Abstract
Background
This study aimed to evaluate incidence risk and adverse clinical outcomes in COVID-19 disease among short-term users of acid-suppressants in South Korea.
Methods
This retrospective cohort study, conducted using a nationwide claims database for South Korea, used data from patients with COVID-19 tested between January 1 and May 15, 2020. Patients aged over 18 years and prescribed proton pump inhibitors (PPI) or histamine-2 receptor antagonist (H2RA) for more than 7 days were identified. Primary outcome was COVID-19 while secondary outcomes were all-cause mortality, hospitalization with respiratory disease, or intensive respiratory intervention. Large-scale propensity scores were used to match patients, while the Cox proportional hazard model was utilized to evaluate any association between exposure and outcome(s). The risk estimates were calibrated by using 123 negative control outcomes.
Results
We identified 26,166 PPI users and 62,117 H2RA users. After propensity score matching, compared to H2RA use, PPI use was not significantly associated with lower risk of COVID-19 (calibrated hazard ratio [HR], 0.81 [95% confidence interval (CI), 0.30–2.19]); moreover, PPI use was not associated with adverse clinical outcomes in COVID-19, namely, hospitalization with respiratory disease (calibrated HR, 0.88 [95% CI, 0.72–1.08]), intensive respiratory interventions (calibrated HR, 0.92 [95% CI, 0.46–1.82]), except for all-cause mortality (calibrated HR, 0.54 [95% CI, 0.31–0.95]).
Conclusions
In this study, we found that the PPI user was not associated with risk of COVID-19 compared to H2RA users. There was no significant relationship between severe clinical outcomes of COVID-19 and exposure to PPI compared with H2RA, except for all-cause mortality.
Similar content being viewed by others
Introduction
Proton pump inhibitors (PPI) are the mainstay in the management of acid-related gastrointestinal disease, including gastroesophageal reflux disease and peptic ulcer disease, and for the prevention of GI bleeding and stress ulcers [1]. While their widespread use has resulted in the improvement of acid-related disorders, concerns about potential complications due to PPI use, such as osteoporosis, dementia, malabsorption, gastrointestinal neoplasia, and increased susceptibility to bacterial infection, have also been rising [2, 3]. Further, it is possible that acid-suppressant drugs could increase susceptibility to respiratory infections because they counter the acidic environment of stomach, thereby allowing bacterial colonization [4, 5]. Even though several studies have evaluated the association between pneumonia and acid-suppressive drugs, the results remain inconclusive [4, 6,7,8,9,10].
Recently, several studies have described the effects of acid-suppressive agent use on the clinical course of and susceptibility to SARS-CoV-2 infection (COVID-19) [11,12,13,14,15]; however, few studies have directly compared incidence and risk of complications in COVID-19 between PPI and histamine-2 receptor antagonist (H2RA) therapy. In 2011, a systematic review and meta-analysis by Eom et al. investigated the association between use of acid-suppressive drugs and risk of pneumonia and found that the overall risk was higher among people using PPIs than H2RAs (adjusted odds ratio [OR] 1.27, 95% confidence interval [CI] 1.11–1.46 vs. 1.22, 95% CI 1.09–1.36) [7]. Nonetheless, the influence of acid-suppressive drugs on the viral pneumonia remains controversial.
To date, only limited data is available on the relationship between acid suppression therapy and clinical course of COVID-19 infection; therefore, we conducted a population-based retrospective cohort study to compare the risk of complications in COVID-19 among Korean patients prescribed PPI and H2RA therapy.
Methods
Data sources
A national claims database in South Korea that included COVID-19 testing data was used in this study [16]. The database was obtained from the Health Insurance Review and Assessment service (HIRA) which is the South Korean national institution for reviewing and assessing national health insurance claims. In response to the COVID-19 pandemic, HIRA collected data on COVID-19 testing by the reverse transcriptase polymerase chain reaction method from 1 January to 15 May, 2020. Notably, the collected data were converted into the Observational Medical Outcomes Partnership (OMOP) common data model (CDM), version 5, and released to the public. Hospitalization records were extracted for all patients involved in the study. This study was approved informed consent waiver by the institutional review board of the Kangdong Sacred Heart Hospital (no. 2020–04-001). All methods were carried out in accordance with relevant guidelines and regulations.
Study population and exposure
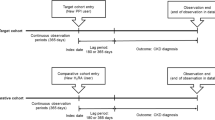
We identified patients aged over 18 years and diagnosed with COVID-19 disease. The cohort comprised patients prescribed acid-suppressants for 7 days or more. The index date was defined as the first day of drug treatment. The PPIs prescribed were defined as rabeprazole, pantoprazole, omeprazole, lansoprazole, ilaprazole, esomeprazole, and dexlansoprazole, while the H2RAs were defined as ranitidine, nizatidine, lafutidine, famotidine, and cimetidine. We excluded all patients prescribed any other primary or secondary medication(s) (i.e., PPIs and H2RAs) within 180 days before the index date. We defined the drug exposures as continuous exposure if the date gap between drug prescriptions was less than 30 days. Acid-suppressant non-users were defined as the patients who were not prescribed acid-suppressants and were not diagnosed with COVID-19 within 180 days before the index date.
Outcomes
The primary outcome was defined as diagnosis of COVID-19 and the secondary outcomes were defined as the complications of COVID-19, namely, (1) all-cause mortality, (2) hospitalization with at least one of the following diagnoses, i.e., pneumonia, acute respiratory disease syndrome (ARDS), sepsis, or acute kidney injury (AKI), and (3) requirement of intensive respiratory interventions such as mechanical ventilation, extracorporeal membrane oxygenation procedure (ECMO), or tracheostomy.
Statistical analyses
We used large-scale propensity score matching (PSM) with regularized logistic regression models to balance baseline characteristics of the study cohorts [17, 18]. Three different methods were used in the analysis, (1) propensity score unadjusted analysis, (2) one-to-four exact PSM with greedy nearest method, and (3) propensity score stratification with five strata [19]. The covariates included age, sex, all medication(s), medical procedure(s), disease history, and comorbidity index in the database. Cox proportional hazards regression models were used to estimate the association between exposures and outcomes. Patients were censored if they were no longer observable in the database. Data analyses were performed for three different cohort comparisons— (1) PPI users versus H2RA users, (2) PPI users versus non-users, and (3) H2RA users versus non-users.
During secondary analysis, only patients with a definite diagnosis of COVID-19 were included to measure complications due to COVID-19 disease among infected patients who had been prescribed acid-suppressive agents (i.e., PPIs and H2RAs). Moreover, we added the hospitalization criteria to investigate the clinical outcomes among COVID-19 patients with severe symptoms. Other analysis settings were identical to that used in primary analysis, i.e., PPI users, H2RA users, and non-users. Overall, six different cohort settings were applied in the secondary analysis (3 analyses among COVID-19 groups + 3 analyses among hospitalized COVID-19 groups).
Even though we utilized large-scale propensity score matching to balance between study groups and to minimize the unmeasured confounders, there still can be the residual bias in the observational studies [18]. To estimate the systematic error in the models, we employed 123 negative control outcomes to estimate systematic error in the models (Supplementary Table 1) [20, 21]. The negative control outcomes were found not to be affected by acid-suppressant use, hence, the negative control outcomes can show whether the model is influenced by unmeasured confounders or not. In this study, the final hazard ratio (HR) and 95% CIs were reported through empirical calibrations to adjust measured systematic errors from the analysis of 123 negative control outcomes [22].
Results
PPI use and risk of COVID-19
Baseline characteristics of the study population
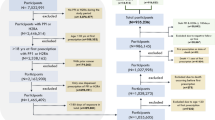
Of the 234,427 patients in the HIRA COVID-19 database, we finally included 26,166 patients prescribed PPI and 62,117 patients prescribed H2RA (Fig. 1) with a person-years follow-up duration of 2361 days for PPI users and 3674 days for H2RA users. Median follow-up days for PPI users was 14 (interquartile range [IQR], 7–75) while it was 7 for H2RA users (IQR, 7–34). Baseline characteristics of the primary analysis are listed in Table 1, which also provides standardized mean differences before and after PSM for the study population. Overall, 20,202 covariates were used for matching (Supplementary Fig. 1), and among overall matched covariates, absolute standardized differences after PSM were less than 0.1 for 96.04% of the covariates, implying that the cohorts were adequately matched and therefore, comparable. Covariates with standardized differences greater than 0.1 after PSM were predominantly medications associated with acid-related disorders and anti-inflammatory products (e.g., bismuth oxide and sucralfate).
Association of PPI use and risk of COVID-19
Table 2 shows the results of the primary analysis that estimated the association between PPI or H2RA usage and risk of COVID-19. PSM-unadjusted analysis showed that PPI use was not significantly associated with risk of COVID-19 infection compared to H2RA use (calibrated HR, 1.49 [95% CI, 0.66–3.36]), moreover, PSM adjusted analyses revealed that PPI use was not significantly associated with lower risk of COVID-19 infection in one-to-four PSM analysis (calibrated HR, 0.81 [95% CI, 0.30–2.19]) and in stratification of propensity scores analysis (calibrated HR, 1.03 [95% CI, 0.51–2.08]).
A comparison of COVID-19 between acid-suppressant users and non-users revealed that PPI use was not significantly associated with lower risk of COVID-19 compared to non-users before adjusted analysis (calibrated HR, 0.43 [95% CI, 0.11–1.61]), after one-to-four matched analysis (calibrated HR, 0.47 [95% CI, 0.17–1.29]), and stratification analysis (calibrated HR, 0.50 [95% CI, 0.17–1.52]). Among H2RA users, medication was associated with the lower risk of COVID-19 compared to non-users during unadjusted analysis (calibrated HR, 0.30 [95% CI, 0.09–0.96]). In other analyses, H2RA use was not significantly associated with infection despite one-to-four PSM (calibrated HR, 0.48 [95% CI, 0.17–1.37]) or propensity score stratification (calibrated HR, 0.46 [95% CI, 0.15–1.43]).
PPI use and complications of COVID-19 disease
Baseline characteristics of the study population
Secondary analysis was performed with data from 1260 patients diagnosed with COVID-19; of these, 410 patients were prescribed PPI and 804 were given H2RA medication (Fig. 2). Subjects were matched based on sex, age groups, medical history (chronic obstructive lung disease and chronic kidney disease), and the Charlson comorbidity index, and the absolute standardized mean difference for all covariates after PSM was less than 0.1. Baseline characteristics are presented in Table 3.
Association between PPI use and complications of COVID-19 disease
Table 4 provides the results of the secondary analysis, which showed no significant association between PPI or H2RA use and all-cause mortality among COVID-19 patients, i.e., (1) unadjusted analysis (calibrated HR, 0.82 [95% CI, 0.47–1.41]), or (2) propensity score stratification (calibrated HR, 0.63 [95% CI, 0.37–1.07]), however, (3) one-to-four PSM analysis showed significant associated between PPI use and all-cause mortality (calibrated HR, 0.54 [95% CI, 0.31–0.95]). Further, hospitalization with pneumonia, ARDS, sepsis, or AKI were not associated with PPI or H2RA use, irrespective of the type of analysis, i.e., (1) unadjusted analysis (calibrated HR, 1.12 [95% CI, 0.87–1.43]), (2) propensity score stratification (calibrated HR, 0.96 [95% CI, 0.79–1.17]), or (3) one-to-four PSM (calibrated HR, 0.88 [95% CI, 0.72–1.08]). Similarly, there was no association between PPI and requirement for intensive respiratory interventions, (1) unadjusted analysis (calibrated HR, 1.28 [95% CI, 0.65–2.50]), (2) propensity score stratification (calibrated HR, 1.01 [95% CI, 0.52–1.97]), and (3) one-to-four PSM (calibrated HR, 0.92 [95% CI, 0.46–1.82]). The results for hospitalized COVID-19 patients are provided in Table 5.
Discussion
This study aimed to estimate and compare risk of incidence and assess outcomes after COVID-19 in Korean patients prescribed PPI or H2RA. Specifically, we evaluated the incidence of COVID 19 in subjects prescribed PPI or H2RA for more than 7 days and show that short-term use of PPI was not associated with incidence of COVID-19 compared to short-term H2RA users or non-users. Moreover, among COVID-19 patients, PPI use for ≥ 7 days was not significantly associated with risk of complications compared to H2RA use except for all-cause mortality in PSM analysis, and it was not associated with complications in hospitalized patients.
Two recent studies have addressed the association between PPI use and incidence of COVID-19 infection [11, 12]. Lee et al. have reported that patients taking PPIs are at increased risk for severe clinical outcomes with COVID-19 but that they are not more susceptible to SARS- CoV-2 infection [12]. They defined current PPI users as patients who took PPIs 1–30 days before the first SARS-CoV-2 test date [12]. Further, they also showed that there was no significant difference in SARS-CoV-2 positivity rates between PPI users and non-users, irrespective of short-term (< 30 days) or long-term (> 30 days) use [12]. Another study, an online survey by Almario et al., reported that individuals using PPIs up to once daily (aOR 2.15; 95%CI, 1.90–2.44) or twice daily (aOR 3.67; 95% CI, 2.93–4.60) had significantly higher odds for testing COVID-19 positive compared to those not taking PPIs [11]. In contrast, we show that PPI use was not associated with the higher risk of COVID-19 infection compared to H2RA use or no acid-suppressant use. This could be due to our use of the Korean national claims database wherein data was converted to the OMOP-CDM format, and this permitted adjustment for many more covariates than previous studies. Additionally, large-scale propensity matching was used to overcome potentially unmeasured confounding factors and we also performed multiple sensitivity analyses. We also calibrated our analysis using 123 negative control outcomes to detect and reduce confounding factors, selection bias, and systematic errors. Thus, of the 48 analyses performed (9 primary and 39 secondary), most results were consistent with the calibrations.
In the secondary analysis, we compared the complication of COVID-19 between PPI and H2RA using multiple sensitivity analyses. The result showed no significant association between PPI and H2RA. Only PSM analysis measuring the association between PPI and all-cause mortality, compared to H2RA, showed significant results, however, the other analyses (i.e., unadjusted and stratification) showed opposite results. We could not perform large-scale PSM in the secondary analysis due to small number of included COVID-19 patients, therefore, there might be biases in the result.
To date, several studies have addressed clinical outcomes in COVID-19; however, most studies only included a small number of patients and were limited by the presence of confounding factors [12,13,14, 23]. Lee et al. found that PPI use led to greater risk of severe clinical outcomes in COVID-19, including intensive care unit admission, requirement of invasive ventilation, or death [12]. However, that study did not consider PPI use after COVID-19 diagnosis, and the comparator group comprised non-PPI users, which could have led to indication bias, i.e., patients in the PPI group could have experienced a more severe course of COVID-19 compared to non-users and the difference might have led to more severe outcomes. Therefore, to avoid indication bias, we compared clinical outcomes between PPI and H2RA users, and consistent with our results, Zhang et al. also reported that PPI use had no effect on the clinical course of COVID-19 [13]. Additionally, Taştemur et al. have suggested that PPIs may be used for both prophylaxis and treatment because hydroxychloroquine and azithromycin may prevent viral spread by accumulating in organelles with acidic content and raising their pH. Thus, given their effects on pH, they concluded that PPIs show similar effects on viral entry and intracellular distribution [15]. Such inconsistent results imply that the risk and benefits of PPI use in viral infection have remained controversial to date [23].
Our study has certain limitations. First, although we used large-scale PSM in the primary analysis, there were a few relatively unmatched covariates that may have affect the results, showing standardized mean difference greater than 0.1. Nonetheless, we measured and adjusted the systematic error in this study through empirical calibration by employing 123 negative control outcomes in the primary analysis. In the secondary analysis, we could not perform large-scale PSM, therefore, the results of clinical outcomes might have many biases. Second, we only included PPI use for 7 days, and therefore, we could not evaluate the effects of long-term PPI use, and as the HIRA database also had data only pertaining to a short period, we could not analyze the long-term effects of acid-suppressants. Third, this was an observational study; therefore, it is not possible to establish causality. Although we could not perform well-designed randomized controlled trial, we performed large-scale PSM and analyzed negative control outcomes to adjust systematic unmeasured confounding factors. Nonetheless, the effects of acid-suppressants on viral infection, especially COVID-19, require further clarification.
Conclusions
In this study, using large-scale PSM and multiple sensitivity analyses, we show that, compared to H2RA use, short-term PPI use is not associated with incidence of COVID-19 infection and severe clinical outcomes. Nevertheless, the effects of long-term PPI use on the incidence and clinical outcomes in COVID-19 disease need to be clearly established.
Availability of data and materials
The data is not publicly available. All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- PPI:
-
Proton pump inhibitor
- COVID-19:
-
SARS-CoV-2 infection
- H2RA:
-
Histamine-2 receptor antagonist
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HIRA:
-
Health Insurance Review and Assessment
- OMOP:
-
Observation Medical Outcomes Partnership
- CDM:
-
Common Data Model
- ARDS:
-
Acute respiratory disease syndrome
- AKI:
-
Acute kidney injury
- ECMO:
-
Extracorporeal membrane oxygenation
- PSM:
-
Propensity score matching
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
References
Savarino V, Tosetti C, Benedetto E, Compare D, Nardone G. Appropriateness in prescribing PPIs: a position paper of the Italian Society of Gastroenterology (SIGE) - study section “digestive diseases in primary care”. Dig Liver Dis. 2018;50(9):894–902. https://doi.org/10.1016/j.dld.2018.07.004.
Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14(12):697–710. https://doi.org/10.1038/nrgastro.2017.117.
Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35–48. https://doi.org/10.1053/j.gastro.2017.04.047.
Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167(9):950–5. https://doi.org/10.1001/archinte.167.9.950 [published Online First: 2007/05/16].
Laheij RJ, Sturkenboom MC, Hassing RJ, et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. Jama. 2004;292(16):1955–60. https://doi.org/10.1001/jama.292.16.1955 [published Online First: 2004/10/28].
Barkun AN, Bardou M, Pham CQ, et al. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107(4):507–20. https://doi.org/10.1038/ajg.2011.474 quiz 21. [published Online First: 2012/02/01].
Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310–9. https://doi.org/10.1503/cmaj.092129 [published Online First: 2010/12/22].
Ho SW, Teng YH, Yang SF, Yeh HW, Wang YH, Chou MC, et al. Association of Proton Pump Inhibitors Usage with risk of pneumonia in dementia patients. J Am Geriatr Soc. 2017;65(7):1441–7. https://doi.org/10.1111/jgs.14813.
MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174(4):564–74. https://doi.org/10.1001/jamainternmed.2013.14673 [published Online First: 2014/02/19].
Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: population based study. BMJ. 2016;355:i5813. https://doi.org/10.1136/bmj.i5813.
Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol. 2020;115(10):1707–15. https://doi.org/10.14309/ajg.0000000000000798 [published Online First: 2020/08/28].
Lee SW, Ha EK, Yeniova A, et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching. Gut. 2021;70(1):76–84. https://doi.org/10.1136/gutjnl-2020-322248 [published Online First: 2020/08/01].
Zhang XY, Li T, Wu H, et al. Analysis of the Effect of Proton-Pump Inhibitors on the Course of COVID-19. J Inflamm Res. 2021;14:287–98. https://doi.org/10.2147/jir.s292303 [published Online First: 2021/02/13].
Luxenburger H, Sturm L, Biever P, Rieg S, Duerschmied D, Schultheiss M, et al. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor? J Intern Med. 2020;289(1):121–4. https://doi.org/10.1111/joim.13121.
Taştemur Ş, Ataseven H. Is it possible to use Proton Pump Inhibitors in COVID-19 treatment and prophylaxis? Med Hypotheses. 2020;143:110018. https://doi.org/10.1016/j.mehy.2020.110018 [published Online First: 2020/07/18].
Rho Y, Cho DY, Son Y, et al. COVID-19 international collaborative research by the health insurance review and assessment service using its nationwide real-world data: database, outcomes, and implications. J Prev Med Public Health. 2021;54(1):8–16. https://doi.org/10.3961/jpmph.20.616 [published Online First: 2021/02/24].
Suchard MA, Simpson SE, Zorych I, et al. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23(1). https://doi.org/10.1145/2414416.2414791 [published Online First: 2013/01/01].
Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005–14. https://doi.org/10.1093/ije/dyy120 [published Online First: 2018/06/26].
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786 [published Online First: 2011/08/06].
Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology (Cambridge, Mass). 2010;21(3):383–8. https://doi.org/10.1097/EDE.0b013e3181d61eeb [published Online First: 2010/03/26].
Voss EA, Boyce RD, Ryan PB, et al. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72–81. https://doi.org/10.1016/j.jbi.2016.12.005 [published Online First: 2016/12/21].
Schuemie MJ, Ryan PB, DuMouchel W, et al. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med. 2014;33(2):209–18. https://doi.org/10.1002/sim.5925 [published Online First: 2013/08/01].
Charpiat B, Bleyzac N, Tod M. Proton pump inhibitors are risk factors for viral infections: even for COVID-19? Clin Drug Investig. 2020;40(10):897–9. https://doi.org/10.1007/s40261-020-00963-x [published Online First: 2020/08/12].
Acknowledgements
The authors thank the healthcare professionals dedicated to treating COVID-19 patients in South Korea, the Ministry of Health and Welfare, and the Health Insurance Review & Assessment Service of Korea for sharing the national health insurance claims data in a prompt manner.
Funding
This research was funded by the Bio Industrial Strategic Technology Development Program (20003883, 20005021) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health &Welfare, Republic of Korea (grant number: HI19C0143, HR16C0001).
Author information
Authors and Affiliations
Contributions
JP, SCY, JC, RWP, SIS, CHP, WGS contributed study concept and design. JP contributed statistical analysis of the study. JP, SCY, JC, SIS, and RWP contributed analysis and interpretation of data, drafting of the manuscript. RWP, SIS, WGS contributed obtaining funding. RWP and SIS contributed supervision of the study. JP and SCY contributed equally to this work. RWP and SIS contributed equally to this work. All author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics and approval and consent to participate
The Institutional review board of the Kangdong Sacred Heart Hospital has approved study and informed consent waiver. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, J., You, S.C., Cho, J. et al. Comparative risk of incidence and clinical outcomes of COVID-19 among proton pump inhibitor and histamine-2 receptor antagonist short-term users: a nationwide retrospective cohort study. BMC Pharmacol Toxicol 23, 9 (2022). https://doi.org/10.1186/s40360-022-00549-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-022-00549-7