Abstract
The aim of this study was to asses in vitro the potential of methacrylate-based adhesive resins containing niobium pentoxide (Nb2O5) for stimulating phosphate deposition. Adhesive resins were obtained by mixing 50 wt% BisGMA, 25 wt% TEGDMA and 25 wt% HEMA, and Nb2O5 was added on 2.5 or 5 wt% to the resin. Discs 6.5 mm in diameter and 1.5 mm in height of the resin without Nb2O5 and of the resins presenting the oxide were obtained by inserting the resin into a silicon matrix, followed by photo activation. Discs were immersed in simulated body fluid at 36°C for 1, 7 and 28 days, and then their surfaces were examined by Raman spectroscopy. Changes of intensity of the 962 cm−1 peak, related to phosphate bond, over the samples’ surfaces were used to assess the potential of adhesive resins to stimulate phosphate deposition. Experimental groups containing 2.5 and 5 wt% niobium pentoxide presented a phosphate-rich layer deposition over their surfaces after 7 and 28 days of SBF immersion, and this deposition increased over time. Incorporation of 2.5 or 5 wt% niobium pentoxide provides the potential to promote phosphate deposition on methacrylate-based adhesive resins.
Similar content being viewed by others
Background
Advances in enamel and dentin bonding enable the predictability of direct restorative treatments [1]. A reliable dentin bond was achieved by the establishment of a hybrid layer, which is defined as a layer composed of collagen fibrillar matrix surrounded by a polymer formed from monomers previously diffused into the collagen matrix [2]. Discrepancies in etching depth and monomer diffusion depth could generate a region of denuded collagen at the bottom of the hybrid layer [2]. Besides, the presence of residual solvent and fluid movement of dentinal tubules into the co-monomer mixtures of adhesives jeopardize the water replacement by resins into the collagen matrix, leading to an incomplete monomer infiltration [3]. With dentin demineralization by acid-etching, matrix metalloproteinases (MMPs) are activated [2],[4], which in association with the susceptibility to hydrolytic degradation of polymers [5], primarily on non-infiltrated collagen regions at the bottom of the hybrid layer [4], could contribute to the degradation of the bonding interface [6].
In order to surpass this problem, one option would be to develop an adhesive system that would induce the release of ions with the goal of filling this exposed collagen fiber region with mineral formation [7]–[11]. Niobium pentoxide (Nb2O5) has shown mineralization induction through mineral deposition in previous studies [12]–[14], demonstrating promising proprieties. Nb2O5 was also evaluated as a filler for adhesive resins and methacrylate-based endodontic cements and enhances the Knoop microhardness and radiopacity of these materials [15],[16]. Besides, Nb2O5 presents the ability to diffuse into the hybrid layer when inserted into an adhesive resin applied following the etching and primer application in a 3-step etch-and-rinse adhesive system [16]. Therefore, the purpose of this study was to asses in vitro the potential of stimulating phosphate deposition of methacrylate-based adhesive resins containing niobium pentoxide.
Methods
Formulation of experimental adhesives
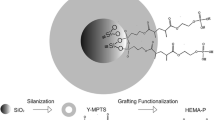
Adhesive resins were obtained by mixing 50 wt% BisGMA, 25 wt% TEGDMA and 25 wt% HEMA (provided by Esstech Inc, Essington, PA, USA). Camphoroquinone (Sigma Aldrich, USA) and ethyl 4-dimethylaminobenzoate (Sigma Aldrich, USA) were added at 1 mol% to the mixture to achieve a photo-activated blend. Niobium pentoxide (Nb2O5, Companhia Brasileira de Metalurgia e Mineração, Araxá, MG, Brazil) was added at 2.5 and 5 wt%, in relation to the mass of the experimental adhesives, and one adhesive without Nb2O5 was used as a control. Nb2O5 was previously silanized using γ-methacryloxypropyltrimethoxysilane (γ-MPTS, Aldrich Chemical Co., Milwaukee, WI, USA) [17]. Reagents were hand mixed and sonicated for 180 s.
Phosphate deposition assay by Raman spectroscopy
Adhesive resin was inserted into a cylindrical silicon matrix of 6.5 mm diameter and 1.5 mm height, covered by a polyester film and photo activated (Radii Cal1200 mW/cm2, SDI LTD., Bayswater, VIC, Australia) for 20 s on each side. Three discs were obtained for each experimental adhesive. Discs were immersed in simulated body fluid (SBF) prepared according to Kokubo and Takadama [18] for 1, 7 or 28 days at 36°C. The SBF prepared had the following ion concentration in mol/m3: Na+ (213.0), K+ (7.5), Mg2+ (2.3), Ca2+ (3.8), Cl− (221.7), HCO3− (6.3), HPO43− (1.5) and SO42− (0.8), and the pH was adjusted to 7.40. After the storage period, samples were washed with 10 ml of distilled water and dried on desiccators at 36°C.
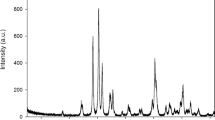
Chemical changes on the sample surface were analyzed by Raman spectroscopy (Senterra, Bruker Optics, Ettlingen, Germany). An area of 200x200μm was irradiated three times for 5 s by a 785-nm laser of 50 mW on 100 equidistant points. Spectra were obtained at 1800 to 440 cm−1 Raman band. Using spectroscopy software (Opus 7.5, Bruker Optics, Ettlingen, Alemanha), the integral of the 962 cm−1 peak absorbance (symmetric stretching ʋ 1 mode of phosphate) [19] and of the 1610 cm−1 peak (vibration mode of carbon-to-carbon stretching of the aromatic ring of BisGMA) [20], as indicated in Figure 1, was calculated. Increases in the ratio between the absorbance of the 962 and 1610 cm−1 peaks (A962cm−1/A1610cm−1) indicate the deposition of calcium phosphates on the samples’ surfaces.
Results and discussion
Bioactive materials should interact with tissues, evoking positive responses to the host body [21], as this stimulates hard tissue formation. Figure 2 shows phosphate content changes over the sample surface after distinct periods immersed in SBF, assessed by variation in the absorbance of the phosphate Raman peak. Phosphate content increases over time for all groups, being more pronounced in the groups containing 2.5 and 5% Nb2O5 than in the control group. Adhesive resins with niobium pentoxide incorporation exhibited phosphate deposition after 7 days of immersion. The presence of niobium pentoxide stimulates the deposition of calcium phosphate from the fluids [12] and allows the material to be bioactive.
Higher content of phosphates (A962cm−1/A1610cm−1) measured at 100 points on each sample after 28 days of SBF immersion reached 0.27 for the control resin, 0.42 for resin with 2.5% Nb2O5 and 0.40 for resin with 5% Nb2O5. Phosphate deposition on the control resin could be attributed to immersion of a solid sample that could be partially solubilized, evoking pH changes, altering local supersaturation of SBF and causing spontaneous precipitation of calcium and phosphate ions [22]. Despite this drawback of SBF testing, the adhesive resins containing Nb2O5 exhibited a more pronounced deposition of phosphates than the control resin. The use of SBF testing to predict bioactivity of biomaterials has been severely questioned [22],[23]. It is stated that use of SBF testing leads to false-positive results and cannot reliably mimic physiological conditions [22],[23]. A review discussing the in vitro and in vivo bioactivity of calcium silicate cements showed that a material that produced an apatite layer over its surface after interacting with the ions derived from SBF did not exhibit a bone bond when inserted in living tissues [24]. Three different mechanisms of interaction with tissues could contribute to the bioactivity of materials: chemical bonds to host tissues, influence on cellular pathways and stimulus to cell differentiation by topographical features of biomaterial. From these mechanisms, the SBF test simulates only the chemical bond to tissues which could be predicted by their apatite-forming ability on samples surface [25]. Nevertheless, the protocol for in vitro testing of implants using SBF was described by ISO 23317 [26]. Recently, Zadpoor [25] systematically reviewed the literature, looking for studies that evaluate the bioactivity of two or more biomaterials by SBF immersion and animal models. Of 33 papers included in the analysis, 25 of the results showed that in vitro testing matched the in vivo results. In three the bioactivity was confirmed by in vitro and in vivo tests; however, the materials’ bioactivity was not ranked in the same way. In the other five papers, the biomaterials showed no apatite layer in an SBF test but bonded to animals’ bone tissue. In this review, no false-positive results were noticed. Thus, the lowcost, rapid speed and ease make the SBF test the method of choice for an initial screening of newly developed materials.
Retention of direct restorative materials currently used is achieved by micromechanical interlocking and/or chemical adhesion [2],[27]. Nowadays, the adhesive procedures have a reliable clinical effectiveness [1]. However, some issues remain challenging in achieving a durable bond to tooth structure. During restorative procedures using a separate etching step, a discrepancy between the demineralization depth and monomer penetration could occur [2]. This fact makes the non-monomer-infiltrated collagen at the bottom of the hybrid layer more prone to degradation by proteolytic enzymes, as dentinal matrix metalloproteinases and, consequently, the resin-dentin bonding interface could be compromised [2]. The incorporation of bioactive materials has been proposed, attempting to backfill the denuded collagen to prevent hybrid layer deterioration [8]–[11]. Calcium silicates and calcium orthophosphates have already been experimentally tested on adhesives aiming at this therapeutic/remineralization effect [28],[29]. These compounds are highly soluble, and their action is due to an initial solubilization followed by a deposition of minerals [28]. This process does not occur for niobium pentoxide; it is expected that an apatite-like phase directly deposits over its surface without an initial dissolution of the mineral due to a Nb-OH bond formed on the oxide surface that induces apatite nucleation from SBF ions [14],[30]. Besides, this does not require the dissolution of filler from the adhesive matrix niobium pentoxide, which enhances the Knoop microhardness and radiopacity of adhesive resins [16]. Thus, we assume that the developed adhesive resins were capable of promoting calcium-phosphate deposition on their surfaces and could contribute to the maintenance of hybrid layer integrity.
Conclusion
Adhesive resins containing 2.5 and 5% niobium pentoxide stimulated the deposition of a phosphate layer over their surfaces.
References
Peumans M, De Munck J, Mine A, Van Meerbeek B (2014) Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent Mater. doi:10.1016/j.dental.2014.07.007
Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A: State of the art etch-and-rinse adhesives. Dent Mater 2011, 27: 1–16. 10.1016/j.dental.2010.10.016
Hashimoto M, Tay FR, Svizero NR, de Gee AJ, Feilzer AJ, Sano H: The effects of common errors on sealing ability of total-etch adhesives. Dent Mater 2006, 22: 560–568. 10.1016/j.dental.2005.06.004
Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjaderhane L, Di Lenarda R, Tay FR, Pashley DH, Breschi L. (2012) MMP activity in the hybrid layer detected with in situ zymography. J Dent Res 91:467–472
Ferracane JL: Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 2006, 22: 211–222. 10.1016/j.dental.2005.05.005
Tjaderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH: Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater 2013, 29: 116–135. 10.1016/j.dental.2012.08.004
Reis A, Carrilho M, Breschi L, Loguercio AD: Overview of clinical alternatives to minimize the degradation of the resin-dentin bonds. Oper Dent 2013, 38: E1-E25. 10.2341/11-436-L
Kim YK, Gu LS, Bryan TE, Kim JR, Chen L, Liu Y, Yoon JC, Breschi L, Pashley DH, Tay FR: Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials 2010, 31: 6618–6627. 10.1016/j.biomaterials.2010.04.060
Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, Pashley DH, Tay FR: Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater 2010, 6: 2740–2750. 10.1016/j.actbio.2009.12.052
Toledano M, Sauro S, Cabello I, Watson T, Osorio R: A Zn-doped etch-and-rinse adhesive may improve the mechanical properties and the integrity at the bonded-dentin interface. Dent Mater 2013, 29: e142-e152. 10.1016/j.dental.2013.04.024
Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, Tay FR: Biomimetic remineralization of dentin. Dent Mater 2014, 30: 77–96. 10.1016/j.dental.2013.07.013
Karlinsey RL, Hara AT, Yi K, Duhn CW: Bioactivity of novel self-assembled crystalline Nb2O5 microstructures in simulated and human salivas. Biomed Mater 2006, 1: 16–23. 10.1088/1748-6041/1/1/003
Obata A, Takahashi Y, Miyajima T, Ueda K, Narushima T, Kasuga T: Effects of niobium ions released from calcium phosphate invert glasses containing Nb2O5 on osteoblast-like cell functions. ACS Appl Mater Interfaces 2012, 4: 5684–5690. 10.1021/am301614a
Miyazaki T, Kim H, Kokubo T, Ohtsuki C, Nakamura T: Apatite-forming ability of niobium oxide gels in a simulated body fluid. J Ceramic Soc Jpn 2001, 109: 929–933. 10.2109/jcersj.109.1275_929
Leitune VC, Takimi A, Collares FM, Santos PD, Provenzi C, Bergmann CP, Samuel SM: Niobium pentoxide as a new filler for methacrylate-based root canal sealers. Int Endod J 2013, 46: 205–210. 10.1111/j.1365-2591.2012.02107.x
Leitune VC, Collares FM, Takimi A, de Lima GB, Petzhold CL, Bergmann CP, Samuel SM: Niobium pentoxide as a novel filler for dental adhesive resin. J Dent 2013, 41: 106–113. 10.1016/j.jdent.2012.04.022
Provenzi C, Leitune VCB, Collares FM, Trommer RM, Bergmann CP, Samuel SM: Interface evaluation of experimental dental adhesives with nanostructured hydroxyapatite incorporation. Appl Adh Sci 2014, 2: 2. doi:10.1186/2196–4351–2-2
Kokubo T, Takadama H: How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27: 2907–2915. 10.1016/j.biomaterials.2006.01.017
Szubert M, Adamska K, Szybowicz M, Jesionowski T, Buchwald T, Voelkel A: The increase of apatite layer formation by the poly (3-hydroxybutyrate) surface modification of hydroxyapatite and beta-tricalcium phosphate. Mater Sci Eng C Mater Biol Appl 2014, 34: 236–244. 10.1016/j.msec.2013.09.023
Collares FM, Portella FF, Leitune VC, Samuel S: Discrepancies in degree of conversion measurements by FTIR. Braz Oral Res 2014, 28: 9–15. 10.1590/S1806-83242013000600002
Terminology for the Bio-Nano Interfaces. British Standards Institution, London, United King; 2007.
Bohner M, Lemaitre J: Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30: 2175–2179. 10.1016/j.biomaterials.2009.01.008
Pan H, Zhao X, Darvell BW, Lu WW: Apatite-formation ability–predictor of “bioactivity”? Acta Biomater 2010, 6: 4181–4188. 10.1016/j.actbio.2010.05.013
Niu LN, Jiao K, Wang TD, Zhang W, Camilleri J, Bergeron BE, Feng HL, Mao J, Chen JH, Pashley DH, Tay FR: A review of the bioactivity of hydraulic calcium silicate cements. J Dent 2014, 42(5):517–533. 10.1016/j.jdent.2013.12.015
Zadpoor AA: Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater Sci Eng C Mater Biol Appl 2014, 35: 134–143. 10.1016/j.msec.2013.10.026
ISO 23317 Implants for surgery - In vitro evaluation for apatite-forming ability of implant materials. International Organization for Standardization, Geneva, Switzerland; 2012.
Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL: State of the art of self-etch adhesives. Dent Mater 2011, 27: 17–28. 10.1016/j.dental.2010.10.023
Profeta AC, Mannocci F, Foxton R, Watson TF, Feitosa VP, De Carlo B, Mongiorgi R, Valdré G, Sauro S: Experimental etch-and-rinse adhesives doped with bioactive calcium silicate-based micro-fillers to generate therapeutic resin-dentin interfaces. Dent Mater 2013, 29: 729–741. 10.1016/j.dental.2013.04.001
Melo MA, Cheng L, Zhang K, Weir MD, Rodrigues LK, Xu HH: Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater 2013, 29: 199–210. 10.1016/j.dental.2012.10.005
Kokubo T, Kim HM, Kawashita M: Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24: 2161–2175. 10.1016/S0142-9612(03)00044-9
Acknowledgments
The authors acknowledge financial support from PET-SESU/MEC (Programa de Educação Tutorial, Secretaria de Educação Superior, Ministério da Educação), and FFP gratefully acknowledges scholarship support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FMC participated in the study design development, manuscript construction and manuscript final review; FFP participated in the study design development, data interpretation and manuscript construction; GCSF participated in the study design development and manuscript construction; SMS, LCBA and ERS participated in laboratory tests; VCBL participated in study design development and manuscript final review; SMWS participated in study design development and manuscript final review. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Collares, F.M., Portella, F.F., da Silva Fraga, G.C. et al. Mineral deposition at dental adhesive resin containing niobium pentoxide. Appl Adhes Sci 2, 22 (2014). https://doi.org/10.1186/s40563-014-0022-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40563-014-0022-0