Abstract
Background
The Rb-Sr isotope system has long been used for radiometric dating and petrogenetic investigation. The concentrations of Rb and Sr could be precisely measured by isotope dilution thermal ionization mass spectrometry combined with chemical purification of these elements. For the simultaneous measurement of Sr isotopic composition and isotope dilution, the contribution from the added spike should be carefully corrected.
Findings
Reliable 87Sr/86Sr ratios of the spike-standard mixed solutions were obtained using the new mass bias factor calculated on the basis of measured Sr isotopic ratios. This correction yielded reasonable 87Sr/86Sr ratios for overspiked standard solutions whose spike fractions reaching 25 wt.%.
Conclusions
The correction procedure described in this study shows that the simultaneous measurement of Sr isotopic composition and isotope dilution is available for the case that the spike proportion in the sample-spike mixture is significantly high (84Sr/86Sr > 3.7). The principle of this correction protocol can also be applied to other isotope systems such as Sm-Nd and Lu-Hf pairs.
Similar content being viewed by others
Findings
Introduction
Rubidium-87 decays to stable 87Sr by emission of β− particle with a decay constant of 1.420 (±0.010) × 10−11 years−1 (Steiger and Jäger [1977]) which corresponds to a half-life of 48.8 × 109 years. It is notable that this International Union of Geological Sciences-accepted decay constant is being considered to be high by 1% to 2% as summarized in Begemann et al. ([2001]). Rubidium and strontium belong to the groups IA and IIA, respectively. They are easily fractionated from each other through geological processes such as partial melting and igneous and metamorphic mineral growth. Therefore, the 87Rb-87Sr isotope system has long been used for radiometric dating and petrogenetic investigation (Faure and Mensing [2005]).
The traditional thermal ionization mass spectrometry (TIMS) is still regarded as the benchmark technique to measure Rb-Sr isotope ratios. By TIMS, the inter-elemental ratio cannot be directly measured but is obtained through isotope dilution (Moore et al. [1973]), a method of analyzing chemical substances by the addition of isotopically enriched spike to the sample. Considering the inhomogeneous distribution of Rb and Sr in most geological samples, simultaneous measurement of Sr isotopic composition and isotope dilution is essential. In this case, inevitable contribution from the spike should be carefully corrected.
This study evaluates the effectiveness of correction protocol to measure 87Sr/86Sr ratio in a series of mixture of 84Sr-enriched spike and standard solution. It has been tested whether a reasonable 87Sr/86Sr ratio could be obtained for overspiked standard solutions of which spike fractions reach 45 wt.%.
Availability and requirements
Preparation of spike and standard solution
The 84Sr-enriched isotope spike and standard solutions were prepared at the Korea Basic Science Institute (KBSI) in Ochang. In the following, all errors are quoted on the basis of 2 σ standard error, unless stated otherwise.
The spike, SrCO3 form, was provided by the Oak Ridge National Laboratory (batch number = 236,201, order number = 65-0056). It was dissolved in hot 10% HNO3 and diluted to about 15 μg/g solution with 5% HNO3. The AnApureTM ICP standard of 1,002 (±10) mg/L Sr (lot no. AEP-130-726) was diluted to 21.480 μg/g solution in 2% HNO3 (the specific gravity of 2% HNO3 = 1.028 g/mL) and then was admixed with the spike solution. Ten mixed solutions were prepared with mixing proportions (spike/standard) ranging from about 1:1.2 to 1:70 in weight.
Instrumental analysis
The Rb-Sr isotopic analysis was conducted by using a Phoenix (Isotopx) TIMS, Cheshire, CW, UK, installed at the KBSI. This instrument is equipped with eight movable Faraday collectors and one fixed axial channel where the ion beam intensities can be measured with either a Faraday collector or an ion counting Daly detector.
In this study, all required isotopes were simultaneously detected on Faraday collectors. The sensitivity on 88Sr was typically around 3 V (1011 Ω resistors). The strontium isotope measurements of the standard and spike-standard mixed solutions were performed with a multi-dynamic mode (Lenz and Wendt [1976]) in which the Faraday collectors were set to simultaneously detect 84Sr (axial), 86Sr (H2), 87Sr (H3), and 88Sr (H4) for the first static run, and 84Sr (L2), 86Sr (H1), 87Sr (H2), and 88Sr (H3) for the second run. One measurement consists of 5 blocks of 12 cycles with an integration time of 10 s. The mass bias was exponentially normalized to 86Sr/88Sr = 0.1194. The mean multi-dynamic 87Sr/86Sr acquired for NBS987 was 0.710252 ± 0.000007 (n = 7) during the course of this study.
The isotopic composition of 84Sr-enriched spike was measured with a multi-static mode (100 cycles, 5 blocks, integration time = 10 s) in which the Faraday collectors were set to detect 84Sr (axial), 86Sr (H2), 87Sr (H3), and 88Sr (H4). In this measurement, the instrumental mass fractionation was not normalized.
Results and discussion
Five measurements for the 84Sr spike yielded an average 84Sr/88Sr of 561.4 ± 0.9, 86Sr/88Sr of 0.7661 ± 0.0010, and 87Sr/88Sr of 0.1819 ± 0.0030, respectively. Therefore, the 84Sr enrichment and atomic weight of the spike are calculated to be 83.9242 and 99.65 at.%, respectively. Although the instrumental mass fractionation was not corrected during the TIMS measurement, this enrichment value is well matched with the suggested value (99.64 ± 0.01%, Tracy [1999]). The exponentially normalized multi-dynamic measurement of the Sr standard yielded an average 87Sr/86Sr ratio of 0.707630 ± 0.000009 (n = 5).
The Sr isotope ratios measured for the spike-standard mixed solutions have been normalized to 86Sr/88Sr = 0.1194. This mass bias correction is not valid because the 86Sr/88Sr of the added spike was significantly shifted from the natural value through the enrichment processes from 0.1194 to 0.7661. The first step for the correction of spike contribution is to determine a new mass bias factor (αnew) from the measured Sr isotope ratios. In the following, ‘s’ denotes the normal Sr standard, ‘t’ denotes the Sr spike, and ‘m’ and ‘c’ represent the measured and corrected values, respectively. The (86t/86s) can be calculated using (84/86)m, (84/86)s, and (84/86)t:
Because the measured 84/86 ratio used in the above calculation has an uncertainty due to incorrect normalization to 86/88 = 0.1194, the 86t/86s should be recalculated after determination of the new mass bias factor (see below).
The (86/88)s can be reduced by using (86/88)m, (88t/88s), and (86/88)t:
The mass fractionation can now be accurately corrected using the calculated (86/88)s. The exponential law (Russel et al. [1978]) yields a mass bias factor before (αold) and after (αnew) the spike correction as the following:
where ‘M’ denotes the mass of the isotope.
The newly corrected (84/86) ratio used for isotope dilution is yielded as the following:
The newly corrected (84/86) ratios of the ten mixed solutions yielded a mean Sr concentration of 15.271 ± 0.031 μg/g for the 84Sr-enriched spike prepared in this study.
Also, the (87/86)c can be newly calculated using measured Sr isotope ratios as the following.
Finally, the (87/86)c(new) is used for the calculation of the (87/86)s: because (it is noted that 86t/86s can be recalculated on the basis of newly corrected 84/86).
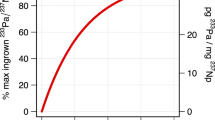
Table 1 summarizes the spike correction results for the ten spike-standard mixed solutions. The corrected 87Sr/86Sr ratios of the mixed solutions are displayed in Figure 1 with the measured values of the unspiked standard. As shown in Figure 1, the protocol described in this study successfully corrected the spike contribution in the case that the spike fraction in the mixture reaches 25 wt.% (84Sr/86Srmixture > 3.7). It is not clear why the corrected 87Sr/86Sr ratio for mix 1 solution (spike fraction = 45.1 wt.%) deviates from the unspiked standard value. The corrected 87Sr/86Sr ratios of nine mixed solutions (mix 2 to mix 10) yield an indistinguishable average of 0.707633 ± 0.000010 from the unspiked value (0.707630 ± 0.000009). There is no trend between the corrected 87Sr/86Sr and the spike fraction (or 84Sr/86Srmixture) in the range from 1.4 to 25.9 wt.%. It is noted that the principle of this correction protocol can also be applied to other isotope systems such as Sm-Nd and Lu-Hf pairs, as briefly interpreted in the study of Cheong and Kwon ([2010]).
Plots of corrected 87 Sr/ 86 Sr as a function of 84 Sr/ 86 Sr and weight fractions of the spike.
Plots of corrected87Sr/86Sr as a function of84Sr/86Sr and weight fractions of the spike. Solid and dashed lines represent an average 87Sr/86Sr and 2 σ standard deviation of the unspiked standard data, respectively. Error bars are based on within-run 2 σ standard error.
References
Begemann F, Ludwig KR, Lugmair GW, Min K, Nyquist LE, Patchett PJ, Renne PR, Shin CY, Villa IM, Walker RJ: Call for an improved set of decay constants for geochronological use. Geochim Cosmochim Acta 2001, 65: 111–121. 10.1016/S0016-7037(00)00512-3
Cheong CS, Kwon ST: Calibration of Sm-Nd mixed spike by Teflon powder method. J Anal Sci Tech 2010, 1: 30–36. 10.5355/JAST.2010.30
Faure G, Mensing TM: Isotopes: Principles and applications. John Wiley & Sons, Hoboken; 2005.
Lenz H, Wendt I: Use of a double collector for high-precision isotope ratio measurements in geochronology. Adv Mass Spectrom 1976, 7A: 565–568.
Moore LJ, Moody JR, Barnes IL, Gramlich JW, Murphy TJ, Paulsen PJ, Shields WR: Trace determination of rubidium and strontium in silicate glass standard reference materials. Anal Chem 1973, 45: 2384–2387. 10.1021/ac60336a014
Russel WA, Papanastassiou DA, Tombrello TA: Ca isotope fractionation on the earth and other solar system materials. Geochim Cosmochim Acta 1978, 42: 1075–1090. 10.1016/0016-7037(78)90105-9
Steiger RH, Jäger E: Subcommission on geochronology: convention on the use of decay constants in geo- and cosmochronology. Earth Planet Sci Lett 1977, 36: 359–362. 10.1016/0012-821X(77)90060-7
Tracy JG: High-purity enrichment of 84Sr. Nucl Instrum Methods Phys Res Sect A 1999, 438: 1–6. 10.1016/S0168-9002(99)00638-5
Acknowledgements
This study was supported by the KBSI grant (G34200).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cheong, Cs., Jeong, YJ. & Kwon, ST. Correction of spike contribution for strontium isotopic measurement by thermal ionization mass spectrometry: a test for spike-standard mixed solutions. J Anal Sci Technol 5, 26 (2014). https://doi.org/10.1186/s40543-014-0026-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40543-014-0026-1