- NLRP3:
-
NLR family pyrin domain containing 3
- AMPK:
-
AMP-activated protein kinase
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
References
Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133:447–54. https://doi.org/10.1161/cir.0000000000000366.
Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379:711–21. https://doi.org/10.1056/NEJMoa1806842.
Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. https://doi.org/10.1161/circulationaha.108.190652.
Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–22. https://doi.org/10.1016/j.annemergmed.2005.05.028.
Herlitz J, Engdahl J, Svensson L, et al. Major differences in 1-month survival between hospitals in Sweden among initial survivors of out-of-hospital cardiac arrest. Resuscitation. 2006;70:404–9. https://doi.org/10.1016/j.resuscitation.2006.01.014.
Mashiko K, Otsuka T, Shimazaki S, et al. An outcome study of out-of-hospital cardiac arrest using the Utstein template--a Japanese experience. Resuscitation. 2002;55:241–6. https://doi.org/10.1016/s0300-9572(02)00207-1.
Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–7. https://doi.org/10.1001/jama.295.1.50.
Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–56. https://doi.org/10.1056/NEJMoa040325.
Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38:674–6. https://doi.org/10.1161/01.Str.0000256294.46009.29.
Laver S, Farrow C, Turner D, et al. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–8. https://doi.org/10.1007/s00134-004-2425-z.
Anderson RJ, Jinadasa SP, Hsu L, et al. Shock subtypes by left ventricular ejection fraction following out-of-hospital cardiac arrest. Crit Care. 2018;22:162. https://doi.org/10.1186/s13054-018-2078-x.
Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–6. https://doi.org/10.1016/s0735-1097(02)02594-9.
Adrie C, Laurent I, Monchi M, et al. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–12.
Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S465–82. https://doi.org/10.1161/cir.0000000000000262.
Bray JE, Stub D, Bloom JE, et al. Changing target temperature from 33 degrees C to 36 degrees C in the ICU management of out-of-hospital cardiac arrest: A before and after study. Resuscitation. 2017;113:39–43. https://doi.org/10.1016/j.resuscitation.2017.01.016.
Salter R, Bailey M, Bellomo R, et al. Changes in Temperature Management of Cardiac Arrest Patients Following Publication of the Target Temperature Management Trial. Crit Care Med. 2018;46:1722–30. https://doi.org/10.1097/ccm.0000000000003339.
Lascarrou JB, Meziani F, Le Gouge A, et al. Therapeutic hypothermia after nonshockable cardiac arrest: the HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial. Scand J Trauma Resusc Emerg Med. 2015;23:26. https://doi.org/10.1186/s13049-015-0103-5.
Vickers A, Zollman C. ABC of complementary medicine. Acupuncture. BMJ. 1999;319:973–6. https://doi.org/10.1136/bmj.319.7215.973.
Hu L, Klein JD, Hassounah F, et al. Low-frequency electrical stimulation attenuates muscle atrophy in CKD--a potential treatment strategy. J Am Soc Nephrol. 2015;26:626–35. https://doi.org/10.1681/asn.2014020144.
Torres-Rosas R, Yehia G, Pena G, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–5. https://doi.org/10.1038/nm.3479.
Cheng CY, Lin JG, Tang NY, et al. Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complement Altern Med. 2015;15:241. https://doi.org/10.1186/s12906-015-0752-y.
Xu H, Zhang YM, Sun H, et al. Electroacupuncture at GV20 and ST36 Exerts Neuroprotective Effects via the EPO-Mediated JAK2/STAT3 Pathway in Cerebral Ischemic Rats. Evid Based Complement Alternat Med. 2017;2017. https://doi.org/10.1155/2017/6027421.
Zheng CX, Lu M, Guo YB, et al. Electroacupuncture ameliorates learning and memory and improves synaptic plasticity via activation of the PKA/CREB signaling pathway in cerebral hypoperfusion. Evid Based Complement Alternat Med. 2016;2016:7893710. https://doi.org/10.1155/2016/7893710.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–12. https://doi.org/10.1111/j.2002.384.doc.x.
Kleinman ME, Brennan EE, Goldberger ZD, et al. Part 5: Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S414–35. https://doi.org/10.1161/cir.0000000000000259.
Link MS, Berkow LC, Kudenchuk PJ, et al. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S444–64. https://doi.org/10.1161/cir.0000000000000261.
Nolan JP, Berg RA, Andersen LW, et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: A Consensus Report From a Task Force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation. 2019;144:166–77. https://doi.org/10.1016/j.resuscitation.2019.08.021.
No authors are listed. A standard international acupuncture nomenclature: memorandum from a WHO meeting. Bull World Health Organ. 1990;68(2):165–9.
Lu L, Zhang XG, Zhong LL, et al. Acupuncture for neurogenesis in experimental ischemic stroke: a systematic review and meta-analysis. Sci Rep. 2016;6:19521. https://doi.org/10.1038/srep19521.
Wang WW, Xie CL, Lu L, et al. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep. 2014;4:3981. https://doi.org/10.1038/srep03981.
Jiang T, Wu M, Kong LH, et al. Effect of pre-acupuncture at Neiguan (PC 6) and Zusanli (ST 36) on exercise-induced fatigue. Zhongguo Zhen Jiu. 2019;39:1063–6. https://doi.org/10.13703/j.0255-2930.2019.10.008.
Zeng R, Zheng Y, Fan R, et al. Si-ni-tang (a Chinese herbal formula) for improving immunofunction in sepsis: study protocol for a pilot randomized controlled trial. Trials. 2019;20:537. https://doi.org/10.1186/s13063-019-3646-3.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. https://doi.org/10.1056/NEJMoa003289.
Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381:2327–37. https://doi.org/10.1056/NEJMoa1906661.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–206. https://doi.org/10.1056/NEJMoa1310519.
Grousset C, Menasche P, Apstein CS, et al. Protective effects of cardioplegia on diastolic function of hypertrophied rat hearts after hypothermic ischaemic arrest. Eur Heart J. 1984;5 Suppl F:347–53. https://doi.org/10.1093/eurheartj/5.suppl_f.347.
Prasad K, Bharadwaj B, Card RT. Effects of blood and crystalloid cardioplegia on cardiac function at organ and cellular levels during hypothermic cardiac arrest. Angiology. 1988;39:23–33. https://doi.org/10.1177/000331978803900104.
Lee BK, Jeung KW, Jung YH, et al. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation. 2017;113:135–41. https://doi.org/10.1016/j.resuscitation.2016.12.002.
Stanger D, Kawano T, Malhi N, et al. Door-to-targeted temperature management initiation time and outcomes in out-of-hospital cardiac arrest: insights from the continuous chest compressions trial. J Am Heart Assoc. 2019;8:e012001. https://doi.org/10.1161/jaha.119.012001.
Arrich J, Holzer M, Havel C, et al. Pre-hospital versus in-hospital initiation of cooling for survival and neuroprotection after out-of-hospital cardiac arrest. Cochrane Database Syst Rev. 2016;3:Cd010570. https://doi.org/10.1002/14651858.CD010570.pub2.
Diao M, Huang F, Guan J, et al. Prehospital therapeutic hypothermia after cardiac arrest: a systematic review and meta-analysis of randomized controlled trials. Resuscitation. 2013;84:1021–8. https://doi.org/10.1016/j.resuscitation.2013.02.003.
Ahn SM, Kim YR, Kim HN, et al. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci Rep. 2016;6:28646. https://doi.org/10.1038/srep28646.
Luo Y, Xu NG, Yi W, et al. Study on the correlation between synaptic reconstruction and astrocyte after ischemia and the influence of electroacupuncture on rats. Chinese J Integr Med. 2011;17:750–7. https://doi.org/10.1007/s11655-011-0754-7.
Kim MW, Chung YC, Jung HC, et al. Electroacupuncture enhances motor recovery performance with brain-derived neurotrophic factor expression in rats with cerebral infarction. Acupunct Med. 2012;30:222–6. https://doi.org/10.1136/acupmed-2011-010126.
Pak ME, Jung DH, Lee HJ, et al. Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Exp Neurol. 2018;300:222–31. https://doi.org/10.1016/j.expneurol.2017.11.014.
Yun YC, Jang D, Yoon SB, et al. Laser acupuncture exerts neuroprotective effects via regulation of Creb, Bdnf, Bcl-2, and Bax gene expressions in the hippocampus. Evid Based Complement Alternat Med. 2017;2017:7181637. https://doi.org/10.1155/2017/7181637.
Han X, Zhao X, Lu M, et al. Electroacupuncture ameliorates learning and memory via activation of the CREB signaling pathway in the hippocampus to attenuate apoptosis after cerebral hypoperfusion. Evid Based Complement Alternat Med. 2013;2013:156489. https://doi.org/10.1155/2013/156489.
Zhang Y, Lin R, Tao J, et al. Electroacupuncture improves cognitive ability following cerebral ischemia reperfusion injury via CaM-CaMKIV-CREB signaling in the rat hippocampus. Exp Ther Med. 2016;12:777–82. https://doi.org/10.3892/etm.2016.3428.
Kim JH, Choi KH, Jang YJ, et al. Electroacupuncture preconditioning reduces cerebral ischemic injury via BDNF and SDF-1alpha in mice. BMC Complement Altern Med. 2013;13:22. https://doi.org/10.1186/1472-6882-13-22.
Liu F, Jiang YJ, Zhao HJ, et al. Electroacupuncture ameliorates cognitive impairment and regulates the expression of apoptosis-related genes Bcl-2 and Bax in rats with cerebral ischaemia-reperfusion injury. Acupunct Med. 2015;33, 478:–484. https://doi.org/10.1136/acupmed-2014-010728.
Zou R, Wu Z, Cui S. Electroacupuncture pretreatment attenuates bloodbrain barrier disruption following cerebral ischemia/reperfusion. Mol Med Rep. 2015;12:2027–34. https://doi.org/10.3892/mmr.2015.3672.
Jung YS, Lee SW, Park JH, et al. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J Biomed Sci. 2016;23:32. https://doi.org/10.1186/s12929-016-0249-0.
Xu H, Zhang Y, Sun H, et al. Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS One. 2014;9:e97488. https://doi.org/10.1371/journal.pone.0097488.
Yuan S, Zhang X, Bo Y, et al. The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res. 2014;1593:19–29. https://doi.org/10.1016/j.brainres.2014.10.005.
Cheng CY, Lin JG, Tang NY, et al. Electroacupuncture-like stimulation at the Baihui (GV20) and Dazhui (GV14) acupoints protects rats against subacute-phase cerebral ischemia-reperfusion injuries by reducing S100B-mediated neurotoxicity. PLoS One. 2014;9:e91426. https://doi.org/10.1371/journal.pone.0091426.
Chen Y, Lei Y, Mo LQ, et al. Electroacupuncture pretreatment with different waveforms prevents brain injury in rats subjected to cecal ligation and puncture via inhibiting microglial activation, and attenuating inflammation, oxidative stress and apoptosis. Brain Res Bull. 2016;127:248–59. https://doi.org/10.1016/j.brainresbull.2016.10.009.
Zeng Q, He H, Wang XB, et al. Electroacupuncture Preconditioning Improves Myocardial Infarction Injury via Enhancing AMPK-Dependent Autophagy in Rats. Biomed Res Int. 2018;2018:1238175. https://doi.org/10.1155/2018/1238175.
Zhang T, Yang WX, Wang YL, et al. Electroacupuncture preconditioning attenuates acute myocardial ischemia injury through inhibiting NLRP3 inflammasome activation in mice. Life Sci. 2020;248:117451. https://doi.org/10.1016/j.lfs.2020.117451.
Zeng R, Ding B, Lai F, et al. Clinical observationof electroacupuncture at zusanli(ST36) on patients with post resuscitation syndrome. JETCM. 2018;27:1560–1563+1566.
Lai F, Ren Y, Lai C, et al. Acupuncture at Zusanli (ST36) for Experimental Sepsis: A Systematic Review. Evid Based Complement Alternat Med. 2020;2020:3620741. https://doi.org/10.1155/2020/3620741.
Acknowledgements
We would like to thank Dr. Zitong Huang, Dr. Tao Yu, and Dr. Zehuai Wen for their excellent assistance with the protocol.
Funding
This work was supported by grants from the Project of Guangzhou Science and Technology Department, China (201803010030), Guangdong Science and Technology Projects, China (2017ZC0164), and Guangdong Provincial Key Laboratory of Research on Emergency in TCM, China (2017B030314176). This trial’s funders have no role in any activities or ultimate authority of study design, data management, statistical analysis, interpretation of data, document writing, or report publication decision.
Author information
Authors and Affiliations
Contributions
Ruifeng Zeng and Banghan Ding drafted this manuscript. Ruifeng Zeng, Fang Lai, and Banghan Ding designed the described study. Decai Zhu, Baijian Chen, Lanting Tao, and Wei Huang will conduct the research. Chengzhi Lai and Manhua Huang will collect the data. Ruifeng Zeng and Chengzhi Lai will perform the statistical analysis. Fang Lai will monitor the process of trial implementation. All authors read and approved this final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Guangdong Provincial Hospital of Chinese Medicine Institutional Review Board (approval number ZF2020-051-01). The investigation will be conducted in accordance with national laws and the Declaration of Helsinki principles. All participants will provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT 2013 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, R., Lai, F., Huang, M. et al. Feasibility of electroacupuncture at Baihui (GV20) and Zusanli (ST36) on survival with a favorable neurological outcome in patients with postcardiac arrest syndrome after in-hospital cardiac arrest: study protocol for a pilot randomized controlled trial. Pilot Feasibility Stud 9, 8 (2023). https://doi.org/10.1186/s40814-023-01239-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-023-01239-9