Abstract
Background
Protein ubiquitylation is an important post-translational regulation, which has been shown to be necessary for life cycle progression and survival of Plasmodium falciparum. Ubiquitin is a highly conserved 76 amino acid polypeptide, which attaches covalently to target proteins through combined action of three classes of enzymes namely, the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligase (E3). Ubiquitin E1 and E2 are highly conserved within eukaryotes. However, the P. falciparum E3 ligase is substantially variable and divergent compared to the homologs from other eukaryotes, which make the E3 ligase a parasite-specific target.
Methods
A set of selected E3 ubiquitin ligase inhibitors was tested in vitro against a chloroquine-sensitive P. falciparum D6 strain (PfD6) and a chloroquine-resistant P. falciparum W2 strain (PfW2). The inhibitors were also tested against Vero and transformed THP1 cells for cytotoxicity. The lead antimalarial E3 ubiquitin ligase inhibitors were further evaluated for the stage-specific antimalarial action and effects on cellular development of P. falciparum in vitro. Statistics analysis was done by two-way ANOVA followed by Tukey and Sidak multiple comparison test using GraphPad Prism 6.
Results
E3 ligase inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 showed prominent antimalarial activity against PfD6 and PfW2. These inhibitors were considerably less cytotoxic to mammalian Vero cells. JNJ 26854165, HLI 373 and Nutlin 3 blocked the development of P. falciparum parasite at the trophozoite and schizont stages, resulting in accumulation of distorted trophozoites and immature schizonts.
Conclusions
Interruption of trophozoites and schizont maturation by the antimalarial E3 ligase inhibitors suggest the role of ubiquitin/proteasome functions in the intraerythrocytic development of malaria parasite. The ubiquitin/proteasome functions may be critical for schizont maturation. Further investigations on the lead E3 ligase inhibitors shall provide better understanding regarding the importance of E3 ligase functions in the malaria parasite as a potential new antimalarial drug target and a new class of antimalarial drug leads.
Similar content being viewed by others
Background
According to the most recent estimates of the World Health Organization (WHO), in 2015 alone malaria caused 438,000 deaths with estimated 214 million new cases [1]. Infections with Plasmodium falciparum are responsible for more than 90% of malaria-related deaths, particularly in African countries [1]. Drug resistance of P. falciparum is the greatest challenge in the fight against malaria, as the parasite has developed resistance to most of the drugs presently used for the treatment of malaria. These factors have led to the earnest need to discover new molecular targets and identify new drug leads against those targets [2].
Post-translational modifications are necessary for the progression of P. falciparum life cycle [3, 4]. Among these modifications, protein ubiquitylation plays an important role. Ubiquitylation of proteins can be proteasome-dependent or independent. Proteasome-dependent ubiquitylation pathway involves various biological processes comprising specific components, which make this pathway a potential therapeutic target to develop new anti-malarial drugs. Ubiquitin system participates in various cellular functions as well as host-parasite interactions [3]. Ubiquitin is a highly conserved 76 amino acids’ polypeptide that covalently attaches to target proteins through the combined action of three classes of enzymes namely, the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2) and the ubiquitin-protein ligase (E3) [5]. At the onset, the ubiquitin-activating enzyme (E1) activates ubiquitin through an ATP-dependent reaction. In this reaction, the terminal glycine residue of ubiquitin is adenylated and transferred to an internal cysteine residue to generate a high-energy thiol ester intermediate. The activated ubiquitin is then transferred to the ubiquitin-conjugating enzyme (E2), forming another thiol-ether intermediate, which then specifically binds to the ubiquitin-protein ligase (E3). Lastly, E3 ligase transfers the activated ubiquitin from E2 to the side chain of a lysine residue of the target protein [3, 5]. Protein ubiquitylation can be monoubiquitylation (attachment of one ubiquitin), multiubiquitylation (several ubiquitin molecules attached to different lysine residues) or polyubiquitylation (polyubiquitin chain generation) [4].
Ubiquitin E1 and E2, the two enzymatic components of ubiquitylation system, are highly conserved within eukaryotes [4]. However, P. falciparum E3 ubiquitin ligases are considerably diverse and thus may serve as promising targets for new antimalarial discovery [5]. The hypothesis behind this study is that targeting the E3 ligases functions of ubiquitin system would selectively kill the malaria parasite and would be less toxic to the mammalian cells. A set of standard E3 ligase inhibitors was tested against the chloroquine (CQ)-sensitive P. falciparum D6 strain (PfD6) and CQ-resistant P. falciparum W2 strain (PfW2) in vitro. The selective antimalarial inhibitors were further investigated to analyze the effect of E3 ligase inhibitors on intraerythrocytic P. falciparum asexual life cycle development. These studies suggest ubiquitin E3 ligases as potential antimalarial drug targets and support previous reports that the ubiquitin proteosomal degradation pathway may be essential for the survival of the malarial parasite [4, 5].
Methods
Cell cultures
CQ-sensitive (D6, Sierra Leone) and -resistant (W2, Indochina) strains of P. falciparum cultures were obtained from Malaria Research and Reference Reagent Resource Center (MR4), USA and maintained at 5% hematocrit in complete culture medium (RPMI 1640 supplemented with 60 μg/mL amikacin, 27 mM NaHCO3 and 10% heat-inactivated normal human type A+ serum). The medium of the culture was replaced every 48 h, flushed with a gas mixture of 90% N2, 5% CO2 and 5% O2 and incubated at 37 °C.
Vero cells and THP1 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37 °C in an incubator with 5% CO2. Both, Vero cells and THP1 cells were obtained from American Type Culture Collection (ATCC), USA having catalogue number ATCC® CCL-81™ and ATCC® TIB-202™ respectively and were subcultured twice a week.
Chemicals
Eight E3 ligase inhibitors namely, thalidomide, proTAME, NSC 66811, Nutlin 3, HLI 373, JNJ 26854165, SMER 3 and NSC 146109 were used in the study. Thalidomide targets cereblon (CRBN), which makes an E3 ubiquitin ligase complex with damaged DNA-binding protein 1 (DDB1) and cullin-4A (Cul4A). Thalidomide binds with CRBN and inhibits the associated ubiquitin ligase activity [6, 7]. ProTAME, a cell-permeable prodrug, converts to an active molecule, tosyl-L-arginine methyl ester (TAME) and inhibits the ubiquitin ligase activity of the anaphase-promoting complex/cyclosome (APC/C) [8, 9]. NSC 66811 and Nutlin 3 have been reported as MDM2 (Murine Double Minute 2) E3 ligase inhibitors [10, 11]. JNJ 26854165 and HLI 373 are HDM2 (Human Double Minute 2) E3 ligase antagonists. SMER 3 is an inhibitor of a yeast SCF family E3 ubiquitin ligase (SCFMet30). Thalidomide, NSC6811, NSC 146109 and SMER 3 were purchased from Tocris Bioscience. Nutlin 3, JNJ 26854165 and ProTAME were purchased from Selleckchem, USA, Axon Medchem, Netherland and Boston Biochem, Inc. USA respectively. Chloroquine (CQ) was used as the positive control, and dimethyl sulfoxide (DMSO) was used as vehicle control in this study. CQ and DMSO were obtained from Sigma-Aldrich, USA.
Antimalarial SYBR Green I-based fluorescence assay
CQ-sensitive P. falciparum D6 strain (Pf D6) and CQ-resistant P. falciparum W2 strain (Pf W2) were grown in RPMI 1640 medium supplemented with amikacin (60 μg/mL), NaHCO3 (27 mM) and 10% heat-inactivated normal human type A+ serum. Only ring stages of the parasite were counted and adjusted to 2% parasitemia with 2% hematocrit. Stock solutions (10 mM) of the test inhibitors were prepared in DMSO. The compounds were serially diluted in serum-free RPMI-1640 medium for testing at eight concentrations. The maximum concentrations of E3 ligase inhibitors and CQ used in the assay were 50 μM and 500 nM respectively. The plates were incubated at 37 °C in 5% CO2, 5% O2, and 90% N2 environment for 48, 72, 96 and 120 h. After incubation, SYBR green with RBC lysis buffer was added to each well, and the plates were incubated for 1 h in the dark at room temperature. The standard antimalarial agent, CQ was tested as the positive control, and DMSO was tested as vehicle control. Standard fluorescence was measured Fluostar Galaxy microplate reader (BMG Lab Technologies) at excitation wavelength of 485 nm and emission wavelength of 535 nm [12]. The anti-malarial activity (expressed as IC50 values) was determined by the dose-response analysis with XLfit©.
In vitro cytotoxicity assays against Vero and transformed THP1 cells
The E3 ligase inhibitors were also tested against Vero cells (African green monkey kidney epithelial cells) and transformed non-dividing human monocytic (THP1) cells. The methods described earlier were used to measure cytotoxicity for Vero and THP1 cells [13, 14]. In brief, four days’ old Vero and THP1 cell cultures were diluted with RPMI medium to 25,000 cells/mL. Vero cells (100 μL per well) were seeded in a clear 96 well culture plate and incubated for 24 h at 37 °C in a 5% CO2 incubator to obtain confluent cells. Phorbol 12-myristate 13-acetate (PMA) (25 ng/mL) was added to THP1 cell culture to transform the cells to adherent macrophages [15]. PMA treated THP1cells were dispensed into clear 96 well culture plates (200 μL/well; 25,000 cells/mL) and incubated at 37 °C in a 5% CO2 incubator for overnight. The test compounds were prepared in DMSO at 10 mM concentration. The compounds were serially diluted in RPMI medium with 2% FBS in the separate daughter plates, for testing at eight concentrations. The cells were washed with culture medium, replaced with the fresh medium and test compounds were added. The maximum concentration of E3 ligase inhibitors and CQ used in these assays were 50 μM and 500 nM respectively. DMSO was tested as vehicle control in these assays. The plates were incubated further in a CO2 incubator at 37 °C for 48 h. After 48 h, 10 μL of AlamarBlue® was added to each well, and the plates were incubated in a CO2 incubator at 37 °C for overnight. Standard fluorescence was measured on a Fluostar Galaxy microplate reader (BMG Lab Technologies) at 544 nm ex, 590 nm em [13]. IC50 values were computed by analyzing dose-response curves with XLfit©.
Analysis of effect of E3 ligase inhibitors on P. falciparum development (stage specific imaging analysis: antimalarial assay)
The stage-specific activity of the lead compounds was tested against Pf D6. The parasite culture was grown in RPMI-1640 medium supplemented with amikacin (60 μg/mL), NaHCO3 (27 mM) and 10% heat-inactivated normal human type A+ serum. The culture was synchronized by 5% sorbitol treatment as previously described [16]. The synchronized culture of PfD6, with ~6% parasitemia and >90% of the parasite in ring stage, was used for these assays. Stock solutions (10 mM) of the test inhibitors were prepared in DMSO. CQ and active antimalarial inhibitors namely, Nutlin 3, JNJ 26854165 and HLI 737 were tested at 50 nM, 35 μM, 5 μM and 5 μM concentration respectively for the stage-specific antimalarial assay. These concentrations were almost equivalent to the IC90 of the inhibitors against PfD6 at 48 h. The effect of the compounds was monitored after every 8 h (0, 8, 16, 24, 32, 40 and 48 h) by preparing thin smears of the infected erythrocytes. The slides were stained with Giemsa, and digital images of the parasite were captured with Nikon Eclipse microscope under bright field using NIS-element imaging software. The images were analyzed for the parasite stages and morphological changes (if any) in the parasite. The levels of parasitemia and differential parasitemia (relative percentage of different parasite stages) counts were also measured. At least one thousand erythrocytes (total- infected & uninfected) were counted from each slide for determination of the the level of parasitemia and differential parasite stage counts.
Statistical analysis
Two-way ANOVA followed by Tukey and Sidak multiple comparison test with GraphPad Prism 6 was used to compare IC50 values of the inhibitors, the parasitemia and also percentage of individual parasite stages.
Results
In vitro antiplasmodial activity of standard E3 ubiquitin ligase inhibitors
The antimalarial activity of a set of E3 ubiquitin ligase inhibitors was evaluated in vitro against PfD6 and PfW2 at eight different concentrations of each compound and at four different time points (48, 72, 96 and 120 h) (Table 1). Among the eight E3 ligase inhibitors tested (Fig. 1), thalidomide and proTAME did not show noticeable antimalarial activity against PfD6 and PfW2 up to 50 μM concentration and 120 h of exposure. E3 ligase inhibitors, namely NSC 66811, SMER 3 and Nutlin 3 showed moderate antimalarial activity against both PfD6 & PfW2. Antimalarial activity of above three inhibitors against PfD6 was not significantly different from the activity against PfW2 except SMER 3. SMER 3 showed significantly better antimalarial activity at 72 h (p < 0.05) against PfW2 as compared to PfD6. NSC 146109, JNJ 26854165 and HLI 373 showed lower IC50 values (below 6 μM) against both PfD6 and PfW2 and exhibited early growth inhibition. The IC50 values of HLI 373 against PfD6 were significantly different and were almost 2 fold higher against PfW2 as compared to PfD6 at 48 (p < 0.05), 72 (p < 0.01), 96 (p < 0.01) and 120 h (p < 0.01). The antimalarial activity of JNJ 26854165 against PfD6 was significantly higher (lower IC50 values) in comparison to PfW2 at 72 h (p < 0.05). Similarly, NSC 146109 showed significantly better antimalarial activity against PfW2 as compared to PfD6 at 48 (p < 0.0001) and 72 h (p < 0.001).
In vitro cytotoxicity of standard E3 ligase inhibitors against Vero and THP1 cells-
The E3 ligase inhibitors tested for in vitro antimalarial activity were also tested against Vero cells (African green monkey kidney epithelial cells) and non-proliferating THP1 (transformed human monocytic) cells for mammalian cell cytotoxicity (Table 1). CQ, thalidomide, proTAME, NSC 66811, Nutlin 3 and HLI 373 were not toxic at the highest concentration tested. SMER 3 and NSC 146109 were toxic against Vero cells, and their IC50 values were not considerably different from corresponding IC50 values against PfD6 and PfW2. However, JNJ-26854165 was significantly less toxic to mammalian cells as compared to PfD6 (p-value <0.001) and PfW2 (p-value <0.001). Therefore Nutlin 3, HLI 373 and JNJ-26854165 were identified as selective antimalarial E3 ligase inhibitors. CQ, thalidomide, and proTAME were not toxic against THP1 cells at the highest concentration tested. NSC 146109 and SMER 3 were toxic against THP1 cells, and IC50 values of NSC 146109 was not considerably different from corresponding IC50 values against PfD6 and PfW2. The IC50 value of SMER 3 was not considerably different from corresponding IC50 values against PfD6, although significantly different than corresponding IC50 values against PfW2 (p-value <0.05). NSC 66811 was not toxic against THP1 cells. Nutlin 3, HLI 373 and JNJ-26854165 were considerably less toxic against THP1 cells as compared to PfD6 (p-value <0.001, <0.001 and <0.001 respectively) and PfW2 (p-value <0.01, <0.001 and <0.001 respectively). Thus Nutlin 3, HLI 373 and JNJ-26854165 were identified as selective antimalarial E3 ligase inhibitors.
Evaluation of antimalarial ubiquitin ligase inhibitors on growth and development of P. falciparum
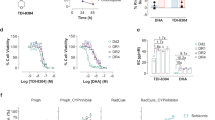
The effects of lead antimalarial ubiquitin ligase inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 were further tested against the asexual intra-erythrocytic development of P. falciparum. These inhibitors showed early effects on the parasite growth (Table 1) and therefore were tested up to 48-h incubation period. The results show the effects of inhibitors on overall levels of the parasitemia (Fig. 2), and relative percentages of different parasite stages namely rings, trophozoites and schizonts (Fig. 3). Any morphological and cellular changes in parasite development were determined by analysis of digital images of the parasite exposed to these inhibitors. The representative digital images of the parasite stages and modifications in parasite morphology are presented in Fig. 4. CQ was tested as a positive control, and DMSO was tested as a vehicle control in this assay. At 0 h the PfD6 cultures had ~ 6% overall parasitemia and > 90% of the parasites were in ring stage. In PfD6 culture treated with vehicle control majority of the parasites were transformed into trophozoites at 8 to 48 h, The ratio of schizonts increased from 0 to 24 h and declined at after 32 h (Fig. 3a). The overall level of parasitemia did not change considerably in vehicle treated PfD6 culture up to 48 h, as compared to 0 h (5.28% vs. 5.98%) (Fig. 2). CQ treatment significantly decreased the overall parasitemia at 40 (p < 0.001) and 48 (p < 0.01) hours, as compared to the vehicle control (Fig. 2a). In CQ treated PfD6 cultures, around 50% of the parasites were arrested at ring stage at 16 h post-treatment. Likewise, more than 50% of the parasites were arrested at ring stage at 40 and 48 h. Only a few parasites matured into schizonts, which further declined after 32 h (Fig. 3b). The change in differential parasite counts in CQ-treated cells, at 48 h post-treatment, were statistically not significant compared to control vehicle treated PfD6 culture, due to large individual variations. However, the rings detected in CQ-treated PfD6 cultures were apparently healthy, but the trophozoites were shrunk (Fig. 4). These results indicate that CQ kills the malarial cells by blocking the growth of parasite at ring/schizont stage. This reduction in parasitemia by JNJ 26854165 was comparable to that of CQ at 40 (1.90% vs 2.40%) and 48 h (1.76% vs 1.92%), and was markedly reduced as compare to control at 40 (1.9 vs 6.90; p < 0.0001) and 48 (1.76% vs 5.28%; p < 0.01) hours of treatment (Fig. 2a and b). The parasites treated with JNJ 2648544858 apparently showed a different life-cycle stage development pattern as compared to control. The majority (~90% at 0 h) of the rings stage parasite matured to trophozoites at 8 h. At 16 and 24 h the majority of P. falciparum parasites were in ring and trophozoite stages, and again trophozoites became dominant at 40 and 48 h (Fig. 3c). The change in differential parasite counts in JNJ2648544858 treated cells at 48 h post-treatment were statistically not significant compared to controls and CQ-treated PfD6 cultures due to large individual variations (Fig. 3). However, distorted rings, trophozoites, and schizonts were seen in PfD6 cultures treatment with JNJ 2648544858 at 40 and 48 h post-treatment (Fig. 4). HLI 373 showed a significant reduction in parasitemia at 40 h (p < 0.05) as compared to control (Fig. 2c). The P. falciparum culture treated with HLI 373 showed ring stages in the majority (~90%) at 0 h, while at the remaining time points after the treatment (8, 16, 24, 32 and 40) majority of the parasite cells were at trophozoite stage. Most of the parasite cells were at schizont stage at 48 h after the treatment with HLI 373 (Fig. 3d). However, the change in differential parasite counts in HLI 373 treated cells at 48 h post-treatment were statistically not significant compared to controls and CQ-treated PfD6 cultures due to large individual variations (Fig. 3). The parasitemia was significantly reduced in Nutlin 3 treated P. falciparum culture as compared to control at 40 h (p < 0.001) (Fig. 2d). In P. falciparum cultures treated with Nutlin 3 trophozoites were in the majority at 8, 16 and 40 h post-treatment, whereas trophozoites and schizonts were the dominating stages at 32 and 48 h post-treatment (Fig. 3e). The change in differential parasite counts in Nutlin 3 treated cells at 48 h post-treatment were statistically not significant compared to controls, and CQ-treated PfD6 cultures due to large individual variations (Fig. 3). However, A large number of distorted trophozoites and schizonts were observed in HLI 373, and Nutlin 3 treated P. falciparum culture at 40 and 48 h post-treatment (Fig. 4).
Effect of selected E3 ligase inhibitors on growth of Plasmodium falciparum in vitro. Each point represents the mean ± SEM of three values. Minimum one thousand erythrocytes were counted for each value. a Chloroquine (CQ) was tested as standard antimalarial drug for positive control and DMSO was used as vehicle control. The ring stage synchronized CQ sensitive P. falciparum D6 strain (Pf D6) culture with high parasitemia (~6%) was treated with the antimalarial E3 ligases inhibitors namely, b JNJ 26854165 (JNJ), c HLI 373 (HLI) and (D) Nutlin-3 (Nutlin). Parasitemia was monitored at 0, 8, 16, 24, 32, 40 and 48 h post treatment as indicated. Statistically significant difference compared to corresponding hours against vehicle control (* p-value <0.05; ** p-value <0.01; ***p-value <0.001; **** p-value <0.0001)
Effect of selected E3 ligase inhibitors on cellular development of Plasmodium falciparum in vitro. a Control [DMSO]; b Chloroquine; c JNJ 26854165; d HLI 373; e Nutlin-3 at 0, 8, 16, 24, 32, 40 and 48 h post-treatment. Each bar represents the mean ± SEM of three observations. Minimum one thousand erythrocytes were counted for each observation. Chloroquine (CQ) and DMSO were tested as positive, and vehicle control respectively. The ring stage synchronized CQ-sensitive P. falciparum D6 strain (Pf D6) culture with high parasitemia (~6%) was treated with the lead E3 ligase inhibitors and differential parasite stages were monitored at 0, 8, 16, 24, 32, 40 and 48 h as indicated. The results on differential parasite stage counts in control, CQ and inhibitors treated P. falciparum cultures were analyzed for statistical comparisons. The change in differential parasite counts in inhibitor treated cells at 48 hors post-treatment were statistically not significant (P values >0.5) compared to controls and CQ treated PfD6 cultures due to large individual variations
Progression of cellular development of asexual Plasmodium falciparum during treatment with antimalarial E3 ligase inhibitors. P. falciparum D6 strain (Pf D6) cultures were treated with vehicle control (DMSO), chloroquine, JNJ 26854165, HLI 373 and Nutlin-3 and digitally monitored at 0, 8, 16, 24, 32, 40 and 48 h’ post-treatment for parasite stages. Chloroquine (CQ) was used as positive control. Only selective digital images from each set of observations are represented here
Discussion
The ubiquitin system of P. falciparum is involved in numerous cellular functions and host-pathogen interaction [3]. The functions of ubiquitin system are essential for progression of the malaria parasites’ life cycle [3]. The P. falciparum genome contains more than 100 genes, which putatively encode for the component associated with the ubiquitin system. The P. falciparum ubiquitin system contains four ubiquitins (PFL0585w, PF14_0027, PF13_0346, and PF08_0067), eight ubiquitin-activating enzymes (specific for ubiquitin and ubiquitin-like moieties), 14 ubiquitin-conjugating enzymes and more than fifty E3 ubiquitin-protein ligases. The proteomic analysis of P. falciparum ubiquitome has shown that the level of ubiquitin was more in rings and trophozoites than in schizonts [4], which suggested that ubiquitylation of proteins occurred more prominently in schizonts’ and might be necessary for schizonts’ maturation and their further differentiation into invasive merozoites [4].
Among the components of ubiquitin pathway, E3 ubiquitin-protein ligase is the most variable and divergent within eukaryotes and highly specific to the P. falciparum parasite [4]. Thus, targeting essential parasite-specific E3 ligases would be a promising approach for molecular target-based new antimalarial drug discovery [5]. E3 ubiquitin-protein ligase catalyzes the transfer of activated ubiquitin to the specific target protein. E3 ubiquitin-protein ligases are divided into three main classes on the basis presence of specific domains namely, Homologous to E6-associated protein C-terminus (HECT), Really Interesting New Gene (RING) and U-box [3]. The standard E3 ligase inhibitors tested in this study showed an early antimalarial effect after the treatment, which was consistent up to 120 h. This suggests that these inhibitors kill the parasites in the first asexual cycle. However, some classes of drugs – specifically, antibiotics like chloramphenicol, clindamycin, rifampin, tetracycline, telithromycin and quinupristin-dalfopristin show delayed killing of the malaria parasite in the second cycle of parasite’s asexual reproduction [17]. These antibiotics inhibit the protein synthesis in general, and the primary target of antibiotic antimalarials in Plasmodium sp. seems to be in the apicoplast. By inhibiting protein synthesis and the apicoplast, these antibiotics cause a delayed death phenomenon [17,18,19,20].
The E3 ligase inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 were found to be active against both CQ-sensitive PfD6 and CQ-resistant PfW2 and were less cytotoxic against Vero and transformed THP1 cells. The two E3 ligase inhibitors namely, JNJ 26854165 and HLI 373 are HDM2 antagonists. HDM2 is a RING finger E3-ubiquitin ligase that acts as the predominant negative regulator of p53 [10, 21,22,23]. JNJ 26854165, also known as Serdemetan, is a tryptamine derivative and interrupts the HDM2-p53 binding and is in phase 1 clinical trial for the treatment of multiple myelomas [21, 22, 24]. Nutlin 3 is an inhibitor of MDM2 (Murine Double Minute 2), an oncogene and a negative regulator of the tumor suppressor protein p53. Nutlin 3 blocks MDM2-p53 interaction with potency in sub-nanomolar range with Ki value 36 nM [10, 25]. Like Nutlin 3, NSC 66811 also is an MDM 2 inhibitor with Ki value 120 nM but showed less prominent activity against PfD6 and PfW2 as compare to Nutlin 3 [11]. ProTAME is a cell-permeable prodrug, which is converted to its active parent molecule TAME. The parent molecule inhibits the ubiquitin ligase activity of the APC/C by preventing its activation by Cdc20 and Cdh1 [8, 9].
The antimalarial active E3 ligase inhibitors arrested the parasite growth at trophozoites stages resulting in accumulation of distorted trophozoites and immature schizonts. These observations are in agreement with previous reports [5], which showed more predominant expression of E3 ligase in trophozoites and early schizonts. The ubiquitin/proteasome function may be essential for maturation of P. falciparum trophozoites.
Conclusions
E3 ligase inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 showed prominent antimalarial activity against both CQ- sensitive PfD6 and -resistant PfW2 strains. JNJ 26854165 showed considerably different activity against PfD6 as compared to PfW2 at 72-h post treatment. Similarly, the antimalarial activity of HLI 373 was considerably different against PfD6 as compared to PfW2 at 48, 72, 96 and 120 h. However, Nutlin 3 did not show considerably different activity against PfD6 as compared to PfW2 at all four-time points. The inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 were selectively active against P. falciparum, and did not show cytotoxicity against Vero and transformed THP1 cells. JNJ 26854165, HLI 373 and Nutlin 3 apparently arrest the development of P. falciparum growth at the trophozoites and schizonts stage and cause accumulation of distorted trophozoites and immature schizonts.
These results suggest that the function of ubiquitin/proteasome may be crucial for schizont maturation in P. falciparum parasite. Antimalarial E3 ligase inhibitors apparently block trophozoites and schizonts maturation and inhibit the intraerythrocytic development of malaria parasite. Further investigations on the lead antimalarial E3 ligase inhibitors would promise new understanding and importance of E3 ligase function in the malaria parasite. It may also provide a viable antimalarial-target and new classes of antimalarial drug leads.
Abbreviations
- APC/C:
-
Anaphase-promoting complex/cyclosome
- CQ:
-
Chloroquine
- CRBN:
-
Cereblon
- Cul4A:
-
Cullin-4A
- DDB1:
-
Damaged DNA binding protein 1
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
fetal bovine serum
- HDM2:
-
Human double minute 2
- HECT:
-
Homologous to E6-associated protein C-terminus
- MDM2:
-
Murine double minute 2
- Pf D6:
-
Chloroquine-sensitive Plasmodium falciparum D6 strain
- Pf W2:
-
Chloroquine -resistant Plasmodium falciparum W2 strain
- RING:
-
Really interesting new gene
- TAME:
-
Tosyl-L-arginine methyl ester
References
Organization WH. World malaria report 2015. In: WHO Global Malaria Programme. Geneva: World Health Organization; 2015.
Sinha S, Medhi B, Sehgal R. Challenges of drug-resistant malaria. Parasite. 2014;21:61.
Ponts N, Yang J, Chung DW, Prudhomme J, Girke T, Horrocks P, Le Roch KG. Deciphering the ubiquitin-mediated pathway in apicomplexan parasites: a potential strategy to interfere with parasite virulence. PLoS One. 2008;3(6):e2386.
Ponts N, Saraf A, Chung DW, Harris A, Prudhomme J, Washburn MP, Florens L, Le Roch KG. Unraveling the ubiquitome of the human malaria parasite. J Biol Chem. 2011;286(46):40320–30.
Chung DW, Ponts N, Prudhomme J, Rodrigues EM, Le Roch KG. Characterization of the ubiquitylating components of the human malaria parasite’s protein degradation pathway. PLoS One. 2012;7(8):e43477.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–50.
Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53.
Aghajan M, Jonai N, Flick K, Fu F, Luo M, Cai X, Ouni I, Pierce N, Tang X, Lomenick B, et al. Chemical genetics screen for enhancers of rapamycin identifies a specific inhibitor of an SCF family E3 ubiquitin ligase. Nat Biotechnol. 2010;28(7):738–42.
Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, Hathaway N, Dimova N, Cuny GD, King RW. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18(4):382–95.
Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–41.
Lu Y, Nikolovska-Coleska Z, Fang X, Gao W, Shangary S, Qiu S, Qin D, Wang S. Discovery of a nanomolar inhibitor of the human murine double minute 2 (MDM2)-p53 interaction through an integrated, virtual database screening strategy. J Med Chem. 2006;49(13):3759–62.
Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51(6):1926–33.
Manda S, Khan SI, Jain SK, Mohammed S, Tekwani BL, Khan IA, Vishwakarma RA, Bharate SB. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-beta-carbolines. Bioorg Med Chem Lett. 2014;24(15):3247–50.
Jain S, Jacob M, Walker L, Tekwani B. Screening North American plant extracts in vitro against Trypanosoma brucei for discovery of new antitrypanosomal drug leads. BMC Complement Altern Med. 2016;16:131.
Jain SK, Sahu R, Walker LA, Tekwani BL. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Vis Exp. 2012;(70):1–474.
Dahl EL. Institution: Department of Medicine. San Francisco: University of California San Francisco.
Dahl EL, Rosenthal PJ. Multiple antibiotics exert delayed effects against the Plasmodium falciparum apicoplast. Antimicrob Agents Chemother. 2007;51(10):3485–90.
Barthel D, Schlitzer M, Pradel G. Telithromycin and quinupristin-dalfopristin induce delayed death in Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52(2):774–7.
Ramya TN, Mishra S, Karmodiya K, Surolia N, Surolia A. Inhibitors of nonhousekeeping functions of the apicoplast defy delayed death in Plasmodium falciparum. Antimicrob Agents Chemother. 2007;51(1):307–16.
Goodman CD, McFadden GI. Targeting apicoplasts in malaria parasites. Expert Opin Ther Targets. 2013;17(2):167–77.
Chargari C, Leteur C, Angevin E, Bashir T, Schoentjes B, Arts J, Janicot M, Bourhis J, Deutsch E. Preclinical assessment of JNJ-26854165 (Serdemetan), a novel tryptamine compound with radiosensitizing activity in vitro and in tumor xenografts. Cancer Lett. 2011;312(2):209–18.
Duffy MJ, Synnott NC, McGowan PM, Crown J, O’Connor D, Gallagher WM. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014;40(10):1153–60.
Kitagaki J, Agama KK, Pommier Y, Yang Y, Weissman AM. Targeting tumor cells expressing p53 with a water-soluble inhibitor of Hdm2. Mol Cancer Ther. 2008;7(8):2445–54.
Stuhmer T, Arts J, King P, Page M, Bommert K, Leo E, Bargou RC. A first-in-class HDM2-inhibitor (JNJ-26854165) in phase I development shows potent activity against multiple myeloma (MM) cells in vitro and ex vivo. ASCO Meeting Abstracts. 2008;26(15_suppl):14694.
Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105(10):3933–8.
Acknowledgments
This work has been partly supported through USDA-ARS cooperative scientific agreement with the NCNPR. The authors are thankful to the National Center for Natural Products Research, University of Mississippi for providing necessary facilities.
Funding
This work was supported by National Center for Natural Products, University of Mississippi, University MS and USDA-ARS Cooperative Scientific Agreement 58-6408-2-0009 with NCNPR.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JJ and BLT designed the study. JJ and SJ performed experiments. JJ and BLT analyzed the results. JJ, BLT, and LW wrote the manuscript. All the authors critically reviewed the results, read and approved the manuscript for submission.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jain, J., Jain, S.K., Walker, L.A. et al. Inhibitors of ubiquitin E3 ligase as potential new antimalarial drug leads. BMC Pharmacol Toxicol 18, 40 (2017). https://doi.org/10.1186/s40360-017-0147-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-017-0147-4