Abstract
Acute ischemic stroke (AIS) is a common neurovascular emergency causing significant burden to society. Currently the main focus of AIS treatment is to restore blood flow to at risk brain tissue. For the last twenty years, intravenous tissue plasminogen activator (tPA) was the only proven therapy for patients with AIS. More recently, five randomized clinical trials established the efficacy of endovascular therapy with or without intravenous tPA in selected patient populations with AIS.
Not all stroke patients benefit from intravenous tPA or endovascular treatment. Nonetheless, the concept of early recanalization of occluded arteries resulting in better clinical outcomes is well established. In this focused review, we will discuss how imaging modalities such as Non-Contrast CT, CT-Angiography, and CT-Perfusion can potentially help physicians determine which patients are likely to recanalize early with intravenous tPA and therefore benefit from this therapy.
Similar content being viewed by others
Background
Stroke is the second leading cause of mortality worldwide [http://www.who.int/mediacentre/factsheets/fs310/en/]. In acute ischemic stroke (AIS), clot lysis and early restoration of blood flow to ischemic brain tissue is the ultimate goal of all reperfusion therapies. Recent data from the ESCAPE trial demonstrated that regardless of treatment modality, early effective restoration of blood supply was associated with smaller infarct volumes and better clinical outcome [1].
The recent endovascular clinical trials established the superiority of endovascular therapy (EVT) over intravenous tissue plasminogen activator (IVT) in patients presenting with large vessel anterior circulation occlusions [2–6]. However, in these trials, 5–12% of patients successfully achieved recanalization (TICI 2b/3) early during first angiography run before EVT (Table 1). An ability to predict which clots will recanalize early with IVT is crucial in the new endovascular era. Identification of this population can lead to better utilization of resources and avoid unnecessary endovascular procedures. This focused review article presents current research on clot characteristics identified using multi-modality CT that can predict early recanalization with IVT alone.
Main text
Theory of clot formation within intracranial arteries
The pathophysiology of clot formation in myocardial infarction is secondary to plaque rupture and thrombosis in situ in 95% [7], while in ischemic stroke it is more diverse and multifactorial (cardio-embolic, arterio-embolic, thrombosis in situ, lacunar, and cryptogenic). The composition of intracranial clots may vary, depending on specific endothelial and blood flow conditions at the source of clot formation (Fig. 1). Old, platelet rich and well-organized clots formed under flow conditions are likely more resistant to thrombolysis than fresh, fibrin- and red blood cell-rich clots formed under conditions of stasis. Time can also affect clot composition: as time passes, extensive fibrin deposition and cross-linking results in complex organized clots that are more resistant to lysis with IVT. The concept of “Time is Brain” is now being qualified by another statement “Time is Clot”. Clot formation within the intracranial tree is therefore a dynamic time-dependent process. The efficacy of IVT potentially depends on the age of the clot. As time passes, extensive fibrin deposition and cross-linking results in complex and organized clots makes them more resistant to lysis with IVT. Muchada et al. [8] found that the effect of IVT on early recanalization decreases over time. Another recent study revealed that clot volume reduction was independently related to time from stroke symptom onset to IVT [9].
Clot analogues showing that the composition of the clot material and clot structure may differ, based on endothelial factors and flow dynamic of the source [46]
To better explain clot formation and organization over time within the intracranial tree in patients with acute ischemic stroke, we recently suggested a theoretical framework wherein intracranial clots have two components [10]. The first is the “original clot” that comes from a proximal source or is formed in-situ due to endo-luminal factors, and the second is new clot that forms over time from stasis of blood flow around the original clot (Figs. 2 and 3). The extent of new clot formation due to stasis around the original clot is dependent on the patient’s collateral status and branching pattern of vessels around the clot (angio-architecture) (Figs. 1 and 2). The original clot can be either RBC- or platelet-rich depending on where it is formed, but the new clot that is formed around the original clot is RBC rich to begin with. Over time, this new clot becomes organized and undergoes changes. In this review, we use this theoretical model to explain how CT-based clot imaging can predict clot lysis with IVT.
The length of a clot depends on pial collateral status, and the branching pattern of arteries (angio-architecture) around the original clot. Panel (a) shows normal internal carotid artery and its branching vessels. (b) is the explanation of the sympoles. We think it is a self-explanatory. Panels (c-f) show varying clot lengths depending on pial collateral status and angio-architecture around the original clot. Panel c shows small clot with minimal stasis due to good collaterals and efficient angio-architecture. Panel (d) shows longer overall clot with good collaterals but inefficient angio-architecture. Panel (e) shows long clot due to poor collaterals. Panel (f) shows a very long clot because of poor collaterals and inefficient angio-architecture. Adapted from Qazi et al. 2015 [10]
A theoretical explanation of recanalization response to IV tPA and intra-arterial therapy based on collateral status, original composition of clot, and efficiency of angio-architecture. All possible combinations are not included. Adapted from Qazi et al. 2015 [10]
Non-contrast computed tomography
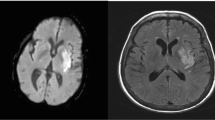
NCCT is quick, safe, non-invasive, widely accessible, and well tolerated by critically ill patients. Given these advantages, NCCT has become the primary neuroimaging modality for the assessment of patients with acute neurological emergencies. In evaluating patients with AIS, NCCT is used to rule out intracranial hemorrhage and to assess early ischemic changes (EIC) (Fig. 4) [11]. NCCT can also be used to evaluate clot characteristics.
ASPECTS scoring scheme. The upper row demonstrates axial CT cuts of the ganglionic ASPECTS level (M1–M3, insula [I], lentiform nucleus [L], caudate nucleus [C], posterior limb of the internal capsule [IC]). The lower row demonstrates CT cuts of the supraganglionic ASPECTS level (M4–M6). As illustrated in the figure, the authors prefer to use cuts in the inferior orbitomeatal line (rather than superior orbitomeatal line). All axial cuts are reviewed for ASPECTS scoring. EIC in the caudate nucleus are scored in the ganglionic level (head of caudate) and supraganglionic level (body and tail of caudate). Adapted from Puetz et al. 2009 [47]
Clot density
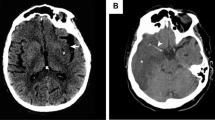
The hyperdense artery sign on NCCT has been shown to predict recanalization. This sign is a marker of clot on NCCT within the cerebral arterial network and normally measures 45–80 Hounsfield units (HU). Post-mortem studies have demonstrated that ischemic stroke can be caused by white, red, or mixed blood cell clots [12]. In in vitro experiments, platelet-enriched plasma and whole blood were mixed to produce samples with varying hematocrit levels ranging from 0 to 0 · 35. These artificial clots were then evaluated by NCCT at various time points from 6 to 144 h. ‘Red clots’, with the highest hematocrit content, showed densities around 70 HU. ‘White clots’, with the highest platelet content, were about 20 HU [13]. Interestingly, clots with lower HU were less likely to recanalize compared to clots with higher HU [14, 15]
Problems with detection of the hyperdense artery sign include false positives and false negatives. The average width of the middle cerebral artery (MCA; the most common site of occlusion in AIS) is 3–4 mm [16]. Scans that exceed this width and are of average signal may potentially miss detecting the hyperdense sign because of the phenomenon of partial volume averaging. Thin slice NCCT (≤2.5 mm) minimizes volume averaging, significantly improving the signal to noise ratio and producing higher object contrast. [17, 18] Thin slice widths (≤2.5 mm) allow for more sensitive and reliable detection of clots occluding the proximal MCA [19]. The clot detectability is slightly reduced in cases with very small clots or if the site of occlusion is superimposed with imaging artifacts [20]. Recent data from the Third International Stroke Trial (IST-3) showed that the sensitivity, but not specificity, of detecting the hyperdense vessel sign improved with thinner NCCT slices. Slice thickness ≤3 mm had a sensitivity 62%, specificity 98%; versus >3 mm slices, sensitivity 41%, specificity 92%, (p = 0.031, p = 0.089, respectively) [21]. A major question to be addressed by future studies involves whether a patient specific threshold, taking the hematocrit level and other patient factors for detecting clot length into consideration, is superior to a priori fixed thresholds.
Clot length on NCCT
The length of the hyperdense sign on NCCT is believed to represent the length of the clot. In a 138-patient study, Reidel et al. showed that clots measuring greater than 8 mm on NCCT had less than 1% chance of recanalization with IVT alone and were associated with worse outcome [22]. Recanalization was assessed on follow up TCD, magnetic resonance angiography (MRA), or CT-angiography (CTA). However, clot length in this study was measured on thin slice NCCT (≤2.5 mm) using a three dimensional semi-automated method, which is very time intensive and currently not used in the clinical world. Nevertheless, a more recent study using CTA and 4D-CTA to calculate the clot length challenges the idea that clot length of 8 mm is the longest clot IV tPA can recanalize, indicating that 11 mm may be a more optimal cut-off value [23].
Computed tomography angiography (CTA)
Although conventionally NCCT is the only requisite before IVT administration, additional information provided by CTA is useful in decision-making. CTA is obtained from aortic arch to vertex and it provides information on presence of clot, characteristics of thrombus, tissue ischemia and collaterals.
Assessment of Clot
The location and extent of clot on CTA is an important predictor of clinical outcome in AIS. EVT is only performed for those with target clots in CTA [24, 25]. Successful recanalization by IVT is dependent on clot length [22, 26] or clot burden as measured by CT angiography [27]. In analyses of predictors of early reperfusion using baseline imaging, where early reperfusion was defined as TICI 2a/2b/3, and median clot length in the early reperfusers was 19 mm (IQR 12.9 mm) compared with the nonreperfusers (34.9 mm, IQR 30.7 mm, P .001) [26]. Early recanalization is an independent factor for good clinical outcome [28]. In 80 patients treated with IVT for acute M1 occlusion, Rohan et al. determined clot length using CTA and temporal maximum intensity projection. They found that length of less than 12 mm was an independent predictor for recanalization and good outcome [23]. Another approach for measuring the clot length on CTA used the distance from the carotid T (origin of MCA) to the proximal end of the clot; this study found that a long distance from the carotid T to the beginning of the clot measure on coronal maximum intensity projection images (i.e. more distal clot) was significantly correlated with a good clinical outcome, defined as mRS ≤2 at 90 days [29].
Measuring clot length is challenging. Another method of quantifying clot characteristics is the clot burden score (CBS), a scoring system to define the extent of thrombus in proximal anterior circulation (Fig. 5). It assigns a score of 0–10, determined by subtracting 2 if thrombus is found in each of the supraclinoid ICAs, the proximal half of the MCA trunk, and the distal half of MCA trunk. A score of 1 is subtracted if thrombus is found in the infraclinoid ICA, anterior cerebral artery (ACA), and for each affected M2 branch. A score of 10 is normal, implying no clot, while a lower score implies greater clot burden. Among 85 patients, those with higher CBS >6 demonstrated smaller final infarct volume and better clinical outcome. A CBS >6 also predicted good recanalization rate with IVT [30]. Analysis of 306 patients with baseline CTA in the Interventional Management of Stroke III (IMS III) Trial was followed by a CTA or MRA after 24 h. It was found that 4.4% of terminal ICA occlusions and 75 –80% of distal M1 and M2 occlusions recanalized with IV tPA [31]. A study of 388 patients with proximal arterial occlusion found that the rates of recanalization were: distal internal carotid artery (ICA) 4.4%; M1-MCA (with or without ICA neck occlusion or stenotic disease) 32.3%; M2-MCA 30.8%; and basilar artery 4% [32].
Illustration of CBS. a, A 10-point score is normal, implying absence of thrombus. Two points (as indicated) are subtracted for thrombus found on CTA in the supraclinoid ICA and each of the proximal and distal halves of the MCA trunk. One point is subtracted for thrombus found in the infraclinoid ICA and A1 segment and for each affected M2 branch. b, Occlusion of infra- and supraclinoid ICAs with a CBS of 7. c, Distal M1 and 2 M2 branch occlusions produce a CBS of 6. d, Occlusion of the terminal ICA, proximal M1, and A1, with a resultant CBS of 5. Adapted from Tan et al. 2009 [30]
Multiphase CT angiography (mCTA) is a time-resolved imaging technique that allows assessment of the pial arterial filling by acquiring temporal information at three data points. Hence, it can give a better estimation of clot length and greater insight into the likelihood of arterial recanalization with IVT (Fig. 6) [33].
Multiphase CT angiography image, with each phase and follow-up CT angiography. a Peak-arterial Phase 1 image showed right M1 MCA occlusion (arrow). b, (c) Peak venous Phase 2 and late venous Phase 3 images demonstrate excellent arterial filling beyond the M1 MCA thrombus and no proximal M2 occlusion which was very short in this example (2 mm). d Follow-up CT angiography after intravenous thrombolysis administration showed complete recanalization of the arterial occlusion
Residual flow on baseline CT angiography along with early reperfusion with IV tPA assessed on the first angiogram of the ipsilesional arterial tree. The top panel shows a patient with a left M1 MCA clot and no residual flow ((a), grade 0 residual flow, yellow arrows, density similar to that of surrounding brain parenchyma). The first angiogram shows no recanalization (b and c). The middle panel shows a left M1 MCA clot with grade 1 residual flow (A, yellow arrows, denser than surrounding brain parenchyma). The first angiogram shows excellent reperfusion (b and c). The bottom panel shows a left M1 MCA clot with grade 2 residual flow (A, yellow arrows, hairline or streak of well-defined contrast across the partial or complete length of the clot). The first angiogram shows excellent reperfusion (b and c). Adapted from Mishra et al. AJNR 2014 [26]
Clot permeability using multiphase CTA/value of CTA in evaluating the clot
Thrombolysis in any vascular bed depends on the ability of blood and thrombolytic agents to permeate clot [34, 35] Permeation of blood flow through the clot or part of it is defined as residual blood flow; this allows the IVT to reach beyond the proximal part of the clot, leading to higher chance of recanalization. Residual blood flow can be observed in CTA source images by clearly visibly increased contrast attenuation through the clot compared with surrounding brain parenchyma [26]. In the TCD studies, this concept was applied to occluded brain arteries [36, 37]. Using CTA, residual flow within clot was graded – grade 0: clot with no contrast permeation and attenuation similar to that in surrounding brain parenchyma; grade I: clot appearing denser than surrounding brain parenchyma, with contrast potentially permeating through the clot; grade II: hairline or streak of well-defined contrast across the partial or complete length of clot (Fig. 7). Patients with residual flow within the clot were 5 times more likely to reperfuse than those without (Fig. 8). Residual flow within the clot may not always be visible to the naked eye due to partial volume effects on CTA. If, however, contrast signals at the proximal and distal clot interfaces (in an appropriate venous-weighted scan) are similar, the similarity is a potential marker of residual flow through the clot or of good collateral status. Accordingly, the authors suggested another indirect marker for residual blood flow on CTA: clot interface ratio in Hounsfield units (cirHU). (Figure 9) This is defined as the ratio of proximal thrombus interface HU to distal clot interface HU. Clot interface ratio >2 implies reduced residual blood flow through the thrombus and is associated with poor recanalization [26].
Clot interface Hounsfield unit ratio calculated by measuring the Hounsfield units in a region of interest selected at the proximal and distal clot interface only in scans that are mid- to late arterial- or appropriate venous-weighted. cirHU is calculated by dividing the proximal clot interface Hounsfield unit by the distal clot interface Hounsfield unit. In (a), a patient with a left M1 MCA clot has a cirHU of 1.05 while in (b), a patient with a left M1 MCA clot has a cirHU of 3.21 Adapted from Mishra et al. AJNR 2014 [26]
With these two markers (visible residual flow and cirHU ≥ 2) related to permeability of clots, the researchers suggested a predictive model for early recanalization of occluded arteries after intravenous thrombolysis, incorporating other characteristics of clots (length, location, and distance from the M1 MCA origin to the proximal clot interface) [26].
In most cases, distinguishing between permeable and non-permeable clot requires time-resolved images; because of this, mCTA and CTP have a clear advantage over single-phase CTA for detecting anterograde blood flow through the clot. In a post-hoc analysis of MR CLEAN trial, using quantitative methods on baseline single-phase CTA and NCCT (seen in 44%), Santos et al. showed that permeable clots were associated with improved functional outcome, smaller final infarct volume, higher recanalization rate, and may be associated with improved response to EVT [38]. Although one research group observed that discrepancy of clot length according to phases of CTA in dual-phase CT was associated with collateral status, anterograde flow on DSA, and poor clinical outcomes, they could not find the meaning of this discrepancy of clot length with respect to permeability of clots [39].
Putting it All together: NCCT + CTA
More recent studies on imaging in stroke have endeavoured to assess early and late recanalization by incorporating information from both NCCT and CTA. Menon et al. at presented an abstract at the Radiological Society of North America meeting in 2014 that used data from the interventional management of stroke trial III (IMS III) and utilized clot characteristics from both NCCT and CTA to develop a Classification And Regression Tree (CART) model to predict early and late recanalization success. The clot characteristics derived from NCCT that were included were hyperdense sign (HDS) location, length, and ratio of maximal HDS Hounsfield Unit (HU) to maximal contralateral MCA HU (rHU). CTA clot characteristics included clot burden score, length, residual flow through clot, and ratio of contrast HU at the proximal and distal clot interfaces (cirHU). Early recanalization was assessed on first run digital subtraction angiography on patients given IV-tPA who also underwent endovascular treatment, while late recanalization was assessed on 24 h CTA in patients only treated with IV-tPa. The results show that patients with a clot burden of >5, cirHU <1.59, rHU < 1.28 and clot length of <13.4 still have an 80% chance of early recanalization with IV-tPA alone (Fig. 8). They also show that 62.5% of patients with clots that are >5.5 mm on NCCT but with a clot burden of >3 recanalize within 24 h (Figs. 9 and 10) [26]. Major limitations to this approach are mainly due to the subjectivity of quantifying the residual blood flow. Another point of critique is that calculating the clot interface ratio is not practical in the acute care setting [40].
68-year-old lady presented with acute left MCA syndrome with NIHSS score of 12 treated with IVT 103 min after symptoms onset after reviewing the NCCT. a. Axial NCCT at ganglionic level with ASPECTS of 10. b. Axial CTA, the yellow arrow points to distal M1-MCA occlusion. c. Axial CTA, the red arrow points to well-defined contrast permeation within the clot. d – f. Multiphase CTA MIP images, showing good collaterals with best arterial backfilling seen in phase 1. g – h. Conventional cerebral angiogram performed immediately after IVT, the first intracranial run demonstrates a successful reperfusion (TICI 3) with IVT. i. Follow-up diffusion-weighted MRI (DWI), obtained 2 days later, shows shows small areas of restricted diffusion in the territory of the MCA. The patient 90 day mRS was zero
Computed tomography perfusion
Although CTP is mainly used to evaluate the perfusion status of brain parenchyma in acute stroke patients, some researchers describe the benefits of 4-dimensional (4-D) CTA from CTP source images using multidetector CT scanners. Recent studies have been able to use 4-D CTA to evaluate collateral status more precisely [41, 42] and demarcate thrombus burden better than was possible using conventional CTA [43]. In addition, anterograde flow across occlusion sites of cerebral arteries could be distinguished from retrograde collateral filling noninvasively by 4-D CTA [44]. In addition to this indirect contribution of CTP to imaging of occluded vessels, a recent study shows a direct parameter of CTP that can be used to evaluate the occult anterograde flow of cerebral artery occlusion in acute stroke patients [45]. Using perfusion maps that measure delay in arrival time of contrast within the intracranial artery tree (T0 maps), a positive sloped regression line of T0 values measured along artery silhouette distal to clot was observed as a marker of occult anterograde flow (permeable clot). This marker was associated with early recanalization of occluded arteries after intravenous thrombolysis (67% for anterograde flow versus 30% for retrograde flow). Moreover, the difference in median T0 value between proximal and distal interface of clot was also related to early recanalization (71% for ≤ 2 s versus 26% > 2 s) – lower difference between two T0 values indicated occult anterograde flow on clots in occluded vessels (Fig. 11). The use of CTP to quantify the occult anterograde flow has limitations, however. It is technically challenging and can only be measured using experts’ input. CTP is also susceptible to motion artifacts.
After identifying a complete occlusion on computed tomographic angiography (CTA; a; white arrow), regions of interest are drawn at the proximal (b), solid white arrow) and distal thrombus interface (B, hollow white arrow) of the thrombus on the CT perfusion (CTP) average map (b). A line profile (white arrow head) is drawn along the silhouette of the artery distal to the thrombus on the CTP-average map. The CTP-average map is then coregistered with the CTP T0 map (c). T0 values vs distance (pixel number) along the line profile are then plotted and the line of best-fit determined (d); T0 values at proximal and distal thrombus interface are also measured. d, In this patient, the presence of a positive artery profile slope suggests the presence of anterograde flow distal to thrombus. Adapted from Ahn et al. Stroke 2015 [45]
Conclusion
Multimodality CT has become an essential tool to evaluate patients with AIS and facilitate treatment decisions. It can provide more details about clot characteristics and can be used to tailor the treatment decision of whether further recanalization with EVT should be attempted. More research effort is needed to help aid the clinical decision about proceeding with EVT or treating with IVT only. Furthermore, neuroimaging of the clot is an important topic in modern stroke management and should be pursued to a greater extent.
Abbreviations
- AIS:
-
Acute ischemic stroke
- CTA:
-
Computed tomography angiography
- CTP:
-
Computed tomography perfusion
- EVT:
-
Endovascular therapy
- IVT:
-
Intravenous tissue plasminogen activator
References
Al-Ajlan FS, Goyal M, Demchuk AM, et al. Intra-arterial therapy and post-treatment infarct volumes: insights from the ESCAPE randomized controlled trial. Stroke. 2016;47(3):777–81.
Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82(3):269–72.
Muchada M, Rodriguez-Luna D, Pagola J, et al. Impact of time to treatment on tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2014;45(9):2734–8.
Kim YD, Nam HS, Kim SH, et al. Time-Dependent Thrombus Resolution After Tissue-Type Plasminogen Activator in Patients With Stroke and Mice. Stroke. 2015;46(7):1877–82.
Qazi EM, Sohn SI, Mishra S, et al. Thrombus Characteristics Are Related to Collaterals and Angioarchitecture in Acute Stroke. Can J Neurol Sci. 2015;42(6):381–8.
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670–4.
Castaigne P, Lhermitte F, Gautier JC, Escourolle R, Derouesne C. Internal carotid artery occlusion. A study of 61 instances in 50 patients with post-mortem data. Brain. 1970;93(2):231–58.
Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;228(1):126–30.
Puig J, Pedraza S, Demchuk A, et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2012;33(1):90–6.
Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44(1):243–5.
Rai AT, Hogg JP, Cline B, Hobbs G. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg. 2013;5(4):371–5.
Barber PA, Demchuk AM, Hudon ME, Pexman JH, Hill MD, Buchan AM. Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemia. Stroke. 2001;32(1):84–8.
Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10(6):419–23.
Riedel CH, Jensen U, Rohr A, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced Computed Tomography reconstructions. Stroke. 2010;41(8):1659–64.
Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 2012;43(9):2319–23.
Mair G, Boyd EV, Chappell FM, et al. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke. 2015;46(1):102–7.
Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42(6):1775–7.
Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke. 2014;45(7):2010–7.
Menon BK, Demchuk AM. Computed Tomography Angiography in the Assessment of Patients With Stroke/TIA. Neurohospitalist. 2011;1(4):187–99.
Menon BK, Campbell BC, Levi C, Goyal M. Role of imaging in current acute ischemic stroke workflow for endovascular therapy. Stroke. 2015;46(6):1453–61.
Mishra SM, Dykeman J, Sajobi TT, et al. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol. 2014;35(12):2265–72.
Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3(4):230–6.
Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254–8.
Friedrich B, Gawlitza M, Schob S, et al. Distance to thrombus in acute middle cerebral artery occlusion: a predictor of outcome after intravenous thrombolysis for acute ischemic stroke. Stroke. 2015;46(3):692–6.
Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525–31.
Demchuk AM, Goyal M, Yeatts SD, et al. Recanalization and clinical outcome of occlusion sites at baseline CT angiography in the Interventional Management of Stroke III trial. Radiology. 2014;273(1):202–10.
Bhatia R, Bal SS, Shobha N, et al. CT angiographic source images predict outcome and final infarct volume better than noncontrast CT in proximal vascular occlusions. Stroke. 2011;42(6):1575–80.
Menon BK, d’Esterre CD, Qazi EM, et al. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology. 2015;275(2):510–20.
Blinc A, Francis CW. Transport processes in fibrinolysis and fibrinolytic therapy. Thromb Haemost. 1996;76(4):481–91.
Diamond SL, Anand S. Inner clot diffusion and permeation during fibrinolysis. Biophys J. 1993;65(6):2622–43.
Saqqur M, Tsivgoulis G, Molina CA, et al. Residual flow at the site of intracranial occlusion on transcranial Doppler predicts response to intravenous thrombolysis: a multi-center study. Cerebrovasc Dis. 2009;27(1):5–12.
Ma M, Berger J. A novel TCD grading system for residual flow in stroke patients. Stroke. 2001;32(10):2446.
Santos EM, Marquering HA, den Blanken MD, et al. Thrombus Permeability Is Associated With Improved Functional Outcome and Recanalization in Patients With Ischemic Stroke. Stroke. 2016;47(3):732–41.
Park M, Kim KE, Shin NY, et al. Thrombus length discrepancy on dual-phase CT can predict clinical outcome in acute ischemic stroke. Eur Radiol. 2015.
Menon BK, Goyal M. Clots, Collaterals, and the Intracranial Arterial Tree. Stroke. 2016;47(8):1972–3.
Smit EJ, Vonken EJ, van Seeters T, et al. Timing-invariant imaging of collateral vessels in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44(8):2194–9.
Frolich AM, Wolff SL, Psychogios MN, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol. 2014;24(2):390–6.
Frolich AM, Schrader D, Klotz E, et al. 4D CT angiography more closely defines intracranial thrombus burden than single-phase CT angiography. AJNR Am J Neuroradiol. 2013;34(10):1908–13.
Frolich AM, Psychogios MN, Klotz E, Schramm R, Knauth M, Schramm P. Antegrade flow across incomplete vessel occlusions can be distinguished from retrograde collateral flow using 4-dimensional computed tomographic angiography. Stroke; a journal of cerebral circulation. 2012;43(11):2974–9.
Ahn SH, d’Esterre CD, Qazi EM, et al. Occult anterograde flow is an under-recognized but crucial predictor of early recanalization with intravenous tissue-type plasminogen activator. Stroke. 2015;46(4):968–75.
NT. I. Advancing the Understanding of Clot no date 2016. http://neuravi.com/neuravi-thromboembolic-initiative/
Puetz V, Dzialowski I, Hill MD, Demchuk AM. The Alberta Stroke Program Early CT Score in clinical practice: what have we learned? Int J Stroke. 2009;4(5):354–64.
Acknowledgements
None.
Funding
No funding to report.
Availability of data and materials
“Data sharing not applicable to this article as no datasets were generated or analysed during the current study. If you do not wish to publicly share your data, please write: “Please contact author for data requests.”
Authors' contributions
FSA, BKM review concept and design, drafting the manuscript, critical revision of the manuscript content. EQ, CKK, EPV, LW drafting the manuscript, critical revision of the manuscript content. All authors read and approved the final manuscript.
Competing interests
All authors declare that they have no competing interests.
Consent for publication
Our manuscript does not contain any individual person”s data in any form (including any individual details, images or videos), or in the case of children, their parent or legal guardian. Copyrights for other figures are attached.
Ethics approval and consent to participate
Our current manuscript is a review article that do not report any studies involving human participants, human data or human tissue.
Declarations
Our current manuscript does not report any studies involving human participants, human data or human tissue.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Al-Ajlan, F.S., Qazi, E., Kim, C.K. et al. Multimodality CT based imaging to determine clot characteristics and recanalization with intravenous tPA in patients with acute ischemic stroke. Neurovasc Imaging 3, 2 (2017). https://doi.org/10.1186/s40809-017-0026-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40809-017-0026-9