Abstract
Background
Intramuscular hemangioma is an uncommon benign tumor found mainly in the limbs of adolescents and young adults. The local recurrence rate is high, ranging from 30 to 50%, necessitating wide local excision of intercostal intramuscular hemangiomas. However, preoperative diagnosis of intramuscular hemangiomas is challenging. Herein, we report a rare case of an intramuscular hemangioma arising from the chest wall.
Case presentation
A healthy 29-year-old asymptomatic man was referred to our hospital after an abnormal shadow was observed on his chest radiography. Computed tomography and magnetic resonance imaging revealed a 30-mm-sized mass in the right second intercostal space. Neoplastic lesions, such as schwannomas or solitary fibrous tumors, were included in the preoperative differential diagnosis. Tumor resection was performed using video-assisted thoracoscopic surgery. The tumor, which had a smooth surface covered with parietal pleura, was dissected from the external intercostal muscle and costal bone. Postoperative histopathological examination revealed proliferation of spindle-shaped endothelial cells arranged in a capillary vascular structure accompanied by entrapped smooth muscle fibers, adipose tissue, and muscle vessels. The final diagnosis was an intramuscular hemangioma with negative surgical margins. There was no evidence of recurrence during the 1-year postoperative follow-up period.
Conclusion
Intramuscular hemangiomas should be considered in the differential diagnosis of chest wall tumors, particularly in young people, owing to their potential for recurrence. Moreover, postoperative follow-up may be necessary for resected intramuscular intercostal hemangiomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Intramuscular hemangioma is a rare benign tumor mainly found in the limbs of adolescents and young adults, often presenting with pain [1, 4]. Hemangioma arising from the chest wall is extremely rare and has only been reported in a few documented cases [2, 4]. Preoperative diagnosis of intramuscular hemangioma is challenging and often relies on postoperative histopathological confirmation. Given its high local recurrence rate (30–50%), resection with enough surgical margin is crucial [3]. We report the case of a 29-year-old man who was incidentally discovered to have a chest wall tumor diagnosed as intercostal intramuscular hemangioma from a specimen resected under video-assisted thoracoscopic surgery (VATS), which was preoperatively unexpected.
Case presentation
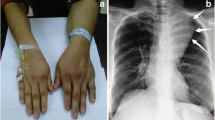
A healthy 29-year-old man with no relevant medical history presented with an abnormal opacity in the right upper field on chest X-ray screening during a routine health check (Fig. 1a). Blood tests, including those for tumor markers, were normal. Plain computed tomography (CT) revealed a 30-mm mass in the second intercostal space (ICS), that was well-circumscribed and homogeneous (Fig. 1b). Plain magnetic resonance imaging (MRI) showed high intensity on T2-weighted images and intermediate intensity on T1-weighted images, with no sign of infiltration into the surrounding tissue, costal bone, or lungs (Fig. 1c and d). Imaging revealed no signs of hemorrhage or calcification on image examinations. Suspecting a schwannoma or solitary fibrous tumor, we performed VATS for diagnosis and treatment. Under general anesthesia, the patient was placed in the left lateral position and three ports were placed. The tumor was identified on the chest wall anterior to the second ICS and had a smooth surface covered with the parietal pleura and internal intercostal muscle (Fig. 2a). The pleural wall was incised along the tumor margin, and the tumor, located between internal and external intercostal muscles, was dissected from the external intercostal muscle and costal bone (Fig. 2b). Gross examination of the surgical specimen revealed that the tumor was a well-circumscribed yellowish-white nodule (Fig. 3a). Histopathological examination showed proliferation of spindle-shaped endothelial cells arranged in a capillary vascular structure, accompanied by entrapped smooth muscle fibers, adipose tissue, and muscle vessels (Fig. 3b and c). The surgical margin was 2 mm and microscopically negative. Immunohistochemically, the endothelial cells were positive for CD34 (Fig. 3d). These pathological features led to a diagnosis of intramuscular hemangioma. The patient’s postoperative course was uneventful, and he was discharged after 5 days. No evidence of recurrence was found at 12 months after surgery.
a Chest radiography showed an abnormal shadow in the right upper lung field (dotted circle). b Chest computed tomography showed a 30-mm mass in the right second intercostal space. c T2-weighted MRI scan showed a high-intensity area in the right second intercostal space without evidence of peritumoral invasion. d T1-weighted MRI scan showed intermediate intensity area in the right second intercostal space. MRI: Magnetic resonance imaging
Gross and histopathological findings. a The tumor was a well-circumscribed yellowish-white nodule. b, c Microscopic findings (H&E stain). The tumor was well-demarcated. Smooth muscle fibers were replaced by dilated vascular channels with focal thrombosis and adipose tissue. d Endothelial cells are positive for CD34
Discussion
Intramuscular hemangioma is defined by the World Health Organization classification as a proliferation of benign vascular channels within skeletal muscle, associated in most instances with variable amounts of mature adipose tissue [4]. The lower limbs are commonly affected, particularly the thigh and calf, followed by the head, neck, and upper limbs, while the tumor rarely arises in the chest wall [4]. Only six cases of resected intercostal intramuscular hemangioma have been reported in the literature (Table 1). Intramuscular hemangioma recurs at a high rate (30–50%) despite the tumor’s benignity [3]. We speculate that intercostal intramuscular hemangiomas also pose a high risk of recurrence. Therefore, regardless of their rarity, these tumors should be included in the differential diagnosis of chest wall tumors, especially in young people.
Clinically, intramuscular hemangiomas often occur in adolescents and young adults, without sex-related differences. Moreover, a previous report has shown that a history of trauma implicates the genesis and growth of intramuscular hemangiomas [7]. The primary symptom of a tumor in the extremities is exercise-induced pain, although only one previously reported case of intercostal intramuscular hemangioma had these symptoms (Table 1) [1, 8]. Hence, the possibility of an intramuscular hemangioma should be considered in asymptomatic chest wall tumors, as in the present case.
Preoperative diagnosis of intercostal intramuscular hemangiomas is challenging (Table 1). Preoperative biopsy did not lead to a correct diagnosis in two previous case reports. In a previously reported case similar to the present case, a neurogenic tumor was also suspected because the plain CT and MRI findings resembled those tumors [10]. Hemorrhage or calcification can sometimes be found in larger hemangiomas on CT and MRI, while neurogenic tumors can present the same in imaging findings, though infrequently. Only two prior cases of dumbbell-shaped tumors with preoperative contrast-enhanced MRI examinations were diagnosed correctly [8, 9]. Dynamic contrast-enhanced MRI findings can be helpful for the diagnosis of hemangiomas, which are reported to have eccentric enhancement with incomplete peripheral filling in the early phase and filling during the delayed phase [9]. Therefore, CT or MRI examinations should be performed with contrast media, even in younger patients.
A retrospective cohort study reported a series of 77 patients with intramuscular hemangiomas who underwent surgical excision over a 25-year period [3]. Most recurrent cases of intramuscular hemangioma showed insufficient surgical margins (21 of 23 cases), suggesting that surgical margin and tumor size are the major determinants for local recurrence in patients with intramuscular hemangioma, indicating the necessity of wide resection with sufficient surgical margins [3]. On the other hand, there are no reports of recurrence in the six previous cases of resected intercostal intramuscular hemangioma (Table 1). Presumably, intercostal hemangiomas grow slowly without infiltration depending on the nature of their benignity. In addition, complete excision was accomplished through rib resection and reconstruction of the chest wall in three previous cases of dumbbell-type tumors arising from the intrathoracic to extrathoracic region, with the ICS as a narrow section [7,8,9, 11].
In our case, marginal excision was performed only with partial resection of the intercostal muscles because the tumor was considered benign owing to intraoperative findings: well-circumscribed, homogeneous, and non-invasive into the surrounding tissue. Consequently, the surgical margin was 2 mm and quite short, but the tumor was completely resected with a negative microscopic surgical margin. A retrospective cohort study reported that patients with completely resected intramuscular hemangiomas had low recurrence rates, with a 92.7% recurrent-free survival rate in 5 years [3]. As mentioned in the previous study, we considered that the possibility of recurrence in our case was low. Therefore, we determined that additional resection was unnecessary.
The previous study above also demonstrated that approximately 10% of cases showed recurrence within 5 years even with wide and complete resection, indicating the necessity of postoperative follow-up after resection of intramuscular hemangioma [3]. In the six previous cases of intercostal intramuscular hemangioma, the mean postoperative observation period was 1.2 years, which may be insufficient (Table 1). Therefore, postoperative follow-up for 5 years may be necessary for patients with resected intercostal intramuscular hemangiomas, even after complete resection, despite the lack of reported recurrences in these cases.
Conclusion
Intramuscular hemangiomas should be considered in the differential diagnosis of chest wall tumors using enhanced-contrast media, particularly in young patients. Wide resection with sufficient surgical margins is preferable for intramuscular hemangioma because of its high recurrence rate despite its benignity. Postoperative follow-up may also be necessary after resection.
Availability of data and materials
The availability of the data used in this case is subject to confirmation by the journal or the authors. For more information on data availability and access procedures, please contact the journal or corresponding author.
Abbreviations
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- VATS:
-
Video-assisted thoracoscopic surgery
- ICS:
-
Intercostal space
References
Matsuoka K, Ueda M, Miyamoto Y. Giant intramuscular hemangioma of the chest wall with osteolytic change. Eur J Cardiothor Surg. 2012;41:1202–3.
Sotoda Y, Hirooka S, Kohi M, Orita H, Mori M. Intramuscular hemangioma in the right ventricle. Gen Thorac Cardiovasc Surg. 2008;56:85–7.
Bella GP, Manivel JC, Thompson RC Jr, Clohisy DR, Cheng EY. Intramuscular hemangioma: recurrence risk related to surgical margins. Clin Orthop Relat Res. 2007;459:186–91.
WHO Classification of Tumors Editorial Board. Soft tissue and bone tumors. 5th ed. Lyon: International Agency for Research on Cancer; 2020.
Winchester DJ, Vicotr TA, Fry WA. Intercostal hemangioma presenting as a chest wall tumor. Ann Thorac Surg. 1992;54:145–6.
Ono N, Yokomise H, Inui K, Wada H, Hitomi S. Intercostal hemangioma. Thorac Cardiovasc Surg. 1996;44:324–5.
Yonehara Y, Nakatsuka T, Ichioka I, Takato T, Matsumoto S, Yamada A. Intramuscular hemangioma of the anterior chest wall. Br J Plast Surg. 2000;53:257–9.
Kubo M, Moriyama S, Nogami T, Kunitomo T, Nawa S. Intramuscular hemangioma. Jpn J Thorac Cardiovasc Surg. 2004;52:435–8.
Elbawab H, Alreshaid F, Hashem T, Alnasser A, Husain R, Aljehani Y. Intercostal hemangioma: case report of a rare chest wall tumor in childhood. Int J Surg Case Rep. 2019;60:319–22.
Ochi T, Sekine Y, Koh E, Hoshino H, Nakazawa T. Diagnosis and management of inteercostal intramuscular hemangioma: an updated review. J Thorac Cardiothorac Surg. 2023;18:210.
Krishnamurthy A, Raghunandhan GC, Majhi U. Dumbbell shaped schwannnoma of the lateral chest wall masquandaering as a soft tissue sarcoma. Indian J Surg Oncol. 2015;6:307–10.
Acknowledgements
We would like to thank Editage (www.editage.jp) for the English-language editing.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Contributions
YN: drafting of the manuscript. TA: critical revision and approval of the manuscript. FK: data collection and approval of the manuscript, MK: data collection and approval of the manuscript. KO: data collection and approval of the manuscript. TH: data collection and approval of the manuscript. TM: data collection and approval of the manuscript. YO: data collection and approval of the manuscript. TT: data collection and approval of the manuscript. MN: final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient prior to treatment.
Consent for publication
The patient provided informed written consent for the publication of this case report and accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakanishi, Y., Akamine, T., Kinoshita, F. et al. Resected intramuscular hemangioma in the chest wall: a case report. surg case rep 10, 225 (2024). https://doi.org/10.1186/s40792-024-02023-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-024-02023-4