Abstract
Mantle cell lymphoma is a relatively new recognized hematological malignant disease, comprising of 2.5–6% non-Hodgkin’s lymphomas. The complexity of its clinical presentations (nodular pattern, diffuse pattern, and blastoid variant), variety in disease progression, and treatment response, make this disease a research focus to both experimental oncology and clinical oncology. Overexpression of cyclin D1 and chromosome t(11,14) translocation are the known molecular biomarkers of this disease. Mantle cell international prognostic index (MIPI), ki-67 proliferation index, and TP53 mutation are emerging as the prognostic biomarkers. Epigenetic profile variance and SOX11 gene expression profile correlate with treatment response. Over the years, the treatment strategy has been gradually evolving from combination chemotherapy to combination of targeted therapy, epigenetic modulation therapy, and immunotherapy. In a surprisingly short period of time, FDA specifically approved 4 drugs for treating mantle cell lymphoma: lenalidomide, an immunomodulatory agent; Bortezomib, a proteasome inhibitor; and Ibrutinib and acalabrutinib, both Bruton kinase inhibitors. Epigenetic agents (e.g. Cladribine and Vorinostat) and mTOR inhibitors (e.g. Temsirolimus and Everolimus) have been showing promising results in several clinical trials. However, treating aggressive variants of this disease that appear to be refractory/relapse to multiple lines of treatment, even after allogeneic stem cell transplant, is still a serious challenge. Developing a personalized, precise therapeutic strategy combining targeted therapy, immunotherapy, epigenetic modulating therapy, and cellular therapy is the direction of finding a curative therapy for this subgroup of patients.
Similar content being viewed by others
Introduction
In 1970s, investigators observed a histologically distinctive subtype of non-hodgkin lymphoma (NHL), which appeared intermediate between well-differentiated (small lymphocytic lymphoma) and poorly differentiated (small cleaved cell lymphoma) and resembled centrocytes of reactive germinal center. This new subtype of NHL was called intermediate lymphocytic lymphoma [1,2,3]. Later investigators identified that this subtype lymphoma originated from mantle zones of secondary follicles and express B cell markers but different from follicular lymphoma [4,5,6]. It was called mantle cell lymphoma and was further stratified into nodular, diffuse and mantle zone subtypes. Mitotic activity, blastic morphology and peripheral blood involvement at diagnosis were recognized as poor prognostic indicators [7, 8].
MCL comprises of 2.5–6% of NHLs [9, 10]. MCL usually have either nodular or diffuse pattern of growth. Approximately 20% MCL cases show blastoid morphology. The MCL cells express surface immunoglobulins, including Ig M and Ig D, CD5, CD19, and CD22; but not CD3, CD23, CD10 and CD11c. Fluorescent in situ hybridization (FISH) reveals translocation t(11; 14) in almost all MCL cells [11]. Mantle cell lymphoma international prognostic index (MIPI) score can divide patients into low, intermediate and high risk groups. Low risk group shows a 5 year overall survival (OS) rate of 60%, intermediate risk group has median OS of 51 months and high-risk group has median OS of 29 months [12]. Ki-67 proliferation index is a prognostic biomarker independent of MIPI score and predicts survival in patients receiving high dose chemotherapy and autologous stem cell transplant (ASCT) [13]. Molecular marker like SOX11 is associated with aggressive phenotype [14].
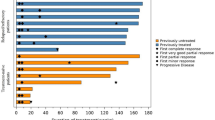
The way to treat MCL has evolved over time, although indolent form of disease may be observed, patients with aggressive variant have different treatment options depending on age, performance status and possibility of bone marrow transplant. New advances including novel targeted therapy and immunotherapy have changed the landscape of treatment (Table 1).
Problem in MCL treatment
In late 1980s, Meusers et al. investigated if centrocytic lymphoma/mantle cell lymphoma was a curable entity. Patients were randomized to COP or anthracycline containing CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone). There were no significant differences with respect to rates of remission induction, probability of progression free survival (PFS) and overall survival (OS). The median survival times for those patients were 33 months. This study suggested that conventional treatment options were not able to improve prognosis at that time [15].
Compared to other NHLs, MCL exhibits shorter durations of response, PFS and OS. In 1995, Teodorovic et al. analyzed two EORTC Lymphoma cooperative group trials and showed that patients with MCL in all grades had shorter duration of response and PFS compared to patients with other lymphomas, though the initial response rates were very similar [16]. Interestingly, adding rituximab to CHOP therapy improved the objective response rate (ORR) to 94–99% [17]. However, this high ORR did not translate into a PFS benefit. Furthermore, Lenz et al. in a GLSG (German Low Grade Lymphoma Study Group) study, showed that addition of rituximab in induction therapy didn’t improve PFS among ASCT patients either [18].
Elderly and transplant ineligible patients
As patients with MCL tend to be elderly with comorbidities, most of the patients are not eligible for intensive induction therapies or ASCT. Bendamustine plus rituximab (BR) emerged as the combination therapy for this group of patients. STiL trial and BRIGHT study demonstrated better CR and improved PFS in comparison to RCHOP/RCVP therapy in elderly patients [19, 20].
Cytarabine has been proved an effective regimen in MCL intensive induction therapy. It was shown that adding low dose cytarabine (500 mg/m2) to BR in treating MCL patients, especially for elderly patients who were ineligible for ASCT, resulted in PET negative CR rate of 91%, PFS rate of 76% at median follow up of 35 months but at the cost of increased myelosuppression, especially thrombocytopenia [21].
Another less intense chemotherapy option for elderly patients was modified Hyper-CVAD (without methotrexate or cytarabine). In 2006, Kahl et al. reported the results of a phase II trial, treating patients with 4 to 6 cycles of modified Hyper-CVAD, followed by 2 years of rituximab maintenance [22]. This study showed overall response rate of 77%, CR rate of 64%, median PFS of 37 months and OS not reached. Two years of rituximab maintenance prolonged PFS rate.
Intensive therapies
In 1998, Khouri et al. demonstrated that 4 cycles of Hyper CVAD/MTX-Ara-C regimen followed by stem cell transplant was superior to standard CHOP-like therapy in previously untreated patients (OS of 92% vs 56% and PFS of 72% vs 28%) [23]. In 2005, Romaguera et al. extended this inductive regimen to 6 to 8 cycles and added rituximab in a phase II trial to young and newly diagnosed aggressive MCL patients, and showed an improved response rate, PFS, and OS [24]. These studies suggested the promising role of intensified induction chemo-immunotherapy followed by transplantation in treating young patients with newly diagnosed aggressive MCL. In 2008, Geiseler et al. demonstrated that combination of intensified chemotherapy with immunotherapy in younger patients would not only yield better response rate but also resulted in an improved long term outcome in a Nordic lymphoma group (NLG) protocol (MCL-2) [25]. Wisconsin Oncology Network trial combining bortezomib with Hyper-CVAD induction regimen without transplant and demonstrated CR of 95% [26, 27]. However, intensified chemotherapy regimen accompanied a significant treatment related toxicity profile including grade 4 neutropenia, thrombocytopenia, severe mucositis, and serious infections.
Efficacy of autologous transplantation was shown to improve with Ara-C containing myeloablative therapy and rituximab maintenance therapy. MCL Younger trial of European mantle cell lymphoma network (MCL net) compared 6 courses of R-CHOP followed by myeloablative radiochemotherapy and ASCT to alternating course of three cycles of CHOP and three cycles of DHAP (dexamethasone, cytarabine and cisplatin or platinum) plus rituximab followed by high dose Ara-C containing myeloablative regimen and ASCT. CR rate and time to treatment failure was significantly higher in arm containing high dose Ara-C followed by ASCT, 36% vs. 25% and 88 months vs. 46 months respectively [28]. In a 2012 update, MCL net showed improved survival with longer median follow up of 27 months in treatment arm that included Ara-C followed by ASCT (not reached vs. 82 months) [29].
Efficacy of rituximab as maintenance therapy after ASCT was investigated by Le Gouill et al. (LyMa trial) [30]. In this trial newly diagnosed patients received R-DHAP based induction, followed by conditioning regimen with BEAM (Carmustine, etoposide, cytarabine and melphalan) and ASCT. Patients who responded went on to receive 3 years of maintenance rituximab. In this trial, patients in rituximab maintenance had 60% reduction of the progression risk and 50% reduction in the death risk. This was the first trial that showed maintenance rituximab after ASCT prolongs EFS (event free survival), PFS and OS.
The above-mentioned trials have established the role of autologous stem cell transplant in patients who respond to first line induction chemo-immunotherapy. Role of allogeneic stem cell transplant in relapsed patients who fail autologous stem cell transplant as been studied as well. Using non-myeloablative conditioning for relapsed MCL patients, investigators at MD Anderson showed a 5 year overall survival and progression free survival of 49% and 37%, respectively [31]. Another retrospective study, using reduced intensity conditioning for relapsed MCL patients, showed median overall survival of 62 months with 32% treatment related mortality in 3 years [32].
Novel agents (Table 2)
Bortezomib
Bortezomib (Valcade), a proteasome inhibitor, has shown efficacy as monotherapy, in relapsed MCL patients with response rate and CR rate reported as 33% and 8% respectively [33]. When combined with R-CHOP in frontline setting, bortezomib has shown ORR of 81% to 91%, with CR of 64% and median PFS of 23 months [34]. Also in first line setting, combination of Bortezomib with rituximab, cyclophosphamide, adriamycin and prednisone (VR-CAP) had resulted in better median PFS in comparing with RCHOP, 24.7 months vs. 14.4 months [35]. Bortezomib maintenance therapy after Bortezomib-RCHOP induction showed that it not only was well tolerated but also improved CR rate to 83% and median PFS to 29.5 months [36].
Combination of bortezomib with intensive therapy has been shown to be safe [37]. Addition of bortezomib to modified R-HyperCVAD or VcR-CVAD (no vincristine on day 11 and no alternating doses of methotrexate/cytarabine) made long-term remission possible. Combined maintenance therapy with rituximab and bortezomib in a post-transplant setting was also shown to result in 2 years DFS and OS of 93.8% and 92.3% respectively [38].
Bruton’s tyrosine kinase (BTK) inhibitors
Early studies in relapsed setting showed that Ibrutinib, a Bruton’s tyrosine kinase inhibitor resulted in response rate and CR of 77% and 33% respectively [39]. In a pooled analysis of Ibrutinib treatment in relapsed and refractory MCL, CR was achieved in 26.5% patients, median PFS was 13 months, PFS with one prior line of chemotherapy was 33.6 months and median OS was 26.7 months [40]. It has been combined with rituximab, bendamustine and RCHOP in treating naïve and refractory cases [41,42,43]. These combinations have resulted in higher responses. When combined with rituximab in relapsed setting, it showed objective response rate and CR of 88% and 44% respectively. Important adverse events noted were fatigue, myalgia, grade 3 nasal bleeding, 12% of patients had grade 3 atrial fibrillation and one patient had grade 3 leukocytosis. In combination with bendamustine and rituximab in phase I/Ib study, 94% patients showed objective response and 76% showed CR. Main adverse events were due to cytopenias and rashes (25%). Early phase study of Ibrutinib in combination with R-CHOP, in treatment naïve setting, showed overall response rate of 94% with grade 4 toxicity of neutropenia.
The emergence of resistance to Ibrutinib has led to development of more specific second generation BTK inhibitors including acalabrutinib (ACP-196) and ONO/GS-4059. A recently published phase II study of acalabrutinib in relapsed/refractory showed 81% overall response rate and 40% CR rate. This new BTK inhibitor is less toxic in phase I trial and better tolerated, it does not cause increased atrial fibrillation and bleeding events were noted in Ibrutinib trials [44, 45].
Recently, combination of Ibrutinib and venetoclax (direct inhibitor of BCL2) in patients with refractory disease showed overall response rate of 71% at 16 weeks as assessed by PET scan. Absence of minimal residual disease was documented in 67% patients according to flow cytometry and 38% according to allele-specific oligonucleotide polymerase chain reaction (ASO-PCR). Majority of side effects were related to diarrhea, nausea or fatigue [46].
Epigenetic agents
Epigenetic dysregulation is a main cause of lymphoma formation and progression. Targeting epigenetic modification mechanisms is a novel approach in treating MCL. Cladribine, a hypomethylating agent that indirectly downregulate DNA methylation, and Vorinostat, a histone deacetylase inhibitor, have been used as one of the combination regimens in treating MCL. A phase I/II trial, combining Vorinostat, Cladribine, and Rituxan, reached a ORR of 97% and CR of 80%, with a 2 year PFS of 70.7% and OS of 86.9% [47]. In other studies combining Velcade, Cladribine, and Rituxan, the ORR and CR for both new and relapsed/refractory MCL were 85% and 77% respectively [48, 49].
Immunomodulatory Agent
Lenalidomide is an immunomodulatory agent with anti-tumor activities. In various early phase trials, lenalidomide monotherapy in relapsed/refractory setting, could result in an OS of 28–57% and CR of 7.5–36% [50,51,52]. These trials show median PFS from 4 to 5.7 months. When lenalidomide and bortezomib combination was used in relapsed patients for induction and maintenance therapy, outcomes were not satisfactory with median PFS and OS of 7 months and 26 months respectively, and ORR and CR of 39.6% and 15.1% respectively [53]. These disappointing results were thought to be due to lenalidomide toxicity related dose reduction and inadequate dosing.
When lenalidomide was combined with rituximab in relapsed setting, PFS and OS improvements were noted, with a median of 11.1 months and 24.3 months respectively [54]. Exceptionally high response rate was achieved when lenalidomide was combined with rituximab in induction and maintenance therapy (ORR 92% and CR rate of 64%) in the first line setting. This combination resulted in grade 3/4 neutropenia in 50% patients, 29% of them experiencing grade 3/4 rashes. Lenalidomide therapy also predisposes patients to secondary cancers. When lenalidomide was combined with rituximab, investigators found higher incidence of non-invasive skin cancers though cases of Merkel cell carcinoma and pancreatic cancer were also reported [55]. Although there is clinical benefit of using lenalidomide and rituximab in first line setting, duration of maintenance therapy is not well defined. A 5 years outcome of this combination was presented at 2017 ASH annual meeting, with median follow up of 58 months, 61% evaluable patients had remained in remission. Median PFS was not reached, but estimated 3 and 4 years OS rates were 80.3% and 69.7% respectively [56]. This data highlights that combination therapy of lenalidomide with rituximab in first line setting can result in long-term remission in MCL patients.
Nordic lymphoma Group looked into efficacy of lenalidomide combining with bendamustine and rituximab as a first line treatment in elderly patients (median age 72 years). This study included 6 cycles of induction therapy followed by 52 weeks maintenance therapy with lenalidomide. After completion of induction therapy, 64% patients had CR and 36% were minimum residual disease (MRD) negative. Median follow up for this study was 31 months, with median PFS and OS were 42 months and 53 months respectively [57]. Major limitation of this combination was high incidence of serious infections, which makes this treatment difficult for elder patients.
Mammalian target of rapamycin (mTOR) inhibitors
Temsirolimus is a specific inhibitor of mTOR kinase, it has been evaluated in refractory/relapsed MCL setting. In a phase III RCT, temsirolimus given 175 mg per week for 3 weeks, followed by weekly dose of 75 mg achieved an objective response rate of 22% and PFS of 4.8 months [58]. Different doses regimen of temsirolimus has been used as monotherapy in relapsed/refractory setting with ORR 38–41%, CR 3–3.7% and median OS 12 months [59, 60]. In a phase I study, temsirolimus also showed to be safe and efficacious when combined with bendamustine and rituximab in refractory setting [61]. When another mTOR inhibitor, everolimus was used in refractory setting, the results were not too encouraging as it only showed modest activity in refractory setting. In that study, the ORR was 8.6% (all partial responses), median PFS and median OS of 4.4 months and 16.9 months respectively [62].
Role of chimeric antigen receptor-engineered T-cells (CART) and Bi-specific T-cells engager (BiTE) therapy
CART offers innovative intervention for MCL patients. CART therapy improves response duration of those refractory/relapsed MCL patients as well [63, 64]. Investigators are modifying various parts of CART therapy to improve the feasibility and efficiency of MCL treatment. One study modified preparatory regimens for CART therapy, another study further optimized CART cells (JCAR-17) [65, 66]. One study even linked chimeric antigen receptor-engineered exosome (CAR-Exo) with membrane fused CD-19 scFV (single chain region of antibody variable region) in order to deliver drug-containing exosome into the lymphoma cells [67]. A multicenter phase 2 study is currently underway to evaluate the role of anti CD-19 CART (axicabtagene ciloleucel) therapy (KTE-C19) in patients with relapsed/refractory MCL (ZUMA-2) [68].
BiTE therapy transiently engages CD3+ T cells with B cells and results in T cell mediated B cell destruction. In a phase I trial for heavily pretreated Non-Hodgkin Lymphoma patients (included 24 MCL patients), Blinatumomab (bispecific CD19/CD3 antibody) showed single agent activity in MCL patients with ORR 71% [69]. A long-term follow-up analysis of 38 patients with relapsed refractory NHL (14 MCL patients), who achieved an objective response to Blinatumomab, showed median overall survival of 1560 days and median progression free survival of 204 days [70]. A recent phase I study presented at 2018 ASH meeting showed clinical efficacy of Mosunetuzumab (bispecific CD20/CD3 antibody) in relapsed refractory NHL (3 MCL patients). Interestingly, responses were also observed in patients thought to be CD20 refractory and who relapsed following CD19 directed CAR-T therapy [71].
Challenges in MCL treatment include pathophysiological variety, high incidence of disease progression and recurrence, shorter disease free interval, advanced patient age and comorbidity. Besides above-mentioned therapies, NK-kB signal pathway blockage studies showed its potential therapeutic significance [72]. The ultimate goal of MCL treatment is to achieve long-term remission without excess toxicities. Developing personalized precise therapeutic strategy is the direction to go.
References
Berard CW, Dorfman RF. Histopathology of malignant lymphomas. Clin Haematol. 1974;3:39–76.
Toksdorf G, Stein H, Lennert K. Morphological and immunological definition of a malignant lymphoma derived from germinal centre cells with cleaved nuclei (centrocytes). Br J Cancer. 1980;41(2):168–82.
Lennert K, Stein H, Kaiserling E. Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer Suppl. 1975;2:29–43.
Weisenburger DD, Kim H, Rappaport H. Mantle-Zone Lymphoma: a follicular variant of intermediate lymphocytic lymphoma. Cancer. 1982;49(7):1429–38.
Lardelli P, Bookman MA, Sundeen J, Longo DL, Jaffe ES. Lymphocytic lymphoma of intermediate differentiation. Morphologica and immunophenotypic spectrum and clinical correlations. Am J Surg Pathol. 1990;14(8):752–63.
Duggan DJ, Weisenburger DD, Ye YL, Bast MA, Pierson JL, Linder J, Armitage JO. Mantle zone lymphoma. A clinicopathological study of 22 cases. Cancer. 1990;66(3):522–9.
Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathological study of 80 cases. Blood. 1997;89(6):2067–78.
Banks PM, Chan J, Cleary ML, Delsol G, De Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES, et al. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic and molecular data. Am J Surg Pathol. 1992;16(7):637–40.
Cheah CY, Seymour JF, Wang ML. Mantle cell lymphoma. J Clin Oncol. 2016;34(11):1256–69.
Zhou Y, Wang H, Fang W, Romaguer JE, Zhang Y, Delasalle KB, Yi Q, Du XL, Wang M. Incidence trends of mantle cell lymphoma in the United States between 1992–2004. Cancer. 2008;113(4):791–8.
Jiang W, Kahn SM, Zhou P, Zhan YJ, Cacace AM, Infante AS, Doi S, Santella RM, Weinstein IB. Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene. 1993;8(12):3447–57.
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, Van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, et al. A new prognostic index (MIPI) for patients with advanced-stage mangle cell lymphoma. Blood. 2008;111(2):558–65.
Geisler CH, Kolstad A, Laurell A, Raty R, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Nilsson-Ehle H, Kuittinen O, Lauritzsen GF, Ralfkiaer E, Ehinger M, et al. The mantel cell lymphoma international prognostic index (MIPI) is superior to the internal prognostic index (IPI) in predicting survival following intensive first line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010;115(8):1530–3.
Balsas P, Palomero J, Eguileor Á, Rodríguez ML, Vegliante MC, Planas-Rigol E, Sureda-Gómez M, Cid MC, Campo E, Amador V. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood. 2017;130(4):501–13.
Meusers P, Engelhard M, Bartels H, Binder T, Fulle HH, Gorg K, Gunzer U, Havemann K, Kayser W, Kong E, et al. Multicentre randomized therapeutic trial for advanced centrocytic lymphoma: anthracycline does not improve the prognosis. Hematol Oncol. 1980;7(5):365–80.
Teodorovic I, Pittaluga S, Kluin-Nelemans JC, Meerwaldt JH, Hagenbeek A, Globbeke MV, Somers R, Bijnens L, Noordijk EM, Peeters DW. Efficacy of four different regimen in 64 mantle cell lymphoma cases: clinicopathologic comparsion with 498 other Non-Hodgkin’s lymphoma subtypes. J Clin Oncol. 1995;13(11):2819–26.
Howard OM, Gribben JG, Neuberg DS, Grassbard M, Poor C, Janicek MJ, Shipp MA. Rituximab and CHOP induction therapy for newly diagnosed mangle cell lymphoma: molecular complete responses are not predictive of progression free survival. J Clin Oncol. 2002;20(5):1288–94.
Lenz G, Dreyling M, Hoster E, Wormann B, Duhresn U, Metzner B, Eimermacher H, Neubauer A, Wandt H, Steinhauer H, Martin S, Heidemann E, Aldaud A, Parwaresch R, Hasford J, Unterhalt M, Hiddemann W. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine and prednisone significantly improves response and time to treatment failure but not long term outcome in patient with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984–92.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kauser U, Weidmann E, Durk H, Ballo H, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first line treatment for patient with indolent and mantle cell lymphomas: an open-label, multicenter, randomized, phase 3 non inferiority trial. Lancet. 2013;381:1203–10.
Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM. Randomized trial of bendamustine-rituximab or RCHOP/RVP in first line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–52.
Visco C, Chiappella A, Nassi L, Patti C, Ferrero S, Barbero D, Evangelista A, Spina M, Tani M, Rocco AD, Pinotti G, Fabbri A, Zambello R, Finotto S, Gotti M, Carella AM, Salvi F, Pileri SA, Ladetto M, Ciccone G, Gaidano G, Ruggeri M, Martelli M. Rituximab, bendamustin, and low dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: a multicenter, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017;4(1):e15–23.
Kahl BS, Longo WL, Eickhoff JC, Zehnder J, Jones C, Blank J, McFarland T, Bottner W, Rezazedeh H, Werndli J, Bailey HH. Maintenance rituximab following induction chemoimmunotherapy may prolong progression free survival in mantle cell lymphoma: a pilot study from the Wisconsin Oncoloty Network. Ann Oncol. 2006;17(9):1418–23.
Kahouri IF, Romaguera J, Kantarjian H, Palmer L, Pugh WC, Korbling M, Hagemeister F, Sammuels B, Rodriguez A, Giralt S, Yaunes A, Przepiorka D, Claxton D, Cabanillas F, Champlin R. Hyper-CVAD and High-dose Methotrexate/Cytrabine followed by stem cell transplantation: an active regimen for aggressive mantle cell lymphoma. J Clin Oncol. 1998;16(12):3803–9.
Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Sarris AH, Dang NH, Wang M, Beasley V, Medeiros LJ, Katz RL, Gabneja H, Samuels BI, Smith TL, Cabanillas FF. High rate of durable remissions after treatment of newly diagnosed aggressive mangle cell lymphoma with rituximab plus Hyper-CVAD alternating with rituximab plus high dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–23.
Geiseler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AB, Kuittinen O, Lauritzsen GF, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo–purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–93.
Romaguera JE, Fayad LE, McLaughlin P, Pro B, Rodriguez A, Wang M, Weaver P, Hartig K, Kwak LW, Feldman T, Smith J, Fort P, Goldberg S, Pecora A, Goy A. Phase I trial of bortezomib in combination with rituximab-hyperCVAD alternating with rituximab, methotrexate and cytarabine for untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;151(1):47–53.
Chang JE, Li H, Smith MR, Gascoyne RD, Paietta EM, Yang DT, Advani RH, Horning SJ, Kahl BS. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an Eastern Cooperative Oncology Group study (E1405). Blood. 2014;123(11):1665–73.
Hermine O, Hoster E, Walewski J, Ribrag V, Brousse N, Thieblemont C, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) is superior to 6 courses CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: results of the MCL younger trial of the European mantle cell lymphoma network (MCL net). Blood 2010;116(21):110.
Hermine O, Hoster E, Walewski J, Ribrag V, Brousse N, Thieblemont C, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) is superior to 6 courses CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: final analysis of the MCL younger trial of the European mantle cell lymphoma network (MCL net). Blood 2012;120(21):151.
Le Gouille S, Thieblemont C, Oberic L, Moreau A, Bouabdullah K, Dartigeas C, Damaj G, Gastinne T, Ribrag V, Feugier P, Cassanovas O, Zerazhi H, Haioun C, Maisonneuve H, et al. Rituximab after autologous stem cell transplantation in mantle cell lymphoma. N Engl J Med. 2017;377(13):1250–60.
Khouri IF, Wang X, Turturro F, Jabbour E, Carballo-Zarate AA, Korbling M, et al. Long-term outcomes of non-myeloablative allogeneic stem cell transplantation (alloSCT) in patients with relapsed mantle cell lymphoma (MCL). Abstract J Clin Oncol. 2016. https://doi.org/10.1200/JCO.2016.34.15_suppl.7551.
Kobrinski DA, Smith SE, Al-Mansour Z, Tsai SB, Martin B, Stiff PJ. Allogeneic hematopoietic stem cell transplantation for mantle cell lymphoma in a heavily pretreated patient population. Abstract J Clin Oncol. 2017. https://doi.org/10.1200/JCO.2017.35.15_suppl.7558.
Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74.
Ruan J, Martin P, Furman RR, Lee SM, Cheung K, Vose JM, LaCasce A, Morrison J, Elstrom R, Ely S, Chadburn A, Cesarman E, Coleman M, Leonard JP. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29(6):690–7.
Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E, Alexeeva J, Pereira J, Drach J, Mayer J, Hong X, Okamoto R, Pei L, Rooney B, van de Velde H, Cavalli F, LYM-3002 Investigators. Bortezomib based therapy for newly diagnosed mangle cell lymphoma. N Engl J Med. 2015;372(10):944–53.
Till BG, Li H, Bernstein SH, Fisher RI, Burack WR, Rimsza LM, Floyd JD, DsSilva MA, Moore DF Jr, Pozdnyakova O, Smith SM, LeBlanc M, Friedberg JW. Phase II trial of R-CHOP plus bortezomib induction therapy followed by bortezomib maintenance for newly diagnosed mantle cell lymphoma: SWOG S0601. Br J Haematol. 2016;172(2):208–18.
Chang JE, Carmichael LL, Kim KM, Peterson CP, Yang DT, Traynor AM, Werndli JE, Huie MS, McFarland TA, Volk M, Blank J, Callander NS, Longo WL, Kahl BS. VcR-CVAD induction chemotherapy followed by maintenance rituximab produces durable remissions in mantle cell lymphoma: a Wisconsin Oncology Network study. Clin Lymphoma Myeloma Leuk. 2018;18(1):e61–7.
Chen RW, Palmer JM, Popplewell L, Alluin J, Chomchan P, Nademanee AP, et al. Phase II trial of bortezomib plus rituximab as maintenance therapy post ASCT for patients with mantle cell lymphoma. J Hematol Oncol. 2018;11(1):87.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(1):88–94.
Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl BS, Hernandex-Rivas JA, et al. Median 3.5-year follow-up of ibrutinib treatment in patients with relapsed/refractory mantle cell lymphoma: a pooled analysis. Blood 2017;130(Suppl 1):151.
Wang MK, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, Fayad L, Samaniego F, Turturro F, Oki Y, Chen W, Badillo M, Nomie K, DeLa Rosa M, Zhao D, Lam L, Addison A, Zhang H, Young KH, Li S, Santos D, Medeiros LJ, Champlin R, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: results from a single-center, open label phase 2 trial. Lancet Oncol. 2016;17(1):48–56.
Maddocks K, Christian B, Jaglowski S, Flynn J, Jones JA, Porcu P, Wei L, Jenkin C, Lozanski G, Bryd JC, Blum K. A phase 1/1b study of rituximab, bendamustine, and ibrutinib in patients with untreated and relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;125(2):242–8.
Younes A, Thieblemont C, Morschhauser F, Flinn I, Friedberg JW, Amorim S, Hivert B, Westin J, Vermeulen J, Banyopadhyay N, Vires RD, Balasubramanian S, et al. Combination of ibrutinib with rituximab, cyclo- phosphamide, doxorubicin, vincristine, and pre- dnisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non- randomised, phase 1b study. Lancet Oncol. 2014;15(9):1010–26.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004) a single-arm, multicenter, phase 2 trial. Lancet. 2018;391(10121):659–67.
Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed refractory B-cell malignancies. Blood. 2016;127(4):411–9.
Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, et al. Ibrutinib plus Venetoclax for the treatment of mantle cell lymphoma. N Engl J Med. 2018;378(13):1211–23.
Spurgeon SE, Sharma K, Claxton DF, Ehmann CW, Gallagher C, Shimko S, Stewart A, Parekh S, Leshchenko VV, Chen Y, Mori M, Pu JJ, Epner EM. Final results of a phase 1-2 STUDY OF VORINOSTAT (SAHA), cladribine, and rituximab (SCR) relapsed B-cell non-Hodgkin’s lymphoma and previously untreated mantle cell lymphoma. Blood. 2014;124:1714.
Pu JJ, Ehmann C, Liao J, Capper C, Levy M, Claxton DF, Rybka WB, Hohl RJ, Epner EM. The results of a phase I study using Velcade, Cladribine and Rituximab (VCR) in treating mantle cell lymphoma. Blood. 2016;128(22):1792.
Puvvada SD, Guillen-Rodriguez J, Kumar A, Inclán L, Heard K, Rivera XI, Anwer F, Schatz JH, Mahadevan D, Persky DO. Phase 2 open-label study of bortezomib, cladribine, and rituximab in advanced, newly diagnosed, and relapsed/refractory mantle-cell and indolent lymphomas. Clin Lymphoma Myeloma Leuk. 2018;18(1):58–64.
Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, Zhang L, Cicero S, Fu T, Witzig TE. Single-agent lenalidomide in patients with mantle cell lymphoma who relpased or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–95.
Habermann TM, Lossos IS, Justic G, Vose JM, Wiernik PH, McBride K, Wride K, Ervin-Hanes A, Takeshita K, Pietronigro D, Zeldis JB, Tuscano JM. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–9.
Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, Bouabdallah R, Haioun C, Tilly H, Guo P, Pietronigro D, Ervin-Haynes AL. An international phase II trial of single agent lenalidomide for relapsed or refractory aggressive B cell non Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–7.
Morrison VA, Jung SH, Johnson J, LaCasce A, Blum KA, Bartlett NL, Pitcher BN, Cheson BD. Therapy with bortezomib plus lenalidmide for relapsed/refractory mantel cell lymphoma: final results of a phase II trial (CALGB 50501). Leuk Lymphoma. 2015;56(4):958–64.
Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, Samaniego F, McLaughlin P, Fanale M, Younes A, Cabanillas F, Fowler N, Newberry JL, Sun L, Young KH, Champlin R, Kwak L, Feng L, Badillo M, et al. Lenalidomide in combination wiht rituximab for patients with relpaesd or refractory mantle cell lymphoma: a phase ½ clinical trial. Lancet Oncol. 2012;13(7):716–23.
Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, Christos P, Rodriguez A, Svoboda J, Lewis J, Katz O, Coleman M, Leonard JP. Lenalidomide plus rituximab as initial treatment for Mantle Cell Lymphoma. N Engl J Med. 2015;373(19):1835–44.
Ruan J, Martin P, Christos PJ, Cerchietti L, Shah B, Schuster SJ, Tam W, Rodriguez A, Hyman D, Calvo Vidal MN, Gonzalez LR, Smith SM, Svoboda J, Furman RR, Coleman M, Leonard P. Initial treatment with lenalidomide plus rituximab for mantle cell lymphoma: 5-year follow-up and correlative analysis from a multi-center phase II study. Blood. 2017;130(Suppl 1):154.
Albertsson-Lindblad A, Kolstad A, Laurell A, Raty R, Gronbeak K, Sundberg J, Pedersen LB, Ralfkiaer E, Karjalainen-Lindsberg ML, Sundstrom C, Ehinger M, Geisler C, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood. 2016;128(14):1814–20.
Hess G, Herbrecht R, Romaguera J, Verhoef G, Crump M, Gisselbrecht C, Laurell A, Offner F, Strahs A, Berenblit A, Hanushevsky O, Clancy J, Hewes B, Moore L, Coiffier B. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27(23):3822–9.
Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, Ansell SM, Luyun R, Flynn PJ, Morton RF, Dakhil SR, Gross H, Kaufmann SH. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347–56.
Ansell SM, Inwards DJ, Rowland KM Jr, Flynn PJ, Morton RF, Moore DF Jr, Kaufmann SH, Ghobrial I, Kurtin PJ, Maurer M, Allmer C, Witzig TE. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113(3):508–14.
Hess G, Keller U, Scholz CW, Witzens-Harig M, Atta J, Buske C, Kirschey S, Ruckes C, Medler C, van Oordt C, Klapper W, Theobald M, Dreyling M. Safety and efficacy of Temsirolimus in combination with Bendamustine and Rituximab in relapsed mantle cell and follicular lymphoma. Leukemia. 2015;29(8):1695–701.
Wang M, Popplewell LL, Collins RH Jr, Winter JN, Goy A, Kaminiski MS, Bartlett NL, Johnston PB, Lister J, Fanning SR, Tuscano JM, Beck JT, Kaya H, Robeva A, Fan J, Klimovsky J, Cheung W, Cherfi A, O’Connor OA. Everolimus for patients with mantle cell lymphoma refractory to or intolerant of bortezomib: multicentre, single-arm, phase 2 study. Br J Haematol. 2014;165(4):510–8.
Chen W, Du X, Luo C, Zhang Q, Wang M. Anti-CD19 chimeric antigen receptor T cells improve responses to chemotherapy-refractory mantle cell lymphoma: a case report. Blood. 2016;128(22):5393.
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–13.
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116.
Abramson JS, Gordon LI, Palomba M, Lunning MA, Arnason JE, Forero-Torres A, Wang M et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018; 36. (suppl; abstr 7505).
Bao H, Bi C, Li W, Zhang X, Zhang H, Meng B, et al. Chimeric antigen receptor-engineered exosome as a drug delivery system in mantle cell lymphoma. Blood. 2017;130:5561.
A phase 2 multicenter study evaluating subjects with relapsed/refractory mantle cell lymphoma (ZUMA-2);2015. https://clinicaltrials.gov/ct2/show/study/NCT02601313?show_locs=Y#locn. (Identification No. NCT0260313). Accessed 5 Jan 2019.
Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34(10):1104–11.
Dufner V, Goebeler ME, Sayheli C, Bargou RC. Bispecific T-cell engager antibody construct blinatumomab shows durable response in a long-term follow-up analysis of 38 NHL patients treated in a phase I trial. Blood 2015;126(23):3974.
Budde LE, Sehn LH, Assouline S, Flinn IW, Isufi I, Yoon SS, et al. Mosunetuzumab, a full-length bispecific CD20/CD3 antibody, displays clinical activity in relapsed/refractory B-cell non-Hodgkin lymphoma (NHL): interim safety and efficacy results from a phase 1 study. Blood 2018;132(Suppl 1):399.
Balaji S, Ahmed M, Lorence E, Yan F, Nomie K, Wang M. NF-κB signaling and its relevance to the treatment of mantle cell lymphoma. J Hematol Oncol. 2018;11(1):83.
Authors’ contributions
Contribution: JJP designed this study. JJP, AL, JZ, and EME wrote this manuscript. All authors read and approved the final manuscript.
Acknowledgements
Would like Ms. Amiara Evelyn Phillips, a dedicated medical student, for proof-reading this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material interests
Not applicable.
Consent for publication
Not applicable.
Ethical approval and consent to participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study is supported by: NIDA/FDA research grant to JJP (P50 DA036107), AA & MDSIF research grant to JJP (146818), American Cancer Society research grant to JJP (124171-IRG-13-043-02), Paige’s Cancer Researcher Fund to JJP (Pu33860), and a SUNY Upstate Medical University research grant to JJP.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ladha, A., Zhao, J., Epner, E.M. et al. Mantle cell lymphoma and its management: where are we now?. Exp Hematol Oncol 8, 2 (2019). https://doi.org/10.1186/s40164-019-0126-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-019-0126-0