Abstract
Background
This study investigated the association between body mass index (BMI) and myopia in the United States.
Methods
This cross-sectional study included 8,000 participants from the 1999 to 2008 National Health and Nutrition Examination Survey (NHANES). BMI was classified into four groups: < 18.5, 18.5 – 24.9, 25–29.9, and > 29.9. Three diagnostic thresholds were used for myopia A\B\C: spherical equivalent ≤ −0.5\−0.75\−1 diopters in the right eye. Multivariate logistic regression analysis and smooth curve fitting were performed to evaluate the association between BMI and myopia.
Results
The incidence of myopia was 39.4%. BMI was correlated with myopia, with each 1 kg/m2 increase in BMI associated with a 1% increase in the risk of myopia (OR, 1.01; 95% CI 1.01 1.02; p < 0.05). In myopia B, after adjusting for confounding factors, compared with the reference group (BMI 18.5–24.9), participants with a BMI of 25–29.9 and greater than 29.9 had a 14% and 25% increased risk of myopia, respectively (OR 1.14; 95% CI 1.01 1.29; p = 0.037, OR 1.25; 95% CI 1.08 1.44; p = 0.003), which was similar to the results for myopic A (OR, 1.15; 95% CI 1.02 1.3; p = 0.027, OR 1.19; 95% CI 1.03 1.37; p = 0.018) and myopia C (OR 1.15; 95% CI 1.01 1.31; p = 0.035, OR 1.18; 95% CI 1.01 1.37; p = 0.032). Moreover, there was a linear relationship between myopia and BMI (p for nonlinearity = 0.767).
Conclusions
Myopia using all three diagnostic thresholds was positively associated with higher BMI. This suggests a potential association between myopia and higher BMI in the American population, warranting further investigations.
Similar content being viewed by others
Background
Myopia is described as light rays entering the eye parallel to the optic axis and coming into focus in front of the retina when the ocular accommodation is relaxed [1]. The prevalence of myopia in the United States increased from 25% in 1971–1972 to 41.6% in 1999–2004 [2]. Two reports from China show that the prevalence of myopia among adolescents is 63.1% and 84.8%, and the incidence of high myopia reaches 9.4% and 19.3%, respectively [3, 4]. According to the forecast, the prevalence of myopia will reach 49.8% in 2050, and the prevalence of high myopia will reach 9.8% [5]. Not only do myopic patients suffer from decreased vision, but they are also at significant risk of developing complications, such as myopic macular degeneration, retinal detachment, open-angle glaucoma, and cataracts, which can severely reduce the quality of life [6, 7]. Globally, myopia has become a major public health concern.
Both genetic and environmental factors have an impact on the development of myopia [8,9,10]. Years of education [11] and hours of outdoor activity [12,13,14] have been shown to have a strong causal association with myopia and inflammation [15, 16] also correlates with myopia. Then, late sleep [17] and high glycaemic load carbohydrate diets [18] may be potentially associated with myopia. According to the carbohydrate–insulin model of obesity, a high glycaemic load carbohydrate diet leads to an increase in adipose tissue and a greater tendency to become obese [19,20,21]. Obese individuals tend to have a higher body mass index (BMI) compared to non-obese individuals. However, the results of existing studies on the association between BMI and myopia are inconsistent and mostly focused on Asian populations. Some studies have associated high BMI with myopia [22, 23], but others have linked it to low BMI [24] or have not found an association between both [25, 26].
Therefore, participants in the National Health and Nutrition Examination Survey (NHANES) database from 1999 to 2008 were selected to explore the association between BMI and myopia in the United States population.
Methods
Study design and participants
The NHANES is a research program designed to assess the health and nutritional status of adults and children in the United States. This survey is conducted annually with a nationally representative sample of approximately 5,000 people. These individuals are located in counties across the country, 15 of which are visited annually. This study was managed by the National Center for Health Statistics of the Centers for Disease Control and Prevention. The study protocol met the requirements of the Declaration of Helsinki and was approved by the institutional review board of the National Center for Health Statistics. Informed consent was obtained from all participants. A more detailed description of the study protocol is provided elsewhere [27].
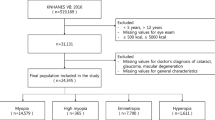
This study was a cross-sectional study using data from the NHANES database from 1999 to 2008. To calculate metabolic equivalents, participants who responded "don't know" for daily activity time (n = 12) and those with daily non-physical activity time less than 5 h (n = 44, because the veracity of the responses was questionable) were excluded. Then, all participants in the survey years 1999–2008 were included (n = 56,505). Duplicate data (n = 6,262) and missing data (n = 29,049) for any variables, and individuals with a history of refractive surgery or do not know (n = 315), cataract surgery or do not know (n = 740), hyperopia (defined as spherical equivalent ≥ 0.5 diopters (D), n = 4,198), unaware of their diabetic status (n = 11), above the age of 25 (n = 7,930) were excluded. Eventually, 8,000 participants were found suitable for inclusion in our study. The inclusion and exclusion processes are illustrated in Fig. 1.
Variables and measurement
We included only the right eye as the evaluation eye, because refractive errors in the right and left eyes have been shown to be highly correlated [13]. Technicians who initially received 8 weeks of training and then underwent updates and remedial training as needed performed the visual examination. The objective refraction (sphere and cylinder) results were obtained by taking the average of three measurements using a Nidek Auto Refractor Model ARK-760 instrument. The spherical equivalent was calculated as the sphere plus half the cylinder. Because the NHANES database does not account for cycloplegia in refractive measurements, to ensure reliable results, myopia was diagnosed using three thresholds: myopia A was defined as spherical equivalent ≤ -0.5 D, myopia B was defined as spherical equivalent ≤ −0.75 D and myopia C was defined as spherical equivalent ≤ -1 D [1, 8, 28, 29].
The professional examiners at the mobile examination center measured the body of each examinee. Information on standing height and weight was collected electronically from the measuring devices to reduce the possibility of data input errors. The US Government Printing Office (https://wwwn.cdc.gov/nchs/nhanes/nhanes3/anthropometricvideos.aspx) offers a specific video technique. BMI was calculated as weight divided by the square of height (BMI = kg/m2) and classified into four groups: < 18.5, 18.5–24.9, 25–29.9, and > 29.9 kg/m2.
Age, sex (male, female), race (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races), and diabetes data were obtained through personal interviews, and the borderline group for diabetes data was considered to have no diabetes. Physical activity was obtained through the NHANES PAQ questionnaire, and metabolic equivalents were calculated with reference to previous papers [30]. High-density lipoprotein cholesterol (HDL-C) was measured using Hitachi 717 and Hitachi 912 (Roche Diagnostics, 9115 Hague Road, Indianapolis, IN 46250) from 1999 to 2006 and a Roche Modular P chemistry analyzer (Roche Diagnostics, 9115 Hague Road, Indianapolis, IN 46250) from 2007 to 2008. Triglycerides, total cholesterol, glucose, iron, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured using Beckman Synchron LX20 and Beckman UniCel® DxC800 Synchron. C-reactive protein (CRP) levels were quantified using latex-enhanced nephelometry.
Missing values were handled by simple deletion, where the proportions of missing values were more than 35% for the following variables: physical activity (44.2%), AST (35.7%), ALT (35.7%), Iron (35.5%), Triglycerides (35.5%), Total cholesterol (35.5%), Glucose (35.5%), cylinder (35.5%). More detailed frequencies and proportions of missing values are shown in Additional file 1: Table S1.
Statistical methods
For the baseline data of the subjects, the measurement data in accordance with the normal distribution were described by the mean ± standard deviation (SD), the measurement data that did not comply with the normal distribution were described by median (first quartile, third quartile), and the count data were described by n (%). A mediation analysis was performed for physical activity and adjusted for age, sex, race, ALT, AST, total cholesterol, triglycerides, HDL-C, glucose, iron, CRP, and diabetes mellitus. Multivariate logistic regression analysis was performed to evaluate the association between BMI and myopia. Model 1 was adjusted for age, sex, physical activity, race, and diabetes mellitus. Model 2 was adjusted for age, sex, physical activity, race, ALT, AST, total cholesterol, triglycerides, and HDL-C. Model 3 was adjusted for age, sex, physical activity, race, ALT, AST, total cholesterol, triglycerides, HDL-C, glucose, iron, CRP, and diabetes mellitus. The smooth curve fitting graph was established and adjusted according to the covariables contained in Model 3. Considering the effect of extreme values, only the middle 95% of BMI data are shown. All analyses were performed using the statistical software package R (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7 (http://www.clinicalscientists.cn/freestatistics/).
Results
Among 8000 participants with a mean age of 16.9 years, 3149 (39.4%) were diagnosed with myopia B (spherical equivalent ≤ −0.75 D). The baseline characteristics are presented in Table 1. The p values for the mediating effect of physical activity on myopia A/B/C were 0.2189/0.184/0.1856, respectively. The results of the multivariate logistic regression analysis of BMI and myopia are presented in Table 2. The trend was the same for all three myopia diagnostic criteria. BMI was correlated with myopia, with each 1 kg/m2 increase in BMI associated with a 1% increase in the risk of myopia (OR 1.01; 95% CI 1.01 1.02; p < 0.05). In myopia B (spherical equivalent ≤ −0.75 D), compared with the reference group (BMI 18.5–24.9), participants with a BMI of 25–29.9 and greater than 29.9 had a 14% and 25% increased risk of myopia, respectively (OR 1.14; 95% CI 1.01 1.29; p = 0.037, OR 1.25; 95% CI 1.08 1.44; p = 0.003), which was similar to the results for myopic A (spherical equivalent ≤ −0.5 D, OR 1.15; 95% CI 1.02 1.3; p = 0.027, OR 1.19; 95% CI 1.03 1.37; p = 0.018) and myopia C (spherical equivalent ≤ -1 D, OR 1.15; 95% CI 1.01 1.31; p = 0.035, OR 1.18; 95% CI 1.01 1.37; p = 0.032) in model 3.Smooth curve fitting showed a linear relationship between BMI and myopia B (p for nonlinearity = 0.767, Fig. 2).
Association between BMI and myopia B odds ratio. Myopia B was defined as spherical equivalent ≤ −0.75 D. Solid and dashed lines represent the predicted value and 95% confidence intervals. They were adjusted for age, sex, physical activity, race, ALT, AST, total cholesterol, triglycerides, HDL-C, glucose, iron, CRP, and diabetes mellitus. Only 95% of the data are shown. ALT alanine aminotransferase, AST aspartate aminotransferase, HDL-C high-density lipoprotein cholesterol, CRP C-reactive protein, BMI body mass index, D diopters
Discussion
In our cross-sectional study that included 8,000 individuals, there was a linear relationship between BMI and myopia (OR 1.01; 95% CI 1.01 1.02; p < 0.05). In a multifactorial analysis, participants with a BMI of 25–29.9 and greater than 29.9 had a 14% and 25% increased risk of myopia (spherical equivalent ≤ 0.75 D), respectively (OR 1.14; 95% CI 1.01 1.29; p = 0.037, OR 1.25; 95% CI 1.08 1.44; p = 0.003), and the trend remained unchanged when the diagnostic threshold was changed to −0.5D (OR 1.15; 95% CI 1.02 1.3; p = 0.027, OR 1.19; 95% CI 1.03 1.37; p = 0.018)or −1 D(OR 1.15; 95% CI 1.01 1.31; p = 0 0.035, OR 1.18; 95% CI 1.01 1.37; p = 0.032).The current focus of myopia and BMI research is on Asian children and adolescents. A cross-sectional study that included 1,359,153 Israeli adolescents aged 16–19 years who underwent medical examinations before mandatory military service showed that the BMI was a j-shaped pattern presented in the form of a bar chart for adolescent myopia and both higher and lower BMI were associated with a higher risk of myopia [22]. The results of our study are linear and presented in the form of smooth curve fitting. In the U.S. population, lower BMI did not appear to be associated with myopia, whereas higher BMI similarly had a higher OR. Similarly, a Korean cross-sectional study that investigated 24,269 participants aged 5–18 years in the KNHANES VII database from 2016 to 2018 found an association between obesity and high myopia in childhood and adolescence and an association between overweight and high myopia in girls [23]. According to the results provided in Table 2, there is not enough evidence to show that BMI and myopia are related, which is inconsistent with our results, and the different populations selected may be the source of the difference. A study using the 2003–2008 NHANES database of 6,855 participants aged 12–25 years showed that BMI was not associated with myopia (R2 = 0, P = 0.79) [26]. Three possible differences that may have contributed to the different results are as follows: first, the association between BMI and myopia was evaluated using multivariate analysis and adjustment was made with physical activity considered as one of the confounding factors; second, in addition to being analyzed as a continuous variable, BMI was also analyzed in groups. The OR of different groups reached different conclusions. Thirdly, the population from 1999 through 2008 was included, and differences in sample size (8,000) may have contributed to this difference. In a study of 19-year-old male consignors in Seoul, Korea, no association was found between myopia and BMI according to quartiles and by logistic regression [31]. In our study, a higher BMI appeared to favor a risk factor over a protective factor. Differences in population selection may account for the differences in results.
The association between BMI and myopia is affected by underlying lifestyle factors. One review found an association between decreased time spent outdoors and worsening myopia by studying children during COVID-19-induced lockdown [32]. Children who spend more time outdoors may have a lower BMI than their peers [33]. This may be a reasonable explanation for our linear results. After mediating role analysis, it was found that physical activity did not mediate the association between BMI and myopia. It may be one of the confounding factors affecting the results, so we included it in the multivariate analysis to adjust for it.
There are some limitations to this study. First, since the study design was cross-sectional, it was not possible to determine a causal relationship between BMI and myopia. In addition, measurement of refractive error in the absence of cycloplegia increases the prevalence of myopia. Therefore, three mainstream myopia diagnostic criteria were adopted to ensure the reliability of the results. Third, this study did not take into account the impact of air pollution and regional differences as confounding factors and further research is needed to explore this aspect. Fourth, due to the fact that BMI does not consider muscle mass, bone density, overall body composition, or racial and sex differences in lifespan, and also because children’s standards for obesity differ from those for adults, this study can only conclude an association between BMI and myopia, rather than a relationship between obesity and myopia. Fifth, NHANES only provided refractive data from 1999 to 2008, which may be outdated (> 15 years), and newer surveys are still needed to reflect current refractive errors, diet, exercise, or lifestyle.
Conclusions
In our study, myopia using all three diagnostic thresholds was positively associated with higher BMI. This suggests a potential association between myopia and higher BMI in the American population, warranting further investigations.
Availability of data and materials
Data supporting the findings of this study are available in the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm). The specific URL for variable acquisition is as follows: 1999–2000. Age, Sex, Race: Demographic Variables & Sample Weights https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&CycleBeginYear=1999. Weight, Standing height, BMI: Body Measures (BMX) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=1999–2000. ALT, AST, Total cholesterol, Glucose, Iron, Triglycerides: Standard Biochemistry Profile & Hormones (LAB18). https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=1999–2000. “Eye surgery for near sightedness?”, “Eye surgery for cataracts?”, sphere, cylinder: Vision (VIX) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=1999–2000. Diabetes: Diabetes (DIQ) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=1999–2000. CRP: C-Reactive Protein (CRP) (LAB11) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=1999–2000. HDL-C: Cholesterol—Total & HDL (Lab13) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=1999–2000. Physical Activity: Physical Activity – Individual Activities (PAQIAF) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=1999–2000 2001–2002. Age, Sex, Race: Demographic Variables & Sample Weights https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&CycleBeginYear=2001. Weight, Standing height, BMI: Body Measures (BMX_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2001–2002. ALT, AST, Total cholesterol, Glucose, Iron, Triglycerides: Standard Biochemistry Profile (L40_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2001–2002. “Eye surgery for near sightedness?”, “Eye surgery for cataracts?”, sphere, cylinder: Vision (VIX_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2001–2002. Diabetes: Diabetes (DIQ_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2001–2002. CRP: C-Reactive protein (CRP), Fibrinogen, Bone Alkaline Phosphatase & Urinary N-telopeptides (L11_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2001–2002. HDL-C: Cholesterol—Total & HDL (l13_b) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2001–2002. Physical Activity: Physical Activity—Individual Activities (PAQIAF_B) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2001–2002 2003–2004. Age, Sex, Race: Demographic Variables & Sample Weights https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&CycleBeginYear=2003. Weight, Standing height, BMI: Body Measures (BMX_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2003–2004. ALT, AST, Total cholesterol, Glucose, Iron, Triglycerides: Standard Biochemistry Profile (L40_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2003–2004. “Eye surgery for near sightedness?”, “Eye surgery for cataracts?”, sphere, cylinder: Vision (VIX_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2003–2004. Diabetes: Diabetes (DIQ_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2003–2004. CRP: C-Reactive Protein (CRP), Bone Alkaline Phosphatase (BAP) & Parathyroid Hormone (PTH) (L11_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2003–2004. HDL-C: Cholesterol—Total & HDL (l13_c) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2003–2004. Physical Activity: Physical Activity—Individual Activities (PAQIAF_C) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2003–2004 2005–2006. Age, Sex, Race: Demographic Variables & Sample Weights https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&CycleBeginYear=2005. Weight, Standing height, BMI: Body Measures (BMX_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2005–2006. ALT, AST, Total cholesterol, Glucose, Iron, Triglycerides: Standard Biochemistry Profile (BIOPRO_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2005–2006. “Eye surgery for near sightedness?”, “Eye surgery for cataracts?”, sphere, cylinder: Vision (VIX_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2003–2004. Diabetes: Diabetes (DIQ_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2005–2006. CRP: C-Reactive Protein (CRP) (CRP_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2005–2006. HDL-C: Cholesterol—HDL (HDL_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2005–2006. Physical Activity: Physical Activity—Individual Activities (PAQIAF_D) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2005–2006 2007–2008. Age, Sex, Race: Demographic Variables & Sample Weights https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics&CycleBeginYear=2007. Weight, Standing height, BMI:Body Measures (BMX_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2007–2008. ALT, AST, Total cholesterol, Glucose, Iron, Triglycerides: Standard Biochemistry Profile (BIOPRO_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2007–2008. “Eye surgery for near sightedness?”, “Eye surgery for cataracts?”, sphere, cylinder: Vision (VIX_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Examination&Cycle=2007–2008. Diabetes: Diabetes (DIQ_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&Cycle=2007–2008. CRP: C-Reactive Protein (CRP) (CRP_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2007–2008. HDL-C: Cholesterol—HDL (HDL_E) https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2007–2008. Physical Activity: Physical Activity (PAQ_E) https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2007.
Abbreviations
- D:
-
Diopters
- MET:
-
Metabolic equivalent
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HDL-C:
-
High-density lipoprotein cholesterol
- CRP:
-
C-reaction protein
- BMI:
-
Body mass index
- SD:
-
Standard deviation
- Q1:
-
First quartile
- Q3:
-
Third quartile
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- NHANES:
-
National Health and Nutrition Examination Survey
References
Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, et al. IMI—defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Investig Opthalmology Vis Sci. 2019;60(3):M20.
Vitale S, Sperduto RD, Ferris FL. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127(12):1632–9.
Dong L, Kang YK, Li Y, Wei WB, Jonas JB. Prevalence and time trends of myopia in children and adolescents in China: a systemic review and meta-analysis. Retina Phila Pa. 2020;40(3):399–411.
Wang J, Ying GS, Fu X, Zhang R, Meng J, Gu F, et al. Prevalence of myopia and vision impairment in school students in Eastern China. BMC Ophthalmol. 2020;20(1):2.
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–42.
Sankaridurg P, Tahhan N, Kandel H, Naduvilath T, Zou H, Frick KD, et al. IMI Impact of Myopia. Investig Opthalmology Vis Sci. 2021;62(5):2.
Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49.
Karthikeyan SK, Ashwini DL, Priyanka M, Nayak A, Biswas S. Physical activity, time spent outdoors, and near work in relation to myopia prevalence, incidence, and progression: an overview of systematic reviews and meta-analyses. Indian J Ophthalmol. 2022;70(3):728–39.
Jacobi FK, Zrenner E, Broghammer M, Pusch CM. A genetic perspective on myopia. Cell Mol Life Sci CMLS. 2005;62(7–8):800–8.
Rose KA, French AN, Morgan IG. Environmental factors and myopia: paradoxes and prospects for prevention. Asia-Pac J Ophthalmol Phila Pa. 2016;5(6):403–10.
Mountjoy E, Davies NM, Plotnikov D, Smith GD, Rodriguez S, Williams CE, et al. Education and myopia: assessing the direction of causality by Mendelian randomisation. BMJ. 2018;6(361):k2022.
Jin JX, Hua WJ, Jiang X, Wu XY, Yang JW, Gao GP, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015;9(15):73.
Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080–5.
He M, Xiang F, Zeng Y, Mai J, Chen Q, Zhang J, et al. Effect of time spent outdoors at school on the development of myopia among children in china: a randomized clinical trial. JAMA. 2015;314(11):1142–8.
Herbort CP, Papadia M, Neri P. Myopia and inflammation. J Ophthalmic Vis Res. 2011;6(4):270–83.
Lin HJ, Wei CC, Chang CY, Chen TH, Hsu YA, Hsieh YC, et al. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine. 2016;10:269–81.
Liu XN, Naduvilath TJ, Wang J, Xiong S, He X, Xu X, et al. Sleeping late is a risk factor for myopia development amongst school-aged children in China. Sci Rep. 2020;10(1):17194.
Cordain L, Eaton SB, Brand Miller J, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand. 2002;80(2):125–35.
Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out.” JAMA Intern Med. 2018;178(8):1098–103.
Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;14(363):k4583.
Lennerz B, Lennerz JK. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin Chem. 2018;64(1):64–71.
Peled A, Nitzan I, Megreli J, Derazne E, Tzur D, Pinhas-Hamiel O, et al. Myopia and BMI: a nationwide study of 1.3 million adolescents. Obesity. 2022;30(8):1691–8.
Lee S, Lee HJ, Lee KG, Kim J. Obesity and high myopia in children and adolescents: Korea National Health and Nutrition Examination Survey. PLoS ONE. 2022;17(3):e0265317.
Rahi JS, Cumberland PM, Peckham CS. Myopia over the lifecourse: prevalence and early life influences in the 1958 British birth cohort. Ophthalmology. 2011;118(5):797–804.
Mori K, Kurihara T, Uchino M, Torii H, Kawashima M, Sasaki M, et al. High myopia and its associated factors in JPHC-NEXT eye study: a cross-sectional observational study. J Clin Med. 2019;8(11):1788.
Harb EN, Wildsoet CF. Nutritional factors and myopia: an analysis of national health and nutrition examination survey data. Optom Vis Sci Off Publ Am Acad Optom. 2021;98(5):458–68.
Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56):1–37. https://pubmed.ncbi.nlm.nih.gov/25078429/.
Burke N, Butler JS, Flitcroft I, McCartney D, Loughman J. Association of total zinc intake with myopia in U.S. children and adolescents. Optom Vis Sci Off Publ Am Acad Optom. 2019;96(9):647–54.
Lyu IJ, Oh SY. Association between age at menarche and risk of myopia in the United States: NHANES 1999–2008. PLoS ONE. 2023;18(5):e0285359.
Fowler JR, Tucker LA, Bailey BW, LeCheminant JD. Physical activity and insulin resistance in 6,500 NHANES adults: the role of abdominal obesity. J Obes. 2020;1(2020):e3848256.
Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in seoul. South Korea Invest Ophthalmol Vis Sci. 2012;53(9):5579–83.
Limwattanayingyong J, Amornpetchsathaporn A, Chainakul M, Grzybowski A, Ruamviboonsuk P. The association between environmental and social factors and myopia: a review of evidence from COVID-19 pandemic. Front Public Health. 2022;10:918182.
Cleland V, Crawford D, Baur LA, Hume C, Timperio A, Salmon J. A prospective examination of children’s time spent outdoors, objectively measured physical activity and overweight. Int J Obes (Lond). 2008;32(11):1685–93.
Acknowledgements
The authors thank Dr. Jie Liu from the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, for his contribution to statistical support, study design consultations, and comments regarding the manuscript. The authors thank Kai Zhang of Bethune Second Hospital of Jilin University for consultation and editing the manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YQ was responsible for data collection, statistical analysis, and writing of most of the manuscript. HH was responsible for organizing the data and writing part of the manuscript. HZ was responsible for proposing the topic and revising and improving this paper. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In 2003, the NHANES Institutional Review Board (IRB) changed its name to the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB). In 2018, the name changed from NCHS Research Ethics Review Board to NCHS Ethics Review Board. This study was approved by the NHANES Health and NCHS ERB. The protocol number and description are as follows: NHANES 2007–2008: Continuation of Protocol #2005–06; NHANES 2005–2006: Protocol #2005–06; NHANES 1999–2004: Protocol #98–12.
Consent for publication
This information can be accessed at this (https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Frequency and proportion of missing values for all variables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qu, Y., Huang, H. & Zhang, H. Association between body mass index and myopia in the United States population in the National Health and Nutrition Examination Surveys 1999 to 2008: a cross-sectional study. Eur J Med Res 28, 561 (2023). https://doi.org/10.1186/s40001-023-01542-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01542-4