Abstract
Macroids and rhodoliths, made by encrusting acervulinid foraminifera and coralline algae, are widely recognized as bioengineers providing relatively stable microhabitats and increasing biodiversity for other species. Macroid and rhodolith beds occur in different depositional settings at various localities and bathymetries worldwide. Six case studies of macroid/rhodolith beds from 0 to 117 m water depth in the Pacific Ocean (northern Central Ryukyu Islands, French Polynesia), eastern Australia (Fraser Island, One Tree Reef, Lizard Island), and the Mediterranean Sea (southeastern Spain) show that nodules in the beds are perforated by small-sized boring bivalve traces (Gastrochaenolites). On average, boring bivalve shells (gastrochaenids and mytilids) are more slender and smaller than those living inside shallow-water rocky substrates. In the Pacific, Gastrochaena cuneiformis, Gastrochaena sp., Leiosolenus malaccanus, L. mucronatus, L. spp., and Lithophaga/Leiosolenus sp., for the first time identified below 20 m water depth, occur as juvenile forms along with rare small-sized adults. In deep-water macroids and rhodoliths the boring bivalves are larger than the shallower counterparts in which growth of juveniles is probably restrained by higher overturn rates of host nodules. In general, most boring bivalves are juveniles that grew faster than the acervulinid foraminiferal and coralline red algal hosts and rarely reached the adult stage. As a consequence of phenotypic plasticity, small-sized adults with slow growth rates coexist with juveniles. Below wave base macroids and rhodoliths had the highest amounts of bioerosion, mainly produced by sponges and polychaete worms. These modern observations provide bases for paleobiological inferences in fossil occurrences.

Similar content being viewed by others
Introduction
Macroids and rhodoliths form extensive beds in marine waters from shallow subtidal and coral reef areas down to outer platforms (e.g., Foster 2001; Amado-Filho et al. 2012; Foster et al. 2013). Macroids are coated grains, larger than 10 mm, made up of encrusting metazoans or protozoans (Hottinger 1983). Coralline red algae are dominant components of free-living nodules which are named rhodoliths (Bosellini and Ginsburg 1971). Macroids and rhodoliths form a habitat for many other organisms, from protists to fish and including boring bivalves that inhabit the nodules themselves, and they contribute to the benthic primary productivity. The nodules can record the biodiversity of the inhabiting boring organisms (e.g., Littler et al. 1991; Foster 2001; Mallela and Perry 2007; Mueller et al. 2014; Adey et al. 2015). Modern nodules therefore can provide a basis for understanding activities of ancient bioeroding organisms in environments where they occur. This paper documents the boring bivalves and their traces found in modern macroids and rhodoliths at six sites, from shallow coral reef to deep-water environments, in the Pacific Ocean and Mediterranean Sea, down to depths of 117 m. This provides a basis for the interpretation of fossil occurrences.
Fossil traces are evidence of the activity of once-living organisms, grouped in different ethological categories (Seilacher 1964; Ekdale et al. 1984; Bromley 1996). These borings (domichnia) include both bioturbation and bioerosion structures (Ekdale et al. 1984; Taylor and Wilson 2003; Gibert et al. 2004). Bioerosion structures, widespread in carbonate stable substrates, are also common in mobile hard substrates represented by macroids and rhodoliths (Bosence 1985; Matsuda and Iryu 2011; Baarli et al. 2012).
Fossil traces have so far been useful tools in palaeoenvironmental analysis for two reasons: (a) they have good preservation potential, and (b) many individual ichnotaxa, ichnocoenoses, and involved taphonomic signatures exhibit reasonably well-constrained environmental distributions (Perry and Bertling 2000; Perry and Hepburn 2008; Checconi et al. 2010; Bassi et al. 2013). However, most knowledge regarding the environmental distribution of boring ichnotaxa and their producers comes from shallow-water coral reefs and rocky shores but little is known about those from deeper settings (Kleemann 1980; Perry 1998; Gibert et al. 2012; Perry et al. 2012; Aguirre et al. 2017a).
Common and abundant macroborer ichnospecies of Gastrochaenolites Leymerie have been described from intertidal rocky settings (e.g., Kelly and Bromley 1984; Bromley and D’Alessandro 1987; Edinger and Risk 1994; Wilson and Palmer 1998; Ekdale and Bromley 2001; Donovan 2002; Kleemann 2009). Little is known about boring gastrochaenids (Gastrochaenidae) and mytilids (Mytilidae) inhabiting macroids and rhodoliths in deeper settings. These boring bivalves, both in organic and inorganic substrates, are exposed to different restrictions in their growth (e.g., Owada 2015; Bagur et al. 2013, 2014; Márquez et al. 2017). These restrictions are phenotypic responses to the environmental conditions. Organisms that grow in different environmental conditions may exhibit behavioral, morphological, ichnological, and physiological differences (e.g., Hollander et al. 2006).
Gastrochaenolites ichno-specimens and related preserved boring bivalves are analyzed in present-day shallow- to deep-water macroids and rhodoliths. Six examples of macroid and rhodolith beds from 0 to 117 m water depth in the Pacific Ocean and the Mediterranean Sea were studied (Table 1): southern Japan (northern Central Ryukyu Islands), eastern Australia (Fraser Island, One Tree Reef, Lizard Island), French Polynesia (Moorea), and southeastern Spain (Cabo de Gata). We compare the bivalve borings and their makers and the growth and taphonomic characteristics of the macroids and rhodoliths in order to decipher the complex interplay between the boring bivalves and their host. Shell-shape diversity of the identified boring bivalves, together with morphological diversity of the ichnospecimens and taphonomic observations, indicate that (1) the mean shell sizes of the boring bivalves are slenderer and smaller than those growing inside shallow-water rocky substrates, and (2) deep-water (> 40 m) boring bivalves grow faster than their hosts. As a consequence of phenotypic plasticity, small-sized adults with slow growth rates might coexist with juveniles.
Geographical settings
Southern Japan: Kikai-jima
This survey area lies southwest of Kikai-jima (Central Ryukyu Islands; Table 1) at water depths between 61 and 105 m in clear oceanic waters with normal marine salinities (34–35). The sea floor is characterized by flat topography and mainly consists of coarse bioclastic carbonate sediments (Arai et al. 2008). Mean seawater annual temperature is ~ 22–24 °C (data from J-DOSS, JODC Data On-line Service System).
Macroids for this study were dredged from the flat sea floor (Bassi et al. 2012a) during a scientific survey cruise carried out by R/V Tansei-maru (KT-09-16, Ocean Research Institute, The University of Tokyo). The nodules consist of various sized macroids, which had been formerly called rhodoliths in the studies of marine sediments and biota around the Ryukyu Islands (Tsuji 1993; Matsuda and Nohara 1994; Iryu et al. 1995). The macroids examined were still living when collected. They were washed and dried before subsequent analysis. Detailed descriptions of the macroid size and shape are reported in Bassi et al. (2012a).
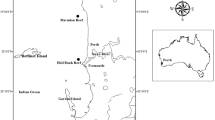
Eastern Australia: Fraser Island, one tree reef, Lizard Island
The samples were collected in 1991 on the continental shelf off Fraser Island in southern Queensland, south of the Great Barrier Reef (Table 1), during a joint survey cruise of the Australian Geological Survey Organization (AGSO) and Japanese National Oil Corporation (JNOC). This area is at the transition from tropical to temperate carbonate deposition (e.g., Marshall et al. 1998; Boyd et al. 2008; Schröder-Adams et al. 2008).
Offshore Fraser Island algal nodules are common in the rhodolith-coral gravelly facies in the mid-shelf (~ 45–100 m) water depths and their abundance decreases both to the outer shelf down to 120 m and to the sandy inner shelf (Marshall et al. 1998; Lund et al. 2000; Fig. 2). Rhodoliths were picked from 31 dredged and grabbed samples along the surveyed depth range.
One Tree Reef is one of the coral reefs in the Capricorn Group in the southern Great Barrier Reef Province (Davies et al. 1976; Table 1). This is a mesotidal region (average tidal range in One Tree Reef is 1.5 m) with predominant southeasterlies (Choukroun et al. 2010). The studied rhodoliths in One Tree Reef extend in the intertidal to shallow subtidal zones of the coralgal rim at the leeward side of the reef. The samples were collected in less than 0.3 m below mean low sea level in October 1992.
Lizard Island is a group of five small granite islands surrounded by coral reefs in the northern Great Barrier Reef (Table 1). Rhodoliths were sampled from shallow subtidal (0.5 to 12 m water depth) sites in the fringing reefs from both eastern and western sides by snorkeling and scuba diving in November 1992.
French Polynesia: Moorea
Moorea, the second youngest volcanic island (~ 1.2 to 2 Ma) within the Society Islands (Table 1), is located 20 km west of Tahiti, has a barrier coral reef intersected by passes, which separate a lagoon from the open ocean (Fajemila et al. 2015, 2020). Water circulation is wind-driven swells that break on the barrier and force mostly unidirectional water into the lagoon from where it then exits through the reef passes (Hench et al. 2008).
Rhodoliths occur in various sites around the island. The rhodoliths used in this study were collected on northwestern Moorea from a carbonate sand in a shallow-water (less than 2 m) channel. This channel separates two motus (shallow islands) at the edge of the lagoon adjacent to the western shore of Motu Tiahura (Table 1). Current speed is up to 6.8 m/s in the sampling site most distant from the motu shore. The rhodolith bed’s extent is greater than 150 × 30 m parallel to Motu Tiahura, with rhodoliths usually separated from one another by a few centimeters to a meter.
Southeastern Spain: Cabo de Gata
The sampled rhodolith beds occur at Rocas del Plomo off Cala del Plomo in Cabo de Gata (Almería, southeastern Spain; Table 1). The rhodoliths mainly grow on bioclastic gravels around volcanic rocks jutting out on the shelf between 2.5 and 50 m water depth. Shorewards of rhodolith beds the sediment is siliciclastic sand with bioclasts while seawards the gravels change to bioclastic muddy sand with dispersed open branching rhodoliths. Waters are oligotrophic to mesotrophic and rhodoliths are only sporadically affected by storm waves. Rhodoliths were collected by dredging from a small boat (AMA 7, Junta de Andalucía) and scuba diving from 2002 to 2004.
Methods/experimental
Macroid and rhodolith shapes were assessed as sphericity index (see Sneed and Folk, 1958; Graham and Midglay 2000) by measuring the short, intermediate, and long axes of each specimen (e.g., Bosence 1983a). Taxonomic composition, nature of the nucleus, inner arrangement, and constructional voids, and outer growth-forms (e.g., Bassi et al. 2012b; Aguirre et al. 2017b) were analyzed on polished slabs and in thin sections (n = 300, ~ 10 μm in thickness). The thin sections were made after embedding the macroid and rhodolith specimens in epoxy resin in a vacuum chamber. The embedded specimens were sectioned along the plane including the center of the specimen. The identified ichnogenera are based on descriptions and illustrations of Bromley and D’Alessandro (1983, 1984, 1989), Kelly and Bromley (1984), Edinger and Risk (1997), and Perry (1996). The ichnotaxa identified in the Kikai-jima and Fraser Island beds have been described and illustrated in Bassi et al. (2011, 2012a). The abundance of bioerosion traces was semi-quantitatively estimated using a binocular microscope (Nebelsick et al. 2011). Boring bivalves were identified according to shell shapes and microstructures of isolated specimens collected from nodules (Owada and Hoeksema 2011). Shell microstructures were observed under scanning electron microscope JEOL JCM-5000 with an accelerating voltage of 15 kV (Department of Biological Sciences, Kanagawa University; Owada 2009; Owada and Hoeksema 2011). Bivalve length and height were assessed along and across the nearly straight ligament, which is the axis of shell opening. These measurements along with the boring traces were evaluated with a digital vernier caliper. The length of the Gastrochaenolites chamber was determined by measuring a very thin iron wire inserted in the burrow.
In macroids and rhodoliths, bioeroding organisms operating with chemical or physical processes destroy the inner nodule structure. The bioerosion index (BI) is an estimation of the degree of bioerosion which ranges from no bioerosion (BI 1) to complete bioerosion (BI 6; undistinguishable arrangement of the inner coated grain; Bassi et al. 2012a). The BI was assessed on macroid polished slabs. BI is similar to the bioturbation index which estimates the percentages of restructuring in clastic- or limey-bedded sedimentary deposits by organisms (Taylor and Goldring 1993).
The studied material from Kikai-jima and Fraser Island is deposited at the Institute of Geology and Paleontology, Graduate School of Science, Tohoku University, while additional material from Fraser Island, and samples from One Tree Reef/Lizard Island, Moorea, and southeastern Spain are stored at the Departamento de Paleontología y Estratigrafía, Universidad de Granada, Spain. Additional material from Moorea is stored in the Museum of Paleontology, University of California. Isolated boring bivalve specimens are housed at the Department of Biological Sciences, Faculty of Science, Kanagawa University.
Results and discussion
Macroid and rhodolith beds
The samples collected in Kikai-jima consist of macroids with minimum and maximum diameters respectively 2.0 cm and 10.4 cm in size (n = 75, mean 6.4 cm, standard deviation s.d. ± 2.0 cm). Macroids from a single locality significantly vary in size from ~ 1.2 cm to 13 cm. Coalescent macroids can be up to 15 cm in size. Spheroidal and sub-spheroidal shapes dominate the studied samples (Fig. 1). Samples from 61–71 m water depth show rare sub-discoidal shapes. Inner macroid arrangement consists of concentrically, commonly asymmetrical, laminated, and superimposed Acervulina inhaerens tests associated with subordinate coralline thalli, serpulids, and bryozoans, and high volumes of constructional voids. Encrusting arborescent foraminifera, such as Homotrema and Miniacina, are also present. The macroid surface shows dominant encrusting foraminifera together with common to rare warty and rare lumpy thin (< 1 mm thick) coralline thalli (Lithothamnion sp., melobesioid indet.; Bassi et al. 2011).
Off Fraser Island in shallow-water settings between 28 and 60 m, rhodoliths comprise algal-coated pebbles and rhodoliths while only rhodoliths were found in deeper settings (> 60 m; see details in Lund et al. 2000). Algal-coated pebbles, up to 9 cm in diameter, range from spheroidal to ellipsoidal to discoidal, with a low percentage of constructional voids. Rhodoliths show a maximum diameter of 4.6 cm and the shape ranges from spheroidal to ellipsoidal, discoidal, and very discoidal (n = 76; Fig. 1). In shallower rhodoliths, melobesioids dominate with subordinate lithophylloids and other corallinaceans, sporolithaceans, and peyssonnelliaceans. Deeper rhodoliths, ranging from ~ 2 to 5 cm in maximum diameter (n = 22), are composed of dominating sporolithaceans, melobesioids, and peyssonneliaceans (Lund et al. 2000). Rhodoliths show a high percentage of constructional voids.
One Tree Reef/Lizard Island rhodoliths, up to 15 cm in diameter (with mean, minimum, and maximum diameters respectively 4.6 cm (n = 7, s.d. ± 1.1 cm), 3 cm, and 6.3 cm in size), consist of coralline algae around coral fragments. The coral nuclei are usually larger than the algal cover, which comprises thick encrusting, lumpy to fruticose thalli of Porolithon gr. P. onkodes (Heydrich) Foslie, Hydrolithon boergesenii (Foslie) Foslie, and various minor amounts of Lithophyllum gr. L. kotschyanum Unger, Lithophyllum gr. L. pustulatum (Lamouroux) Foslie, Spongites sp., and Neogoniolithon sp. (Figs. 2 and 3).
The Moorea rhodoliths, ranging in size from 2.7 to 9.2 cm (n = 29, mean 4.5 cm, s.d. ± 1.3 cm), have a wide variety of shapes (Fig. 1). They typically consist of several thick coralline algal thalli encrusting coral fragments (Fig. 4). In many cases, the coral nucleus is larger than the algal covering and controls the nodule shape. Other rhodoliths are mainly made up of coralline algae and are sub-spheroidal to sub-discoidal/sub-ellipsoidal in shape, with a low constructional void percentage. Coralline growth forms tend to be encrusting in the inner part of the algal covering and pass outwards to warty/lumpy and fruticose. Thick encrusting to lumpy thalli of Porolithon gr. P. onkodes (Heydrich) Foslie are the most common components, followed by branching growths of Neogoniolithon sp. and Lithophyllum gr. L. kotschyanum Unger. Hydrolithon boergesenii (Foslie) Foslie and Lithophyllum gr. L. prototypum (Foslie) Foslie also occur as minor elements.
Slab surfaces of Moorea algal nodules (< ~ 2 m water depth) showing encrusting (en) and fruticose (fr) coralline growth forms and the boring bivalve traces (G, Gastrochaenolites). Within the algal nodules, serpulids and encrusting/arborescent foraminifera (min, Miniacina; hr, Homotrema) are present. Note that most of the nodules consist of coral fragments
Rhodoliths in southeastern Spain are mainly fruticose and boxwork structures (from 2.3 to 4.9 cm in size, mean 3.3 cm, s.d. ± 0.7 cm, n = 18; Fig. 1), developed around relatively small bioclastic nuclei (Figs. 5 and 6). Monospecific fruticose rhodoliths can be formed by Lithophylllum gr. L. racemus (Lamarck) Foslie, Phymatolithon gr. P. calcareum (Pallas) Adey and McKibbin, Lithothamnion valens Foslie, Lithothamnion minervae Basso, Lithothamnion gr. L. corallioides (Crouan and Crouan) Crouan and Crouan, and Spongites fruticulosus Kützing. Complex, multispecific rhodoliths with boxwork structure can include combinations of any of these species and other Lithophyllum, Lithothamnion, Mesophyllum, Phymatolithon, and Sporolithon species, as well as Peyssonneliacean algae. Below 40 m they are mainly small loose-branching rhodoliths made of L. gr. L. corallioides and P. gr. P. calcareum.
Slab surfaces of rhodoliths from Cabo de Gata showing complex boring patterns and bioerosion traces from 24.5, 30, 40, and 50 m water depth. Symbol points out the split rhodoliths (see Fig. 6). E, Entobia; G, Gastrochaenolites; en, encrusting; lu, lumpy; fr, fruticose; bn, bioeroded nucleus
Slab surfaces of two splitted rhodoliths showing their inner arrangement and the trace fossils; Cabo de Gata. Each rhodolith was cut in two parts through its center. Note that the Gastrochaenolites specimen (G; comparable to G. lapidicus Kelly and Bromley; dashed lines) still contains its producer boring bivalve (Gastrochaena sp.), which grew in encrusting (en) and lumpy (lu) coralline growth forms. The rhodolith shape is not influenced by the boring-bivalve shape
Distribution and relative abundance of the ichnotaxa
Five ichnogenera were identified: Entobia Bronn, Gastrochaenolites Leymarie, Trypanites Mägdefrau, Maeandropolydora Voigt, and Rogerella de Saint-Seine, the latter present only off Fraser Island. Trypanites/Maeandropolydora is used when distinguishing ichnocharacters are not preserved. Micro-endolithic traces also occur. The borings often match exactly the shape of the boring endolithic invertebrate (Fig. 6), although similar borings are sometimes produced by different organisms. The bioerosion structures are distributed in two tiers relative to the substrate surface: shallow and deep. All the ichnotaxa occur as rare to abundant (Fig. 7).
Distribution of the ichnogenera, their relative abundance in the studied areas, and the bioerosion index (BI). The distribution of samples is plotted according the different sampling methods (see text for details). The sampled bathymetric range of the studied beds is also reported (depth intervals are dark gray for macroids and gray for rhodoliths). E, Entobia; G, Gastrochaenolites; T, Trypanites; T/M, Trypanites/Maeandropolydora; R, Rogerella; r, rare; c, common; a, abundant
In the studied sites, the bioerosion index (BI) ranges from 1 to 4, being higher in the deeper water settings (Fig. 7). The shallowest studied rhodoliths show BI 1 (i.e., One Tree Reef/Lizard Island, Moorea; Fig. 7). From ~ 3 to ~ 10 m water depth, rhodoliths have a BI of 1 with a single case of B2 in Cabo de Gata. Below 10 m to 60–70 m water depth, macroids and rhodoliths show BI 2 (Kikai-jima, Fraser Island, Cabo de Gata). BI 3 was recognized in macroids and rhodoliths down to ~ 115 m (Kikai-jima, Fraser Island), with locally BI 4 in the deepest samples (below 90 m).
Gastrochaenolites: size, location, and boring bivalves
In general, the identified Gastrochaenolites specimens are 3.5–16.7 mm in length. Length of Gastrochaenolites ichnospecimens shows a significant correlation with bathymetry (correlation coefficient R = 0.44, p < 0.001; Fig. 8a). On the other hand, a very weak but statistically significant correlation (R = 0.16, p < 0.05) was observed between macroid/rhodolith size and depth (Fig. 8b). There is no significant correlation between macroid/rhodolith size and Gastrochaenolites length (R = 0.06, p > 0.05).
Plots of (a) length of Gastrochaenolites ichnospecimens and water depth (R = 0.44, p = 0.001, total n = 57; Fraser Island n = 21; Kikai-jima, n = 11; Cabo de Gata, n = 12; Moorea, no = 7; One Tree Reef/Lizard Island, n = 6), (b) Macroid and rhodolith sizes and water depth (R = 0.16, p = 0.0145, n = 234) from the six study cases. The sampled bathymetric ranges of the studied beds are also reported for each studied locality (vertical bars). OTR/LI, One Tree Reef/Lizard Island
In our samples, remains of the bivalve producers of Gastrochaenolites may still be found in situ inside most of the related perforations. However, some boring bivalves do not preserve enough diagnostic shell characters for species determination. The identified boring bivalves belong to four genera (Gastrochaena, Gregariella, Leiosolenus, Lithophaga) and eleven species (Table 2). When diagnostic shell characters for distinguishing the genera Leiosolenus and Lithophaga (family Mytilidae) are not preserved, Lithophaga/Leiosolenus sp. is used. The bivalves are represented by adults of small sizes and juveniles with approximately the same size ranges of the small adults. Juveniles and adults were distinguished according to the size ranges reported in literature as well as comparing the growth increments marked by the growth lines (Carter 1978; Savazzi 1999; Carter et al. 2008; Owada and Hoeksema 2011).
In Kikai-jima, Gastrochaenolites commonly occurs in the outer parts of the larger macroids, but below 85 m water depth Gastrochaenolites occur both in the inner and outer parts. Boring bivalves, represented by juvenile specimens of Lithophaga spp. and Lithophaga/Leiosolenus sp. (Table 2, Fig. 9), are chemical borers (e.g., Savazzi 1999). These macroids are subspheroidal in shape and about 8 cm in diameter.
Boring mytilid bivalves from deep-water macroids off Kikai-jima, Japan. a Leiosolenus sp., inner and outer shell surface. b, c SEM photos illustrating shell microstructures of Lithophaga sp. (b) and Leiosolenus sp. (c). HO, homogeneous structure; SN, sheet nacreous; ISP, irregular simple prisms; ISpP, irregular spherulitic prisms
Off Fraser Island Gastrochaenolites generally occurs perpendicular to the rhodoliths’ outer surface. They can also occur just beneath or some millimeters below the surface where they are overlain by encrusting coralline algal thalli (Fig. 10). This ichnotaxon does not occur in rhodoliths smaller than 3 cm; rare boring bivalves were preserved in the holes. In the shallower water rhodoliths (see Bassi et al. 2013), the identified boring bivalves are juvenile individuals of Gastrochaena cuneiformis Spengler, Leiosolenus malaccanus (Reeve), L. mucronatus (Philippi), and Leiosolenus spp. Leiosolenus malaccanus (up to 2.5 cm in length) and L. mucronatus (up to 1.2 cm in length) also occur as adults. The boring bivalves Lithophaga/Leiosolenus sp. and Lithophaga sp. were identified in the deep-water rhodoliths (Table 2).
Thin section photomicrographs showing Gastrochaenolites (G), Trypanites (T), and Trypanites/Maeandropolydora (T/M) in rhodoliths off Fraser Island. a Cylindrical tubes of Trypanites bore successive coralline thallial growth increments. b Encrusting coralline thallus (arrow) overlying Gastrochaenolites
In the shallow-water One Tree Reef, Lizard Island, and Moorea rhodoliths, Gastrochaenolites is rare to common. Very small ovate main chambers with a very long neck region, circular in cross section throughout, occur in the rhodolith nuclei (generally a coral fragment), and the long necks (tubes) reach up to the last growth stage constructed by coralline thalli (Figs. 4, 5, and 6). These ichnospecimens show similarities to G. turbinatus Kelly and Bromley, 1984. No boring bivalves were found within the traces (compare with Kleemann 1995).
Gastrochaenolites was recognized throughout the Cabo de Gata rhodolith beds being abundant at 30 to 40 m water depth. Some ichnospecimens show smooth, elongate ovate chambers circular in cross section with a distinct neck region (Figs. 5 and 6). These characteristics of the ichnospecimens are comparable to G. lapidicus Kelly and Bromley, 1984. Gastrochaenolites specimens commonly contain the producing boring bivalves, which are Gregariella petagnae (Scacchi), possible juvenile forms, and Gastrochaena sp. The studied specimens show keel shells in shape (~ 7 mm in length), hairs on the shell surface, and prominent dysodont teeth in the anterior and posterior margins. These characters are considered diagnostic of Griegariella petagnae (Huber, 2010). The siphons of large boring Gastrochaena sp. can be encrusted by very thin coralline thalli (Fig. 6). The shape of a rhodolith with a bivalve boring in the nucleus is not influenced by the boring-bivalve shape (Figs. 5 and 6).
Ecological evidence and constraints in Gastrochaenolites bathymetric distribution
In the studied macroids and rhodoliths, the occurrence of boring bivalves and related traces (Gastrochaenolites) is constrained by their ecology. These constraints, ranging from the relationship with other boring and encrusting organisms (i.e., associated ichnotaxa and bioerosion index BI) through the sizes of the bivalves and the host (i.e., macroids and rhodoliths) to the nature and stability of the substrate, can be assessed even in fossil material.
In our samples, Gastrochaenolites is rarely affected by other traces if it kept up with the growth of the outer nodule surface (Moorea, Cabo de Gata; Figs. 4 and 5). By contrast, if Gastrochaenolites is buried in the nodule, its last and upper part can be perforated by other borings (Kikai-jima, fig. 6 in Bassi et al. 2012a; One Tree Reef/Lizard Island, Fig. 3). Gastrochaenolites producers, when still alive, therefore, do not have any active competitor for space (e.g., Carter 1978).
In the settings above 10 m water depth, Gastrochaenolites is rare to common (e.g., One Tree Reef/Lizard Island, Moorea) and becomes abundant along with Entobia below 20 m (Cabo de Gata) and 30 m water depth (Fraser Island; Fig. 7). Our studied cases show that the bioerosion increases below ~ 40 m water depth as evidenced by the BI. High BI (i.e., 3–4) at 40–50 m water depth in the Cabo de Gata rhodoliths and below 60 m water depth in the Kikai-jima macroids and Fraser Island rhodoliths (Fig. 7) indicate high bioerosion intensity, related to a lower macroid and rhodolith growth rate and to long-lasting exposure on the sea floor. Influence of high boring speed and population density cannot be ruled out as bioerosion factors. On the Brazilian shelf in high nutrient settings, delayed burial favors intense boring of rhodoliths (Brasileiro et al. 2018). In high-energy intertidal and reef-crest/shallow reef-front settings, macroboring assemblages are typically dominated by sponges while boring bivalves decrease below ~ 30 m water depth (e.g., Perry 1998; Perry and Hepburn 2008).
The recorded Gastrochaenolites specimens show a significant correlation between chamber-length and bathymetry (Fig. 8a). The largest specimens occur in Kikai-jima macroids (> 60 m), while the smallest specimens were recorded in Moorea, One Tree Reef, and Fraser Island rhodoliths. In Cabo de Gata and Fraser Island rhodoliths (< 60 m), large and small individuals occur throughout. Very weak but statistically significant correlation occurs between nodule size and water depth. However, the absence of correlation between nodule size and Gastrochaenolites length suggests that stability of the nodules rather than their size favors boring bivalve growth.
Boring bivalves need a relatively stable substrate with currents to avoid burial. Above the fair-weather wave base, the overturn rate of the rhodoliths constrains the growth of the boring bivalves which, then, may not reach the adult stage. In the deeper settings, the higher stability of the macroids and rhodoliths, which only occasionally overturn (e.g., Matsuda and Iryu 2011; Bassi et al. 2012a, 2013), allows the juvenile boring bivalves to thrive longer. The absence of correlation between the nodule size and Gastrochaenolites length confirms that nodule size does not affect boring bivalve colonization and growth (e.g., Fig. 6; Fig. 6, Bassi et al. 2012a; Fig. 5a and 5c, Bassi et al. 2013).
The average shell sizes of boring bivalve individuals in the macroids and rhodoliths are slenderer and smaller than those growing inside rocky substrates. All our Gastrochaenolites specimens are at least ten times smaller than the known Gastrochaenolites spp. (Kelly and Bromley 1984; Donovan 2002, 2013; Donovan and Hensley 2006; Santos et al. 2011; Donovan et al. 2014; Silva et al. 2019). This is clear for G. lapidicus identified in the Spanish rhodoliths collected at 30–40 m water depth (Fig. 6). This ichnospecies has also been identified so far in clasts and cobbles from palaeoshores (Santos et al. 2011; Donovan 2013). The largest boring bivalves up to ~ 85 mm long producing large Gastrochaenolites were described in shallow-water rocky substrates (Santos et al. 2011; Bagur et al. 2016; Somaya et al. 2018). Although in larger bivalves and corals Gastrochaenolites spp. show large sizes (Perry 1998, 2000; Blanchon and Perry 2004; Domènech et al. 2014), they are much smaller than the ichnospecimens found in rocky substrates (Cachão et al. 2011). Different types of substrates generate different restrictions on the growth of Leiosolenus patagonicus individuals (as Lithophaga patagonica in Bagur et al. 2013). Boring bivalves in larger ostreid shells cause an increase of the metabolic energy costs to the host which produces extra conchiolin to seal off the holes (Diez et al. 2014). Leiosolenus patagonicus changes its phenotype depending on the oyster’s anti-parasitism responses by physical compression (Márquez et al. 2017). The chemical inducement or deterrent of acervulinid and coralline species to boring bivalve settlement is unknown.
According to these ecological constraints related to the nature and stability of the substrate, the boring bivalves and especially their traces (i.e., Gastrochaenolites) are more likely to be preserved and, hence, are useful proxies in palaeoecological studies. The distinction of Gastrochaenolites ichnospecies is based on the chamber shape and the occurrence of one or two tubes in the neck region (Bromley and D’Alessandro 1987; Donovan 2013; Bassi et al. 2017). Because the bored substrate no longer represents a possible diagnostic character of the ichnogenus (Donovan and Ewin 2018), the ichnotaxonomy of Gastrochaenolites ichnospecies needs a re-assessment which would provide hints to decipher the boring bivalve palaeobiodiversity in common fossil macroid and rhodolith deposits.
Boring bivalves and growth rates
The identified boring gastrochaenid and mytilid species are commonly found at depths shallower than 20 m (Carter et al. 2008; Owada and Hoeksema 2011; Printrakoon et al. 2016). In our samples, they also occur deeper than 40 m (Table 2). In particular, Lithophaga spp. and Leiosolenus spp. identified in the Kikai-jima macroids (> 75 m) are the deepest-living boring mytilids reported so far. Adult gastrochaenids and mytilids boring deep-water macroids and rhodoliths are smaller than their shallow-water counterparts in rocky-shelly substrates as a phenotypic response to size and substrate stability. Lithophaga and Leiosolenus are monophyletic genera (Owada 2007), whose species show shell shapes that vary according to the different thickness of the substrate (Owada 2015). These variations appear to be an ecophenotypic response to substrate thickness, although a genetic component is possible based on the separation of species by depth. Genetic studies of these species’ populations, however, have not yet been done. Another alternative hypothesis is that food supply could be a factor in bivalve size. While the small size of the shallowest (< 10 m; Lizard Island, One Tree Reef, Moorea) boring bivalves can be related to nutrient depletion in coral reefs, no oligotrophic conditions prevail in the rest of sampling sites, and, therefore, nutrient availability does not seem to be a major controlling factor on boring-bivalve size. For the moment, we accept the first hypothesis of ecophenotypic response to be most likely pending further information.
The small Gastrochaenolites ichnospecimens represent both juvenile boring bivalves that could not reach the adult stage and young adults (Fig. 11). Most of the identified boring bivalves were, in fact, live juveniles of chemical borers (Kikai-jima, Fraser Island, Cabo de Gata; Table 2). These juveniles likely start boring after reaching ~ 1.5 mm in length, like shallow-water juveniles of Barnea manilensis (e.g., < 5 m, Ito 1999). The identified adults are very small in size compared with the shallow-water counterparts in rocky shelly substrates (Kleemann 1984). While the adults of Leiosolenus mucronatus are rather smaller than those of the other species even in shallow-water settings (Kleeman and Maestrati 2012), the ones of identified Lithophaga spp. are very small in size compared with the shallow-water counterparts in rocky shelly substrates (Kleeman 1984). Comparing the length of the studied Gastrochaenolites with the previously reported length to growth-rate ratio of gastrochaenid and mytilid boring bivalves (Carter 1978; Bagur et al. 2013; Peharda et al. 2015), our ichnospecimens are likely to be only up to a few years in age or small-sized adults. The small-sized adults grew obviously with slower growth rate than the shallower counterparts. The adaptation to the substrate (macroids and rhodoliths) constrained the bivalve growth rate or, vice versa, the bivalve grew slower to adapt to the substrate.
Schematic summary of development and preservation of bivalve borings (Gastrochaenolites) in deep-water macroids and rhodoliths. a The juvenile bivalve growth rate is as rapid as the encrusting foraminiferal and coralline red algal growth rate. b In young bivalves, growth rate is higher than the host’s rate; these individuals cannot be embedded by the host and, therefore, die. c No adults are preserved unless phenotypic plasticity promoted small individuals. General growth curve of age-at-length boring bivalves after Bagur et al. (2013) and Peharda et al. (2015)
Gastrochaenids and mytilids perform different chemical boring activity. Adapted to live in rapidly eroded and broken coral margins, gastrochaenids can impede or escape coral overgrowth by elongating their siphonal burrow (Carter 1978). The siphons are especially extensible and retractable, thereby enabling the organism to survive coral overgrowth or erosion. A similar strategy seems to be performed by some boring bivalves inhabiting macroids and rhodoliths (e.g., Gastrochaena sp. in Cabo de Gata; Fig. 6). The Mytilidae, such as Gregariella, Leiosolenus, and Lithophaga, do not possess siphons (Savazzi 1999).
When the host substrate is fouled by epibionts or overgrows the opening of the borehole, these bivalves react by boring backward (Savazzi 1999). This backward migration can be interrupted if the host growing rate is slower than the boring bivalve rate or if macroids and rhodoliths are moved or overturned, as is likely in the studied case. Because macroids and rhodoliths do not constantly grow (e.g., Matsuda and Iryu 2011), the juveniles colonized the host surfaces and grew until a subsequent turning or burial of the host. The turning or burial commonly result in the death of the bivalves.
As in large bivalve shells and corals (Perry and Hepburn 2008; Bagur et al. 2014), boring mytilids colonize both living and dead macroids and rhodoliths. However, since these bivalves do not elongate their siphonal burrow and they need protection during their growth, bivalves in dead/inactive macroids and rhodoliths may be killed in a few years. In this case, the preservation of the bivalve shell inside their traces is very rare.
Deep-water (> 20 m) boring bivalve growth rates are unknown. Shallow-water boring bivalves show high variations in growth rates among individuals, during different seasons and with different substrate hardness (Peharda et al. 2015; Bagur et al. 2013; Wizemann et al. 2018). Longevity and growth vary among Lithophaga species, which thrive in hard carbonate substrates. In Lithophaga lithophaga living at less than 3 m water depth in Croatia, ontogenetic ages of shells varied from 10 to 54 years with a growth rate ranging from ~ 3.3 mm/year (for individuals 10 years old, the youngest one analyzed in the study) to ~ 1.7 mm/year (50 years old; Peharda et al. 2015). In southwest Argentina, intertidal rock-boring Leiosolenus patagonicus populations longevity ranged between 6.5 and 15 years with a growth rate from ~ 3.6 mm/year (4 years old) to ~ 2.2 mm/year (13 years old; Bagur et al. 2013).
Many studies report shallow-water rhodolith growth rates of < 1 mm/year (Bosence 1983b; De Grave et al. 2000; Frantz et al. 2000; Rivera et al. 2004; Steller et al. 2007; Kamenos et al. 2008). Annual growth rates for rhodoliths from the Santa Catalina Island, California were calculated at 1.25 ± 0.62 mm/year (Tompkins and Steller 2016). Although some tropical coralline species grow faster than 1 mm/year (Matsuda 1989), the majority of coralline species have growth rates < 0.4 mm/year (Figueiredo et al. 2012).
Considering a calibrated age of about 3500 cal. year BP, the deep-water macroids off Kikai-jima have an apparent growth rate of about 0.02 mm/year (Bassi et al. 2019). These macroids did not grow continuously; rather their growth is commonly intermittent, probably as a result of repeated burial and exposure, as commonly known from many macroids and rhodoliths in various settings (e.g., Matsuda and Iryu 2011). This apparent macroid growth rate is comparable to those for deep-water foraminiferal-algal nodules of 0.02–0.09 mm/year at 30–60 m depth (Reid and Macintyre 1988) and 0.01–0.05 mm/year at 61–91 m depth (Littler et al. 1991). Comparable growth rates have been found for deep-water rhodoliths on the Brazilian Shelf (0.04 mm/year, Brasileiro et al. 2018).
In shallow-water (< 12 m), rocky and sandy coquina substrates Lithophaga growth rates of ~ 1.6–3.0 mm/year (Peharda et al. 2015) are higher than those reported for our deeper macroids/rhodoliths (0.01–0.05 mm/year). Considering that substrate composition and hardness along with water temperature seem to largely influence boring bivalve growth parameters (Bagur et al. 2014; Peharda et al. 2015), in deep-water macroids and rhodoliths, the boring bivalves likely grow slower than the shallow-water counterparts, although still higher than the host’s growth. In the deep-water macroid and rhodolith beds, the identified juvenile boring bivalve specimens grew as fast as their host just during the early growth stages (Fig. 11). Since the boring bivalves grow faster than the macroid and rhodolith host, most of them died or reached an early adult stage characterized by a small size due to a slow growth rate. For other macroids and rhodoliths that might grow faster, these problems do not develop. Where deep-water macroids and rhodoliths might overturn, the juveniles may be choked and die.
Concluding remarks
Six study cases of macroid and rhodolith beds from 0 to 117 m water depth of the Pacific Ocean (northern Central Ryukyu Islands, Fraser Island, One Tree Reef, Lizard Island, Moorea) and Mediterranean Sea (southeastern Spain) were analyzed. Distribution of the domichnia Gastrochaenolites and its producers (i.e., gastrochaenid and mytilid boring bivalves) were assessed. Four core conclusions underscore unusual aspects of the studied macroid and rhodolith beds.
-
1.
The gastrochaenid and mytilid boring bivalves do not show competition for space with other borers such as etching sponges and polychaete worms. The highest bioerosion recorded in the macroids and rhodoliths below wave base is mainly produced by sponges and polychaete worms.
-
2.
The boring bivalves belong to four genera (Gastrochaena, Gregariella, Leiosolenus, Lithophaga) and eleven species. For the first time, living individuals of Gastrochaena cuneiformis, G. cf. turbinatus, Leiosolenus (L. malaccanus, L. mucronatus, L. spp.; Fraser Island, deeper than 40 m), and Lithophaga/Leiosolenus sp. (Kikai-jima, deeper than 75 m) were found in waters deeper than 20 m.
-
3.
On average, the boring bivalves are slenderer and smaller than those growing inside shallow-water rocky substrates. In free-living nodules, the boring bivalve growth is constrained by the acervulinid foraminiferal/coralline algal host growth rate and by the host-overturn and burial rate. In shallow-water settings, the higher rhodolith-overturning rate causes juvenile mortality. In deep-water (> 40 m) higher macroid and rhodolith stability allows the boring bivalves to reach an adult stage.
-
4.
During the juvenile stage, the bivalves can equal the host growth but, due to their faster growth, rarely they reach the early adult stage. Although the growth rate of deeper gastrochaenids and mytilids is presumably slower than that of their shallow-water rocky-substrate counterparts, it is faster than that of the acervulinid foraminiferal/coralline algal hosts. As a consequence of phenotypic plasticity, small-sized adults with slow growth rates might coexist with juveniles.
-
5.
These observations provide a basis for palaeoecological interpretation of fossil macroid and rhodolith deposits in terms of boring bivalve morphologic variation, ichno- and palaeo-biodiversity, and palaeobathymetry. The use of Gastrochaenolites ichnospecies as palaeobathymetric indicators must be treated with caution due to the lack of taxonomic and ecological (phenotypic plasticity) data about present-day deep-water boring bivalves.
Availability of data and materials
All data generated and analyzed during this study are included in this published article. Please contact the corresponding author regarding data requests.
Abbreviations
- BI:
-
Bioerosion index
- E :
-
Entobia Bronn
- G :
-
Gastrochaenolites Leymarie
- M :
-
Maeandropolydora Voigt
- s.d.:
-
Standard deviation
- T :
-
Trypanites Mägdefrau
- R :
-
Rogerella de Saint-Seine
References
Adey W, Halfar J, Humphreys A, Suskiewicz T, Belanger D, Gagnon P, Fox M (2015) Subartic rhodolith beds promote longevity of crustose coralline algal buildups and their climate archiving potential. Palaios 30:281–293. https://doi.org/10.2110/palo.2014.075
Aguirre J, Braga JC, Bassi D (2017b) The role of rhodoliths and rhodolith beds in the rock record and their use in palaeoenvironmental reconstructions. In: Riosmena-Rodriguez R, Nelson W, Aguirre J (eds) Rhodolith/maerl beds: a global perspective. Springer, Berlin, spec vol, pp 105−138. https://doi.org/10.1007/978-3-319-29315-8_5
Aguirre J, Doménech R, Martinell J, Mayoral E, Santos A, Pérez-Asensio JN (2017a) Witnesses of the early Pliocene Sea-level rise in the Manilva Basin (Málaga, S Spain). Span J Palaeontol 32:35–52
Amado-Filho GM, Moura RL, Bastos AC, Salgado LT, Sumida PY, Guth AZ, Frncini-Filho RB, Pereira-Filho GH, Abrantes DP, Brasileiro PS, Bahia RG, Leal RN, Kaufman L, Kleypas JA, Farina M, Thompson FL (2012) Rhodolith beds are major CaCO3 bio-factories in the tropical touth West Atlantic. PLoS One 7(4):e35171. https://doi.org/10.1371/journal.pone.0035171
Arai K, Inoue T, Matsuda H, Machiyama H, Sasaki K, Iryu Y, Sugihara K, Fujita K, Nara M (2008) Shallow seismic profiling survey on postglacial fore-reef near the present-day northern limit of coral reef formation in the northwestern Pacific. Proc 11th Int Coral Reef Symp 4:9–52.
Baarli BG, Santos A, da Silva CM, Ledesma-Vázquez J, Mayoral E, Cachão M, Johnson ME (2012) Diverse Macroids and Rhodoliths from the Upper Pleistocene of Baja California Sur, Mexico. J Coast Res 28:296–305. https://doi.org/10.2112/11T-00010.1
Bagur M, Gutiérrez JL, Arribas LP, Palomo MG (2014) Endolithic invertebrate communities and bioerosion rates in southwestern Atlantic intertidal consolidated sediments. Mar Biol 161:2279–2292. https://doi.org/10.1007/s00227-014-2505-8
Bagur M, Gutiérrez JL, Arribas LP, Palomo MG (2016) Complementary influences of co-occurring physical ecosystem engineers on species richness: insights from a Patagonian rocky shore. Biodivers Conserv 25:2787–2802. https://doi.org/10.1007/s10531-016-1203-x
Bagur M, Richardson CA, Gutiérrez JL, Arribas LP, Socorro Doldan M, Palomo MG (2013) Age, growth and mortality in four populations of the boring bivalve Lithophaga patagonica from Argentina. J Sea Res 81:49–56. https://doi.org/10.1016/j.seares.2013.04.003
Bassi D, Humblet M, Iryu Y (2011) Recent ichnocoenosis in deep water macroids, Ryukyu Islands, Japan. Palaios 26:232–238. https://doi.org/10.2110/palo.2010.p10-093r
Bassi D, Iryu Y, Braga JC, Takayanagi H, Tsuji T (2013) Bathymetric distribution of ichnocoenoses from recent subtropical algal nodules off Fraser Island, eastern Australia. Palaeogeogr Palaeoclimatol Palaeoecol 369:58–66. https://doi.org/10.1016/j.palaeo.2012.10.003
Bassi D, Iryu Y, Humblet M, Matsuda H, Machiyama H, Sasaki K, Matsuda S, Arai K, Inoue T (2012a) Recent macroids on the Kikai-jima shelf, Central Ryukyu Islands, Japan. Sedimentology 59:2024–2041. https://doi.org/10.1111/j.1365-3091.2012.01333.x
Bassi D, Iryu Y, Humblet M, Matsuda H, Machiyama H, Sasaki K, Matsuda S, Arai K, Inoue T (2019) Deep-water macroid beds of the Ryukyu Islands, Japan: encrusting acervulinids as ecosystem engineers. J Coast Res 35:463–466. https://doi.org/10.2112/JCOASTRES-D-18-00049.1
Bassi D, Iryu Y, Nebelsick JH (2012b) To be or not to be a fossil rhodolith? Analytical methods for studying fossil rhodolith deposits. J Coast Res 28:288–295. https://doi.org/10.2112/11T-00001.1
Bassi D, Posenato R, Nebelsick JH, Owada M, Domenicali E, Iryu Y (2017) Bivalve borings in lower Jurassic Lithiotis fauna from northeastern Italy and its palaeoecological interpretation. Hist Biol 29:937–946. https://doi.org/10.1080/08912963.2016.1265956
Blanchon P, Perry CT (2004) Taphonomic differentiation of Acropora palmata facies in cores from Campeche Bank reefs, Gulf of México. Sedimentology 51:53–76. https://doi.org/10.1046/j.1365-3091.2003.00610.x
Bosellini A, Ginsburg RN (1971) Form and internal structure of recent algal nodules (rhodolites) from Bermuda. J Geol 79:669–682
Bosence DWJ (1983a) Description and classification of rhodoliths (rhodoids, rhodolites). In: Peryt TM (ed) Coated grains. Springer, Berlin, pp 217–224
Bosence DWJ (1983b) The occurrence and ecology of recent rhodoliths – a review. In: Peryt TM (ed) Coated grains. Springer, Berlin, pp 225–242
Bosence DWJ (1985) The “Coralligene” of the Mediterranean - a recent analogue for tertiary coralline algal limestones. In: Toomey DF, Nitecki MH (eds) Contemporary research and applications. Springer, Berlin, pp 216–225
Boyd R, Ruming K, Goodwin I, Sandstrom M, Schroder-Adams C (2008) Highstand transport of coastal sand to the deep ocean: a case study from Fraser Island, Southeast Australia. Geology 36:15–18. https://doi.org/10.1130/G24211A.1
Brasileiro PS, Braga JC, Amado-Filho GM, Leal RN, Bassi D, Franco T, Bastos AC, Moura RL (2018) Burial rate determines Holocene rhodolith development on the Brazilian shelf. Palaios 33:464–477. https://doi.org/10.2110/palo.2017.109
Bromley RG (1996) Trace fossils: biology, taphonomy and applications. Chapman and Hall, London, 361 pp
Bromley RG, D’Alessandro A (1983) Bioerosion in the Pleistocene of southern Italy: ichnogenera Caulostrepsis and Maeandropolydora. Riv Ital Paleontol Strat 89:283–309
Bromley RG, D’Alessandro A (1984) The ichnogenus Entobia from the Miocene, Pliocene and Pleistocene of southern Italy. Riv Ital Paleontol Strat 90:227–296
Bromley RG, D’Alessandro A (1987) Bioerosion of the Plio-Pleistocene transgression of southern Italy. Riv Ital Paleontol Strat 93:379–442
Bromley RG, D’Alessandro A (1989) Ichnological study of shallow marine endolithic sponges from the Italian coast. Riv Ital Paleontol Strat 95:279–314
Cachão M, Redweik P, Barreira E, Dinis J, Catita C, da Silva CM, Santos A, Mayoral E, Linder W (2011) Photogrammetric and spatial analysis of a bioeroded Early Miocene rocky shore, western Portugal. Facies 57:417–429. https://doi.org/10.1007/s10347-010-0248-7
Carter JG (1978) Ecology and evolution of the Gastrochaenacea (Mollusca, Bivalvia) with notes on the evolution of the endolithic habitat. Bull Peabody Mus Nat Hist 41:1–92
Carter JG, McDowell T, Namboodiri N (2008) The identity of Gastrochaena cuneiforms Spengler, 1783, and the evolution of Gastrochaena, Rocellaria, and Lamychaena (Mollusca, Bivalvia, Gastrochaenoidea). J Paleontol 82:102–117. https://doi.org/10.1666/04-066.1
Checconi A, Bassi D, Monaco P, Carannante G (2010) Re-deposited rhodoliths in the middle Miocene hemipelagic deposits of Vitulano (southern Apennines, Italy): coralline assemblage characterization and related trace fossils. Sediment Geol 225:50–66. https://doi.org/10.1016/j.sedgeo.2010.01.001
Choukroun S, Ridd PV, Brinkman R, McKinna LIW (2010) On the surface circulation in the western Coral Sea and residence times in the great barrier reef. J Geophys Res 115(C6):C06013. https://doi.org/10.1029/2009JC005761
Davies PJ, Radke BM, Robison C (1976) The evolution of one tree reef, southern great barrier reef, Queensland. BMR J Aust Geol Geophys 1:231–240
De Grave S, Fazakerley H, Kelly L, Guiry MD, Ryan M, Walshe J (2000) A study of selected maërl beds in Irish waters and their potential for sustainable extraction. Mar res Ser 10. The Marine Institute, Dublin http://hdl.handle.net/10793/209
Diez ME, Vázquez N, Urteaga D, Cremonte F (2014) Species associations and environmental factors influence activity of borers on Ostrea puelchana in northern Patagonia. J Molluscan Stud 80:430–34. https://doi.org/10.1093/mollus/eyu035
Domènech R, Farinati EA, Martinell J (2014) Crassostrea patagonica (d’Orbigny, 1842) shell concentrations from the late Miocene of Rio Negro province, NE Patagonia, Argentina. Spanish J Paleontol 29:165–182
Donovan SK (2002) A new ichnospecies of Gastrochaenolites Leymerie from the Pleistocene port Morant formation of Southeast Jamaica and the taphonomy of calcareous linings in clavate borings. Ichnos 9:61–66. https://doi.org/10.1080/10420940190034085
Donovan SK (2013) A recent example of the boring Gastrochaenolites lapidicus Kelly and Bromley and its producing organism in north Norkfolk, eastern England. Bull. Mizunami Fossil Mus 39:69–71
Donovan SK, Ewin TAM (2018) Substrate is a poor ichnotaxobase: a new demonstration. Swiss J Palaeontol 1:103–107. https://doi.org/10.1007/s13358-018-0146-0
Donovan SK, Harper DAT, Portell RW, Renema W (2014) Neoichnology and implications for stratigraphy of reworked upper Oligocene oysters, Antigua, West Indies. Proc Geologists’s Ass 125:99–106. https://doi.org/10.1016/j.pgeola.2013.10.002
Donovan SK, Hensley C (2006) Gastrochaenolites Leymerie in the Cenozoic of the Antillean region (review). Ichnos 13:11–19. https://doi.org/10.1080/10420940500511629
Edinger EN, Risk MJ (1994) Oligocene–Miocene extinction and geographic restriction of Caribbean corals: roles of turbidity, temperature, and nutrients. Palaios 9:576–598
Edinger EN, Risk MJ (1997) Sponge borehole size as a relative measure of bioerosion and paleoproductivity. Lethaia 29:275–286. https://doi.org/10.1111/j.1502-3931.1996.tb01660.x
Ekdale AA, Bromley RG (2001) Bioerosional innovation in the early Ordovician of Sweden: a revolutionary adaptation for living in carbonate hardgrounds. Lethaia 34:1–12. https://doi.org/10.1080/002411601300068152
Ekdale AA, Bromley RG, Pemberton SG (1984) Ichnology. SEPM Short Course 15, Tulsa, 317 pp
Fajemila O, Langer MR, Lipps JH (2020) Atlas of shallow-water tropical benthic Foraminifera from Moorea (Society Archipelago, French Polynesia). Cushman Found Foraminifer Res Spec Publ 48. In press
Fajemila OT, Langer MR, Lipps JH (2015) Spatial patterns in the distribution, diversity and abundance of benthic foraminifera around Moorea (Society Archipelago, French Polynesia). PLoS One 10(12): e0145752. https://doi.org/10.1371/journal.pone.0145752
Figuiredo MAO, Coutinho R, Villas-Boas AB, Tâmega FTS, Mariath R (2012) Deep-water rhodolith productivity and growth in the southern Atlantic. J Appl Phycol 24:487–493. https://doi.org/10.1007/s10811-012-9802-8
Foster MS (2001) Rhodoliths: between rocks and soft places. J Phycol 87:659–667. https://doi.org/10.1046/j.1529-8817.2001.00195.x
Foster MS, Amado-Filho GM, Kamenos NA, Riosmena-Rodríguez R, Steller DL (2013) Rhodoliths and rhodolith beds. In: Lange M (ed) Smithsonian contributions to the marine sciences 39. Smithsonian Institution Scholarly Press, Washington DC, pp 143–155
Frantz B, Kashgarian M, Coale KH, Foster MS (2000) Growth rate and potential climate record from a rhodolith using 14C accelerator mass spectrometry. Limnol Oceanogr 45:1773−1777. https://doi.org/10.4319/lo.2000.45.8.1773
Gibert JM, de Domènech R, Martinell J (2004) An ethological framework for animal bioerosion trace fossils upon mineral substrate with proposal of a new class, Fixichnia. Lethaia 37:429–437. https://doi.org/10.1080/00241160410002144
Gibert JM, de Domènech R, Martinell J (2012) Rocky shorelines. In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments, Developments in sedimentology, vol 64. Springer, Berlin, pp 441–462
Graham DJ, Midglay NG (2000) Graphical representation of particle shape using triangular diagrams: an excel spreadsheet method. Earth Sur Proc Land 25:1473–1477. https://doi.org/10.1002/1096-9837(200012)25:13<1473::AID-ESP158>3.0.CO;2-C
Hench JL, Leichter JJ, Monismith SG (2008) Episodic circulation and exchange in a wave-driven coral reef and lagoon system. Limnol Oceanogr 53:2681–2694. https://doi.org/10.2307/40058355
Hollander J, Collyer ML, Adams DC, Johannesson K. 2006. Phenotypic plasticity in two marine snails: constraints superseding life history. J Evol Biol 19(6):1861–1872. https://doi.org/10.1111/j.1420-9101.2006.01171.x
Hottinger L (1983) Neritic macroid genesis, an ecological approach. In: Peryt TM (ed) Coated grains. Springer, Berlin, pp 38–55. https://doi.org/https://doi.org/10.1007/978-3-642-68869-0_5
Huber M (2010) Compendium of bivalves. ConchBooks Vorm, Hackenheim, 904 pp
Iryu Y, Nakamori T, Matsuda S, Abe O (1995) Distribution of marine organisms and its geological significance in the modern reef complex of the Ryukyu Islands. Sediment Geol 99:243–258. https://doi.org/10.1016/0037-0738(95)00047-C
Ito Y (1999) Ontogenetic changes in boring behavior by the rock-boring bivalve, Barnea manilensis. Veliger 42(2):157–168
J-DOSS, JODC Data On-line Service System, Japan. https://www.jodc.go.jp/service.htm. Accessed 01 March 2020
Kamenos NA, Cusack M, Moore PG (2008) Red coralline algae are global paleothermometers with bi-weekly resolution. Geochim Cosmochim Acta 72:771–779. https://doi.org/10.1016/j.gca.2007.11.019
Kelly SRA, Bromely RG (1984) Ichnological nomenclature of clavate borings. Palaeontology 27:793–807
Kleemann K, Maestrati P (2012) Pacific Lithophaga (Bivalvia, Mytilidae) from recent French expeditions with the description of two new species. Boll Malacol 48:73–102
Kleemann KH (1980) Boring bivalves and their host corals from the Great Barrier Reef. J. Molluscan Stud 46:13–54. https://doi.org/10.1093/oxfordjournals.mollus.a065519
Kleemann KH (1984) Lithophaga (Bivalvia) from dead coral from the Great Barrier Reef, Australia. J Molluscan Stud 50:192–230. https://doi.org/10.1093/oxfordjournals.mollus.a065864
Kleemann KH (1995) Association of coral and boring bivalves: Lizard Island (Great Barrier Reef, Australia) versus Safaga (N Red Sea). Beitr Paläontol 20:31–39
Kleemann KH (2009) Gastrochaenolites hospitium isp. Nov., trace fossil by a coral-associated boring bivalve from the Eocene and Miocene of Austria. Geol Carpath 60:339–342. https://doi.org/10.2478/v10096-009-0025-0
Littler MM, Littler DS, Hanisak MD (1991) Deep-water rhodolith distribution, productivity, and growth history at sites of formation and subsequent degradation. J Exp mar biol Ecol 150:163−182. https://doi.org/10.1016/0022-0981(91)90066-6
Lund M, Davies PJ, Braga JC (2000) Coralline algal nodules off Fraser Island, eastern Australia. Facies 42:25–34. https://doi.org/10.1007/BF02562564
Mallela J, Perry CT (2007) Calcium carbonate budgets for two coral reefs affected by different terrestrial runoff regimes, Rio Bueno, Jamaica. Coral Reefs 26:129–145. https://doi.org/10.1007/s00338-006-0169-7
Márquez F, Frizzera AC, Vázquez N (2017) Environment-specific shell shape variation in the boring mytilid Leiosolenus patagonicus. Mar Biol Res 13:246–252. https://doi.org/10.1080/17451000.2016.1248848
Marshall JF, Tsuji Y, Matsuda H, Davies PJ, Iryu Y, Honda N, Satoh Y (1998) Quaternary and tertiary subtropical carbonate platform development on the continental margin of southern Queensland, Australia. In: Camoin GF, Davies PJ (eds) Reefs and carbonate platforms in the Pacific and Indian oceans. Spec Pub Int Ass Sediment 25:163–195. https://doi.org/10.1002/9781444304879.ch9
Matsuda S (1989) Succession and growth rates of encrusting crustose coralline algae (Rhodophyta, Cryptonemiales) in the upper fore-reef environment off Ishigaki Island, Ryukyu Islands. Coral Reefs 7:185–195. https://doi.org/10.1007/BF00301597
Matsuda S, Iryu Y (2011) Rhodoliths from deep fore-reef to shelf areas around Okinawa-jima, Ryukyu Islands, Japan. Mar Geol 282:215–230. https://doi.org/10.1016/j.margeo.2011.02.013
Matsuda S, Nohara M (1994) Radiocarbon ages of rhodoliths on the deep forereef to insular shelves around Okinawa-jima, Ryukyu Islands. Bull Coll Edu Univ Ryukyus 44:185–193 (in Japanese with English abstract)
Mueller B, de Goeij JM, Vermeij MJA, Mulders Y, van der Ent E, Ribes M, van Duyl FC (2014) Natural diet of coral-excavating sponges consists mainly of dissolved organic carbon (DOC). PLoS One 9(2): e90152. https://doi.org/10.1371/journal.pone.0090152
Nebelsick JH, Bassi D, Rasser M (2011) Microtaphofacies: exploring the potential for taphonomic analysis in carbonates. In: Allison PA, Bottjer DJ (eds) Taphonomy. Process and bias through time. Topics in Geobiology 32. Springer, Berlin, pp 337–374. https://doi.org/10.1007/978-90-481-8643-3_9
Owada M, (2007) Functional morphology and phylogeny of the rock-boring bivalves Leiosolenus and Lithophaga (Bivalvia: Mytilidae): a third functional clade. Marine Biology 150 (5):853–860.
Owada M (2009) Organic sheets in the shells of endolithic mytilids (Bivalvia: Mytilidae). Paleont Res 13:159–166. https://doi.org/10.2517/1342-8144-13.2.159
Owada M (2015) Functional phenotypic plasticity of the endolithic mytilid Leiosolenus curtus (Lischke, 1874) (Bivalvia: Mytilidae). Molluscan Res 35:188–195. https://doi.org/10.1080/13235818.2015.1052129
Owada M, Hoeksema BW (2011) Molecular phylogeny and shell microstructure of Fungiacava eilatensis Goreau et al. 1968, boring into mushroom corals (Scleractinia: Fungiidae), in relation to other mussels (Bivalvia: Mytilidae). Contrib Zool 80:169–178
Peharda M, Puljas S, Chauvaud L, Schöne BR, Ezgeta-Balić D, Thébault J (2015) Growth and longevity of Lithophaga lithophaga: what can we learn from shell structure and stable isotope composition? Mar Biol 162:1531–1540. https://doi.org/10.1007/s00227-015-2690-0
Perry CT (1996) Distribution and abundance of macroborers in an upper Miocene reef system, Mallorca, Spain: implications for reef development and framework destruction. Palaios 11:40–56. https://doi.org/10.2307/3515115
Perry CT (1998) Macroborers within coral framework at Discovery Bay, North Jamaica: species distribution and abundance, and effects on coral preservation. Coral Reefs 17:277–287. https://doi.org/10.1007/s003380050129
Perry CT (2000) Macroboring of Pleistocene coral communities, Falmouth formation, Jamaica. Palaios 15:483–491. https://doi.org/10.2307/3515517
Perry CT, Bertling M (2000) Temporal and spatial patterns of coral reef macroboring since the Mesozoic. In: Insalaco E, Skelton P, Palmer T (eds) Carbonate platform systems: components and interactions. Geol Soc London Spec Publ 178:33–50. https://doi.org/10.1144/GSL.SP.2000.178.01.04
Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ (2012) Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31:853–868. https://doi.org/10.1007/s00338-012-0901-4
Perry CT, Hepburn LJ (2008) Syn-depositional alteration of coral reef framework through bioerosion, encrustation and cementation: taphonomic signatures of reef accretion and reef depositional events. Earth-Sci Rev 86:106–144. https://doi.org/10.1016/j.earscirev.2007.08.006
Printrakoon C, Yeemin T, Valentich-Scott P (2016) Ecology of endolithic bivalve mollusks from Ko Chang, Thailand. Zool Stud 55:e50. https://doi.org/10.6620/ZS.2016.55-50
Reid PR, Macintyre IG (1988) Foraminiferal-algal nodules from the eastern Caribbean: growth history and implications on the value of nodules as paleoenvironmental indicators. Palaios 3:424–435. https://doi.org/10.2307/3514788
Rivera MG, Riosmena-Rodriguez R, Foster MS (2004) Age and growth of Lithothamnion muelleri (Corallinales, Rhodophyta) in the southwestern Gulf of California, Mexico. Cienc mar 30:235−249. https://doi.org/10.7773/cm.v30i12.104
Santos A, Mayoral E, Bromley RG (2011) Bioerosive structures from Miocene marine mobile-substrate communities in southern Spain, and description of a new sponge boring. Palaeontology 54:535–545. https://doi.org/10.1111/j.1475-4983.2011.01040.x
Savazzi E (1999) Boring, nestling and tube-dwelling bivalves. In: Savazzi E (ed) Functional morphology of the invertebrate skeleton. Wiley, Chichester, pp 205–237
Schröder-Adams CJ, Boyd R, Ruming K, Sandstrom M (2008) Influence of sediment transport dynamics and ocean floor morphology on benthic foraminifera, offshore Fraser Island, Australia. Mar Geol 254:47–61. https://doi.org/10.1016/j.margeo.2008.05.002
Seilacher A (1964) Biogenic sedimentary structures. In: Imbrie J, Newell N (eds) Approaches to palaeoecology. Wiley, New York, pp 296–316. https://doi.org/10.1007/978-1-4020-3609-5_29
Silva CM, da Cachão M, Rebelo AC, Johnson ME, Baarli BG, Santos A (2019) Paleoenvironment and taphonomy of lower Miocene bivalve and macroid assemblages: the Lagos biocalcarenite (Lagos-Portimão formation, southern Portugal). Facies 65:6. https://doi.org/10.1007/s10347-018-0550-3
Sneed ED, Folk RL (1958) Pebbles in the lower Colorado River, Texas: a study in particle morphogenesis. J Geol 66:114–150
Somaya MT, Abdel Razek FA, Khafage AR, Omar HA, El-Deeb RS (2018) Biometric variables and relative growth of the date mussel Lithophaga lithophaga (L., 1758) (Bivalvia: Mytilidae) from the eastern Mediterranean Sea, Egypt. Egypt J Aquat Biol Fish 22(5):241–248. https://doi.org/10.21608/EJABF.2018.22062
Steller DL, Hernandez M, Riosmena-Rodriguez R, Cabello-Pasini A (2007) Effect of temperature on photosynthesis, growth and calcification rates of the free-living coralline alga Lithophyllum margaritae. Cienc Mar 33:441–456. https://doi.org/10.7773/cm.v33i4.1255
Taylor, AM, Goldring, R (1993) Description and analysis of bioturbation and ichnofabric. J Geol Soc Lond 150:141–148. https://doi.org/10.1144/gsjgs.150.1.0141
Taylor PD, Wilson MA (2003) Palaeoecology and evolution of marine hard substrate communities. Earth-Sci Rev 62:1–103. https://doi.org/10.1016/S0012-8252(02)00131-9
Tompkins PA, Steller DL (2016) Living carbonate habitats in temperate California (USA) waters: distribution, growth, and disturbance of Santa Catalina Island rhodoliths. Mar Ecol Progr Ser 560:135–145. https://doi.org/10.3354/meps11919
Tsuji Y (1993) Tide influenced high energy environments and rhodolith-associated carbonate deposition on the outer shelf and slope off Miyako Islands, southern Ryukyu Island arc, Japan. Mar Geol 113:255–271. https://doi.org/10.1016/0025-3227(93)90021-M
Wilson MA, Palmer TJ (1998) The earliest Gastrochaenolites (early Pennsylvanian, Arkansas, USA): an upper Paleozoic bivalve boring? J Paleontol 72:769–772. https://doi.org/10.1017/S0022336000040464
Wizemann A, Nandini SD, Stuhldreier I, Sánchez-Noguera C, Wisshak M, Westphal H, Rixen T, Wild C, Reymond CE (2018) Rapid bioerosion in a tropical upwelling coral reef. PLoS One 13(9):e0202887. https://doi.org/10.1371/journal.pone.0202887
Acknowledgements
Sincere thanks are expressed to the Museum of Natural History and to the Graduate Program in Earth and Environmental Sciences (GP-EES), Tohoku University, for inviting DB to Sendai, Japan. JHL thanks the support and encouragement of the Directors of the Gump Biological Research Station (GBRS) of the University of California, Berkeley located on Moorea, Directors George Roderick and Dr. Neil Davies for permitting JHL to work at the Station, and the station managers Mr. Frank Murphy, Drs. Stephen Strand, and John Woodward, and the staff of GBRS for logistic and living support. This work was done under permit No. 568/BCO from Haut-Commissariat de La Republique en Polynesie Francaise. JHL is grateful for the collection support from the Museum of Paleontology, Berkeley. This is UC Museum of Paleontology Publication 3000, and a GBRS contribution in science. This paper is a scientific contribution of the Project MIUR–Dipartimenti di Eccellenza 2018–2022 and of the PRIN 2017 RX9XXXY. The authors are grateful to two anonymous reviewers and the PEPS editor K. Endo for constructive comments.
Authors’contributions
DB, JCB, and YI proposed and designed the study. JCB, JA, JHL, and YI conducted the field sampling. DB, JCB, JA, and YI described the macroids and the rhodoliths. DB identified the ichnospecimens. MO identified the boring bivalves and discussed their ecology. MO and HT illustrated the studied specimens. All authors interpreted the data and read and approved the final manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI (Grant-in-Aid for Scientific Research), grant number 25247083 to YI. This investigation was financially supported in part by local research funds (Erasmus+, FAR2012–2017, FIR2016, FIR2018, PRIN 2017RX9XXXY “Biotic resilience to global change: biomineralization of planktonic and benthic calcifiers in the past, present and future” and the BioMed Central-Prepay Membership at the University of Ferrara, DB). JCB and JA were supported by Junta de Andalucía Research Group RNM 190. JHL thanks the Committee on Research, the Museum of Paleontology and the Department of Integrative Biology, UC Berkeley, and the UC Pacific Rim Project for financial support of his field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bassi, D., Braga, J.C., Owada, M. et al. Boring bivalve traces in modern reef and deeper-water macroid and rhodolith beds. Prog Earth Planet Sci 7, 41 (2020). https://doi.org/10.1186/s40645-020-00356-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40645-020-00356-w