Abstract
Hepatocellular carcinoma (HCC) accounts for the vast majority of primary liver cancer and constitutes a major global health challenge. Tumor ablation with either radiofrequency ablation (RFA) or microwave ablation (MWA) is recommended as a curative-intent treatment for early-stage HCC. Given the widespread use of thermal ablation in routine clinical practice, accurate evaluation of treatment response and patient outcomes has become crucial in optimizing individualized management strategies. Noninvasive imaging occupies the central role in the routine management of patients with HCC. Magnetic resonance imaging (MRI) could provide full wealth of information with respect to tumor morphology, hemodynamics, function and metabolism. With accumulation of liver MR imaging data, radiomics analysis has been increasingly applied to capture tumor heterogeneity and provide prognostication by extracting high-throughput quantitative imaging features from digital medical images. Emerging evidence suggests the potential role of several qualitative, quantitative and radiomic MRI features in prediction of treatment response and patient prognosis after ablation of HCC. Understanding the advancements of MRI in the evaluation of ablated HCCs may facilitate optimal patient care and improved outcomes. This review provides an overview of the emerging role of MRI in treatment response evaluation and prognostication of HCC patients undergoing ablation.
Clinical relevance statement
MRI-based parameters can help predict treatment response and patient prognosis after HCC ablation and thus guide treatment planning.
Key points
-
1.
ECA-MRI provides morphological and hemodynamic assessment of ablated HCC.
-
2.
EOB-MRI provides more information for tumor response prediction after ablation.
-
3.
DWI improve the characterization of HCC and optimize treatment decision.
-
4.
Radiomics analysis enables characterization of tumor heterogeneity guidance of clinical decision-making.
-
5.
Further studies with multiple radiologists and sufficient follow-up period are needed.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Liver cancer is the second most lethal malignancy globally and its incidence is on the rise, with more than 1 million worldwide new cases by 2025 [1, 2]. Hepatocellular carcinoma (HCC) accounts for the vast majority of primary liver cancer and constitutes a major global health challenge [1]. Tumor ablation with either radiofrequency ablation (RFA) or microwave ablation (MWA) is recommended as a curative-intent treatment for early-stage HCC, and frequently used to downsize or control tumor burden prior to liver transplantation [2, 3]. Particularly, ablation could be given priority over hepatectomy for patients with HCC ≤ 3 cm owing to the merits of comparable survival benefits, less invasiveness and cost-effectiveness [4]. Given the increased application of thermal ablation in routine clinical practice, accurate evaluation of treatment response and patient outcomes has become crucial in optimizing personalized management strategies.
Noninvasive imaging plays a critical role in the therapeutic response assessment and risk stratification of HCC, and magnetic resonance imaging (MRI) exhibits a particularly promising prospect. Conventional extracellular contrast agent-enhanced MRI (ECA-MRI) has been widely used for qualitative evaluation of tumor morphology and hemodynamics. With tremendous progress in MRI techniques, and the introduction of hepatospecific agents such as gadoxetate disodium (Eovist/Primovist; Bayer HealthCare, Berlin, Germany)-enhanced MRI (EOB-MRI), the diagnosis and characterization of HCC has improved significantly [5,6,7]. On the basis of this, EOB-MRI combined with diffusion-weighted imaging (DWI) can further improve the capabilities of HCC characterization and enabled accurate guidance of ablation treatment plan by providing images with high tumor-to-liver contrast and good depiction of intrahepatic vascular and biliary structures [8,9,10]. With accumulation of liver MR imaging data, radiomics analysis has recently emerged as a promising strategy that enables characterization of tumor heterogeneity and guidance of clinical decision-making by extracting high-throughput quantitative imaging features from digital medical images, including signal intensity, histogram-based features, and textural feature [11,12,13]. Encouraging studies have been published on the potential utility of novel MRI characteristics, including qualitative features, quantitative parameters and radiomic signatures, for noninvasively estimating therapeutic efficacy and providing prognostication in HCC patients treated with ablation (Figs. 1 and 2, Tables 1 and 2). In this context, understanding the usefulness of MRI for evaluation of ablated HCCs may contribute to the optimal clinical decision-making and improved patient outcomes. The purpose of this review is to overview of the emerging role of MRI in treatment response evaluation and prognostication of HCC patients undergoing ablation therapy.
Extracellular contrast agent-enhanced MRI
Conventional imaging markers
Conventional imaging plays an important role in assessing and predicting the efficacy of ablative therapies for HCC because the images are stable and easily accessible, as well as the ease with which the image features obtained can be validated. A few attempts have been made to explore the potential of pretreatment ECA-MRI in evaluating the prognosis of HCC patients receiving ablation. In a retrospective, single-center study of 35 patients, Sheng et al. [14] demonstrated that multiplicity, tumors with no or disrupted periablational enhancement, persistent hyperintensity in the central ablative zone on T1 weighted imaging and serum albumin < 3.5 g/dL, were independently associated with intrahepatic distant metastasis after RFA of HCC. Moreover, another two retrospective studies showed that small steatotic HCC, identified on pretreatment MRI, was associated with a less aggressive tumor phenotype and improved overall survival [15, 16] (Fig. 3).
Gadobenate dimeglumine–enhanced axial MRI scans in a 57-year-old man with hepatitis B virus (A-H). A 1.7-cm steatotic mass is detected in segments VII. The mass shows T1 hypointensity (A), signal intensity reduced on out-of-phase TIWI (arrow in B), moderate T2 hyperintensity (C), marked hyperintensity on DWI (b = 1000 s/mm2) (D), rim enhancement on arterial phase image (E), “wash-out” appearance on portal venous phase (F) and equilibrium phase (G), marked hepatobiliary phase hypointensity (H). The recurrence-free survival for this patient was 150 days
Liver imaging reporting and data system treatment response algorithm
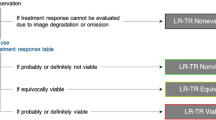
Liver Imaging Reporting and Data System (LI-RADS) Treatment Response Algorithm (TRA) provides a comprehensive scheme to standardize the evaluation of treatment response after local–regional therapy, thereby guiding management decisions. As per LI-RADS TRA, treated lesions are categorized as viable, nonviable, or equivocal [17] (Fig. 4). An ECA-MRI-based study comprising 36 patients, Chaudhry et al. [18] showed that the LI-RADS TRA performed well in the prediction of both complete (negative predictive value range for residual tumors, 89%–90%) and incomplete (positive predictive value range for residual tumors, 70%–87%) tumor necrosis when equivocal estimates were treated as viable or nonviable, respectively. In addition, the majority of ablated tumors categorized as LR-TR equivocal were confirmed as incompletely necrotic at histopathology, suggesting that sensitivity for incompletely necrotic lesions might be increased if equivocal lesions were regarded as viable. Furthermore, post-treatment nodular, mass-like, or irregular thick tissue in or along the treated lesion with arterial phase hyperenhancement was the most powerful predictor of histopathological necrosis (odds ratio: 142.86).
Images in a 56-year-old man with chronic hepatitis B virus infection. A–D Preoperative gadoxetate disodium–enhanced axial MRI scans show a 2.0-cm mass (NHHN) in segment VII, which shows moderate T2 hyperintensity (A), marked hyperintensity on DWI (b = 800 s/mm2) (B), without obvious enhancement on arterial phase image (C), marked hepatobiliary phase hypointensity (D). E–G Enhanced abdominal CT images of the patient 50 days after radiofrequency ablation treatment of tumor. A low-density ablation area was shown (E), and there was not any enhancement tissue in or along the margin of the treated lesion (F, G). Diagnosis was agreed upon by the 2 readers (LR-TR nonviable). H–K Gadoxetate disodium–enhanced axial MRI scans 67 days after tumor ablation, which shows T2 hyperintensity (H), marked hyperintensity on DWI (b = 800 s/mm2) (I), irregular thickened enhanced tissue area was found along the margin of the treated lesion (* in J), and marked hepatobiliary phase hypointensity (K). The recurrence-free survival for this patient was 60 days
In summary, several conventional ECA-MRI features depicting either intra- or peri-tumoral alterations may convey prognostic information for HCC patients treated with thermal ablation. As for the application of LI-RADS TRA on ECA-MRI, although potentially promising, further multicenter prospective validation is needed to confirm these clinically meaningful results.
Gadoxetate disodium-enhanced MRI
Conventional imaging markers
Hepatobiliary phase (HBP) images obtained by hepatobiliary-specific contrast-enhanced MRI can visualize the impairment of hepatocyte function and provide vital information for tumor grading before ablation and assessment of tumor activity after treatment. Several studies investigated the prognostic implications of imaging features on pre-ablation EOB-MRI in patients with HCC. For example, in a retrospective, single-center study of 183 patients, Bae et al. [19] reported that satellite nodules on HBP images were independently associated with poor disease-free survival and overall survival, whereas HBP peritumoral hypointensity was predictive of poor overall survival. Another study by Cha et al. [20] assessed the early recurrence patterns in 349 patients after RFA. The authors observed that tumors with arterial rim enhancement plus other targetoid appearances showed significantly higher rates of local tumor progression, intrahepatic distant metastasis and extrahepatic metastasis within 2 years compared with those without arterial rim enhancement. Whereas no differences in outcomes were observed between tumors with arterial rim enhancement only and those without arterial rim enhancement. In addition, Kang et al. retrospectively constructed a risk score for the prediction of local tumor progression after RFA of HCC based on tumor size, tumor margin and HBP peritumoral hypointensity [21].
LI-RADS TRA based on EOB-MRI
The first EOB-MRI-based study evaluating the performance of LI-RADS TRA after thermal ablation of small HCC was conducted on a retrospective cohort of 45 patients. The authors reported that LR-TRA after thermal ablation had high interrater reliability (90% agreement, Cohen’s ĸ = 0.75) but unsatisfactory sensitivity (30%) in detecting residual viable tumors [22]. The low accuracy might be attributed to the disruption of local blood flow in the ablated tissue, which could impact the arterial enhancement and washout seen at MRI. These findings emphasized the importance of incorporating histopathology as the gold standard for estimation of ablated HCCs in future studies.
Nonhypervascular HBP hypointense nodule
Nonhypervascular HBP hypointense nodule (NHHN) refers to the borderline hepatocellular nodules with the absence of arterial phase hyperenhancement and presence of hypointensity on HBP images (Fig. 4), because the decrease in organic anion transporting polypeptide 8 expression occurs at an earlier step of hepatocarcinogenesis than the typical dynamic vascular alterations of progressed HCC [23]. Previous studies have reported that pre-existing NHHN could develop into hypervascular HCCs during follow-up [24, 25]. The association between the presence of NHHNs and tumor recurrence after RFA of HCC has been explored. Lee et al. [26] retrospectively investigated 139 patients from a single center and reported that the presence of NHHNs was an independent risk factor of HCC recurrence after RFA. Notably, the 5-year cumulative incidences of intrahepatic distant recurrence were significantly higher in patients with NHHNs than in those without, whereas no significant difference was observed in the 5-year cumulative incidences of local tumor progression and extrahepatic metastasis. These findings were in good accordance with other three studies, which demonstrated that the presence of NHHNs was a predictive factor of recurrence [27] or intrahepatic distant recurrence [28, 29]. This could be partly explained by the fact that RFA stimulates distant tumor growth by immunomodulatory processes and proangiogenic pathway [30,31,32]. Specifically, the “off-target” effect of RFA, which might be secondary to the elevation of cytokines (e.g., interleukin-6 and hepatocyte growth factor) in a response to hepatic regeneration following tissue injury, could possibly promote the acceleration of carcinogenesis in NHHNs [33]. More recently, Lee et al. [34] investigated whether the presence of NHHNs could assist in the decision-making between hepatectomy and RFA in 345 HCC patients. The researchers observed that the presence of NHHNs was a significant predictor of HCC recurrence after both RFA and surgical resection. Moreover, patients without NHHNs achieved better RFS after hepatectomy compared to RFA, whereas patients with NHHNs obtained similar outcomes after the two treatments. These findings highlighted the promising role of MRI in directing the curative treatment selection for early HCCs.
Ablation margin
A sufficient ablation margin (AM) surrounding the index tumor is another critical element affecting progression-free survival and overall survival of HCC patients [35,36,37,38]. The first study evaluating the utility of post-ablation EOB-MRI in assessing AM was conducted on a retrospective cohort of 95 patients. The authors categorized AM on HBP images into three grades, including AM( +), low-intensity area with continuous high-intensity rim; AM zero, low-intensity area with discontinuous high-intensity rim; and AM( −), low-intensity area extends beyond the high-intensity rim. The cumulative local tumor progression rates in AM( +) HCCs were significantly lower than those in AM zero HCCs [39]. More recently, in a retrospective study of 29 patients, Takeyama et al. [40] reported that the ablation margin status (AM( +), ablation margin completely surrounding the tumor vs. AM zero, a partially discontinuous ablation margin without protrusion of HCC) assessed using fusion images of pre- and post-ablation HBP series was an independent predictor for local tumor progression.
Compared to ECA-MRI, EOB-MRI provide additional information in the prediction and assessment of tumor response after ablation (i.e., HBP images features including the tumor and the peritumoral characteristics). In addition, the ablation margin status assessed by EOB-MRI is a significant predictor of local tumor progression. It is because most HCCs after ablation are not pathologically confirmed, thus EOB-MRI may be an alternative method to determine whether the residual tumor is still viable.
Contrast enhanced-MRI in combination with contrast enhanced-computed tomography
Several studies investigated the effectiveness of contrast-enhanced MRI in combination with contrast-enhanced computed tomography (CT) in the estimation of recurrence and survival of HCC patients undergoing ablation. For example, in a recent study of 467 patients, Lee et al. [41] retrospectively evaluated the 10-year overall survival and local tumor progression of RFA for single small (< 3 cm) HCCs. The 5- and 10-year overall survival rates were 83.7% and 74.2%, respectively, and the 5- and 10-year local tumor progression rates were 20.4% and 25.1%, respectively. In addition, local tumor progression was an independent risk factor for overall survival, while periportal and subphrenic locations of HCC and tumor size were independently associated with local tumor progression. Periportal HCC is more prone to recurrence, which is mainly related to the heat-sink effect [42]. This effect prevents a sustained accumulation of heat in the tumor area during the ablation process. In addition, the surgeon may choose a less energetic ablation needle for the procedure to avoid damage to the adjacent vessel wall, which may also lead to inadequate ablation margins [43]. For tumors in specific locations, such as subphrenic HCC (Fig. 5), the increased risk of local tumor progression may be due to the difficulty in placing electrodes along the surface of the liver to obtain sufficient ablation margins for subphrenic tumors [44].In another western cohort of 238 patients, Hermida et al. [16] identified several clinical and radiological factors, including tumor size, multiplicity, steatotic HCC, serum alpha-fetoprotein (AFP) > 100 ng/mL, treatment naivety, Ultrasound (US) guidance, American Society of Anesthesiologists score > 2, for predicting tumor recurrence and/or overall survival after percutaneous thermal ablation. Moreover, a retrospective study by Kawamura et al. [45] demonstrated that enhancement pattern type (heterogeneous enhancement pattern with a septum-like structure and irregularly shaped ring structure enhancement pattern), treatment procedure (touch ablation), and serum AFP ≥ 30 μg/L were independently predictive of intrasubsegmental recurrence. Furthermore, in a prospective study comprising 589 HCC patients, Kondo et al. [46] assessed the prognostic impact of thermal injuries to intrahepatic bile duct after RFA. The authors reported that the bile duct dilatation affecting two or more subsegments after RFA was significantly associated with recurrence and death, suggesting the need for careful evaluation of such posttreatment complication. What’s more, a prospective study comprising 33 patients showed that MRI outperformed multidetector-row CT in the differentiation of ablation margin and index tumor immediately after RFA of HCC. The cumulative incidence of local tumor progression was significantly lower in AM( +) tumors (AM completely surrounding the tumor) on MRI [47]. The above findings suggested that combined enhanced MRI and CT can better characterize the tumor composition and the aggressive biological behavior of HCC, but MRI shows better ability to identify whether the treated lesion with arterial phase hyperenhancement is a treatment response or early tumor recurrence. Despite the clinical relevance, these findings remain to be further validated in multicenter prospective cohorts.
Images in a 62-year-old man with chronic hepatitis B virus infection. A–F Gadoxetate disodium–enhanced axial MRI scans show a 2.4-cm mass in segment VII (subphrenic location), which shows T1 hypointensity (A), mild to moderate T2 hyperintensity (B), marked hyperintensity on DWI (b = 800 s/mm2) (C), mild inhomogeneous enhancement on arterial phase image (D), obvious enhancement on portal venous phase (E), and demonstrates marked hepatobiliary phase hypointensity (F). The recurrence-free survival for this patient was 50 days
Diffusion-weighted imaging and diffusion kurtosis imaging
DWI represents a functional MR imaging modality that can characterize water molecule diffusion in tissues by the apparent diffusion coefficient (ADC). It has been recognized that ADC values can reflect the number and proliferation activity of tumor cells [48]. Recent data have shown promising results of DWI in HCC prognostication after ablation. In a study analyzing 136 patients with small HCC, Mori et al. [49] reported that the hypointensity on the apparent diffusion coefficient (ADC) map was independently associated with tumor recurrence and survival after RFA. In addition to this, ADC values also enable quantitative evaluation of RFA efficacy in HCC. For example, Ma et al. [50] retrospectively analyzed 64 patients using ADC histogram analysis and reported that the baseline ADC values could be used as imaging markers for predicting progression-free survival in HCC patients treated with RFA. Another study by Barat et al. [51] showed that a low ADC value at 1 month after RFA was an independent predictor of early local recurrence of HCC, with good predictive accuracy (area under the receiver operating characteristic curve (AUC), 0.860). Moreover, in a retrospective cohort of 105 patients, Hu et al. reported that ADC value and rim enhancement were independently associated with local tumor progression after RFA. By incorporating the above two predictors, a nomogram yielded a concordance index of 0.782 [52]. According to the literature, low ADC values are associated with aggressive tumor behaviors (e.g., poor differentiation and MVI), which may partly explain why HCCs with lower ADC values had poorer clinical outcomes [48, 53]. To better characterize the water diffusion properties in biologic tissues with non-Gaussian form, diffusion kurtosis imaging (DKI) has emerged as a useful technology for assessing the tissue microstructure abnormalities [54]. In a retrospective cohort consisting of 107 HCC patients, Yuan et al. compared the performance between DKI and DWI in prediction of tumor recurrence after RFA. The authors demonstrated that mean kurtosis showed significantly higher accuracy than that of ADC for tumor recurrence prediction (AUC, 0.956 vs. 0.842; p < 0.05) [55].
Fusion imaging
Fusion imaging, which refers to the combination of two different imaging modalities via registration software, has been recently introduced to treatment response assessment for ablative HCC [56]. Compared with traditional visual side-by-side inspection, fusion of pre- and post-RFA images enables more accurate estimation of ablative margin. Recently, Kobe et al. [57] conducted a retrospective study on 43 HCCs to evaluate the ability of fusion of pre-ablation MRI with post-ablation perfusion–CT in assessment of local tumor progression after RFA of HCC. The authors demonstrated that the difference (ablation zone − tumor) of perfusion parameters (normalized peak enhancement, arterial liver perfusion, blood flow, and blood volume) enabled an accurate prediction of local tumor recurrence within 24 h after RFA. Another prospective study by Yoon et al. [58] enrolled 68 patients with 88 HCCs who underwent pre-ablation MRI and post-ablation CT, demonstrating that ablation margin assessment using registration software-assisted inspection was superior to visual evaluation for predicting local tumor progression after RFA. Moreover, Wang et al. [59] explored the ability of EOB-MRI/US fusion imaging in improving the prognosis of HCC patients after RFA, with the ablation area covering two HBP imaging findings (peritumoral hypointensity and irregular protruding margin). The authors showed that HCCs with HBP imaging findings had significantly higher recurrence rates than those without HBP imaging findings. Notably, in HCCs with HBP imaging findings, RFA guided by EOB-MRI/US fusion imaging produced a significantly lower recurrence rate than contrast-enhanced US/US.
Radiomic analysis
Radiomics is a newly emerging technique of imaging analysis that performs the high-throughput extraction of quantitative features from standard-of-care medical imaging to obtain predictive or prognostic information. Combined with other patient data (e.g., clinical, pathological or genetic characteristics), radiomics displayed potential power to improve prediction accuracy and optimize therapeutic decision-making in various clinical settings [11,12,13]. Recent research has shown promising results of the radiomics analysis in predicting treatment response and patient outcomes after RFA of HCC. For instance, a pilot study of 34 patients demonstrated that the MRI-based textural features may serve as useful biomarkers for sustained complete response to RFA. In particular, the second-order features (Gray Level Dependence Matrix and Gray Level Co-occurrence Matrix) extracted from equilibrium phase provided the optimal discriminatory performance (AUCs > 0.7) [60]. In a retrospective study of 65 patients, Petukhova-Greenstein et al. [61] reported that multifocality, the appearance of capsular continuity, and a higher radiomic signature based on nodular and perinodular features were associated with poorer PFS in HCC after RFA. Moreover, Wen et al. [62] retrospectively constructed a nomogram for the prediction of early recurrence based on pretreatment platelet count and radiomics signature, with excellent predictive performance (AUC, 0.98). Concurrently, in a retrospective study of 58 HCC patients, Lv et al. [63] developed a model based on tumor shape, ADC value, DWI signal intensity, ΔSI (signal intensity enhancement rate), and radiomics signature for predicting aggressive intrasegmental recurrence after RFA, with good predictive accuracy in both the training (AUC, 0.941) and test (AUC, 0.818) cohorts.
In general, the application of radiomics analysis in predicting treatment response and patient outcomes after RFA of HCC consists mainly of deeper mining of traditional imaging features, and exploration of quantitative MRI features, with the latter being a trend for future research. Despite the great promise, to our knowledge, no radiomic signatures have yet been in widespread clinical use. Current major obstacles for radiomics analysis lie in the standardized data collection and biologic rationales explanation[64]. Future prospective multicenter research is warranted to validate its clinical utility and promote its translation into practice.
Conclusions and perspectives
There is ample evidence to suggest the potential role of MRI-based qualitative and quantitative parameters in prediction of treatment response and patient prognosis after ablation of HCC, thereby directing a personalized clinical care. Although these initial reports are promising, there are still some shortcomings in the present studies, such as the fact that most studies were single-center or retrospective studies with small sample size. Moreover, there are limited data on MRI-based LI-RADS TRA in the prediction of histologic response and is of limited use in interpreting and validating radiological-pathological correlations. Therefore, future prospective studies in the large multicenter with rigorous designs (e.g., including multiple radiologists with various training and experience levels to assess the interreader reliability and sufficient follow-up period) are needed to look at this issue.
Availability of data and materials
The figure and table are available from the corresponding author, Prof. Bin Song, upon reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AFP:
-
Alpha-fetoprotein
- AM:
-
Ablation margin
- AUC:
-
Area under the receiver operating characteristic curve
- CT:
-
Computed tomography
- DKI:
-
Diffusion kurtosis imaging
- DWI:
-
Diffusion-weighted imaging
- ECA-MRI:
-
Extracellular contrast agent-enhanced magnetic resonance imaging
- EOB-MRI:
-
Gadoxetate disodium-enhanced magnetic resonance imaging
- HBP:
-
Hepatobiliary phase
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MRI:
-
Magnetic resonance imaging
- MWA:
-
Microwave ablation
- NHHN:
-
Nonhypervascular hypointense nodule
- RFA:
-
Radiofrequency ablation
- TRA:
-
Treatment response algorithm
- US:
-
Ultrasound
References
Llovet JM, Kelley RK, Villanueva A et al (2021) Hepatocellular carcinoma. Nat Rev Dis Prim 7(1):6. https://doi.org/10.1038/s41572-020-00240-3
EASL Clinical Practice Guidelines (2018) Management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Marrero JA, Kulik LM, Sirlin CB, et al. (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723–750. https://doi.org/10.1002/hep.29913
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 76(3):681–693. https://doi.org/10.1016/j.jhep.2021.11.018
Feng Z, Zhao H, Guan S, Wang W, Rong P (2021) Diagnostic performance of MRI using extracellular contrast agents versus gadoxetic acid for hepatocellular carcinoma: a systematic review and meta-analysis. Liver Int 41(5):1117–1128. https://doi.org/10.1111/liv.14850
Semaan S, Vietti Violi N, Lewis S et al (2020) Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur Radiol. 30(2):1020–30. https://doi.org/10.1007/s00330-019-06458-4
Liu X, Jiang H, Chen J, Zhou Y, Huang Z, Song B (2017) Gadoxetic acid disodium-enhanced magnetic resonance imaging outperformed multidetector computed tomography in diagnosing small hepatocellular carcinoma: a meta-analysis. Liver Transplant 23(12):1505–1518. https://doi.org/10.1002/lt.24867
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR Imaging of hepatocellular carcinoma in the cirrhotic liver: challenges and controversies. Radiology 247(2):311–330. https://doi.org/10.1148/radiol.2472061331
Lee VS, Lavelle MT, Rofsky NM et al (2000) Hepatic MR imaging with a dynamic contrast-enhanced isotropic volumetric interpolated breath-hold examination: feasibility, reproducibility, and technical quality. Radiology 215(2):365–372. https://doi.org/10.1148/radiology.215.2.r00ma16365
Bathe OF, Mahallati H (2007) MR-guided ablation of hepatocellular carcinoma aided by gadoxetic acid. J Surg Oncol 95(8):670–673. https://doi.org/10.1002/jso.20768
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. https://doi.org/10.1038/ncomms5006
Sheng RF, Zeng MS, Ren ZG, Ye SL, Zhang L, Chen CZ (2015) Intrahepatic distant recurrence following complete radiofrequency ablation of small hepatocellular carcinoma: risk factors and early MRI evaluation. Hepatobil Pancreat Dis Int 14(6):603–612. https://doi.org/10.1016/s1499-3872(15)60390-3
Hermida M, Preel A, Assenat E et al (2021) Small steatotic HCC: a radiological variant associated with improved outcome after ablation. Hepatol Commun 5(4):689–700. https://doi.org/10.1002/hep4.1661
Hermida M, Cassinotto C, Piron L, Aho-Glélé S, Guillot C, Schembri V, et al. Multimodal Percutaneous Thermal Ablation of Small Hepatocellular Carcinoma: Predictive Factors of Recurrence and Survival in Western Patients. Cancers (Sao Paolo) 2020;12(2). doi: https://doi.org/10.3390/cancers12020313.
Chernyak V, Fowler KJ, Kamaya A, et al. (2018) Liver imaging reporting and data system (LI-RADS) Version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 289(3):816–830. https://doi.org/10.1148/radiol.2018181494
Chaudhry M, McGinty KA, Mervak B et al (2020) The LI-RADS Version 2018 MRI Treatment Response Algorithm: Evaluation of Ablated Hepatocellular Carcinoma. Radiology 294(2):320–326. https://doi.org/10.1148/radiol.2019191581
Bae JS, Kim JH, Lee DH, Kim JH, Han JK (2021) Hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with HCC: prognostic features before resection, ablation, or TACE. Eur Radiol 31(6):3627–3637. https://doi.org/10.1007/s00330-020-07499-w
Cha DI, Lee MW, Jeong WK et al (2021) Rim-arterial enhancing primary hepatic tumors with other targetoid appearance show early recurrence after radiofrequency ablation. Eur Radiol 31(9):6555–6567. https://doi.org/10.1007/s00330-021-07769-1
Kang TW, Rhim H, Lee J et al (2016) Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur Radiol 26(10):3437–3446. https://doi.org/10.1007/s00330-015-4190-5
Cools KS, Moon AM, Burke LM, McGinty KA, Strassle PD, Gerber DA (2020) Validation of the liver imaging reporting and data system treatment response criteria after thermal ablation for hepatocellular carcinoma. Liver Transplant 26(2):203–214. https://doi.org/10.1002/lt.25673
Joo I, Kim SY, Kang TW et al (2020) Radiologic-pathologic correlation of hepatobiliary phase hypointense nodules without arterial phase hyperenhancement at gadoxetic acid-enhanced MRI: a multicenter study. Radiology 296(2):335–345. https://doi.org/10.1148/radiol.2020192275
Yamamoto A, Ito K, Tamada T et al (2013) Newly developed hypervascular hepatocellular carcinoma during follow-up periods in patients with chronic liver disease: observation in serial gadoxetic acid-enhanced MRI. AJR Am J Roentgenol 200(6):1254–1260. https://doi.org/10.2214/ajr.12.9136
Hyodo T, Murakami T, Imai Y et al (2013) Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology 266(2):480–490. https://doi.org/10.1148/radiol.12112677
Lee DH, Lee JM, Lee JY et al (2015) Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol 62(5):1122–1130. https://doi.org/10.1016/j.jhep.2014.12.015
Toyoda H, Kumada T, Tada T, Sone Y, Maeda A, Kaneoka Y (2015) Non-hypervascular hypointense nodules on Gd-EOB-DTPA-enhanced MRI as a predictor of outcomes for early-stage HCC. Hep Intl 9(1):84–92. https://doi.org/10.1007/s12072-014-9553-5
Iwamoto T, Imai Y, Igura T et al (2017) Non-hypervascular hypointense hepatic nodules during the hepatobiliary phase of gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI as a risk factor of intrahepatic distant recurrence after radiofrequency ablation of hepatocellular carcinoma. Dig Dis 35(6):574–582. https://doi.org/10.1159/000480185
Inoue M, Ogasawara S, Chiba T et al (2017) Presence of non-hypervascular hypointense nodules on Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 32(4):908–915. https://doi.org/10.1111/jgh.13622
Rozenblum N, Zeira E, Bulvik B et al (2015) Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology 276(2):416–425. https://doi.org/10.1148/radiol.15141918
Rozenblum N, Zeira E, Scaiewicz V et al (2015) Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology 276(2):426–432. https://doi.org/10.1148/radiol.2015141695
Nijkamp MW, Borren A, Govaert KM et al (2010) Radiofrequency ablation of colorectal liver metastases induces an inflammatory response in distant hepatic metastases but not in local accelerated outgrowth. J Surg Oncol 101(7):551–556. https://doi.org/10.1002/jso.21570
Kim TH, Woo S (2020) Hepatobiliary phase hypointense nodule without arterial phase hyperenhancement: are they at risk of HCC recurrence after ablation or surgery? A systematic review and meta-analysis. Eur Radiol 30(3):1624–1633. https://doi.org/10.1007/s00330-019-06499-9
Lee DH, Lee JM, Yu MH et al (2019) Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MR can help determine the treatment method for HCC. Eur Radiol 29(6):3122–3131. https://doi.org/10.1007/s00330-018-5941-x
Shiina S, Tateishi R, Arano T et al (2012) Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 107(4):569–577. https://doi.org/10.1038/ajg.2011.425
Nakazawa T, Kokubu S, Shibuya A et al (2007) Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 188(2):480–488. https://doi.org/10.2214/ajr.05.2079
Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY (2010) The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol 195(3):758–765. https://doi.org/10.2214/ajr.09.2954
Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS (2008) Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol 190(6):1544–1551. https://doi.org/10.2214/ajr.07.2798
Koda M, Tokunaga S, Okamoto T et al (2015) Clinical usefulness of the ablative margin assessed by magnetic resonance imaging with Gd-EOB-DTPA for radiofrequency ablation of hepatocellular carcinoma. J Hepatol 63(6):1360–1367. https://doi.org/10.1016/j.jhep.2015.07.023
Takeyama N, Mizobuchi N, Sakaki M et al (2019) Evaluation of hepatocellular carcinoma ablative margins using fused pre- and post-ablation hepatobiliary phase images. Abdom Radiol (NY) 44(3):923–935. https://doi.org/10.1007/s00261-018-1800-0
Lee MW, Kang D, Lim HK (2020) Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol 30(4):2391–2400. https://doi.org/10.1007/s00330-019-06575-0
Lu DS, Raman SS, Limanond P et al (2003) Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 14(10):1267–1274. https://doi.org/10.1097/01.rvi.0000092666.72261.6b
Kettenbach J, Kostler W, Rucklinger E et al (2003) Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol 180(6):1537–1545. https://doi.org/10.2214/ajr.180.6.1801537
Song KD, Lim HK, Rhim H et al (2019) Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol 11(3):227–237. https://doi.org/10.4251/wjgo.v11.i3.227
Kawamura Y, Ikeda K, Shindoh J et al (2019) No-touch ablation in hepatocellular carcinoma has the potential to prevent intrasubsegmental recurrence to the same degree as surgical resection. Hepatol Res 49(2):164–176. https://doi.org/10.1111/hepr.13254
Kondo Y, Shiina S, Tateishi R et al (2011) Intrahepatic bile duct dilatation after percutaneous radiofrequency ablation for hepatocellular carcinoma: impact on patient’s prognosis. Liver Int 31(2):197–205. https://doi.org/10.1111/j.1478-3231.2010.02415.x
Kim SM, Shin SS, Lee BC et al (2017) Imaging evaluation of ablative margin and index tumor immediately after radiofrequency ablation for hepatocellular carcinoma: comparison between multidetector-row CT and MR imaging. Abdom Radiol (NY) 42(10):2527–2537. https://doi.org/10.1007/s00261-017-1146-z
Surov A, Pech M, Omari J et al (2021) Diffusion-weighted imaging reflects tumor grading and microvascular invasion in hepatocellular carcinoma. Liver Cancer 10(1):10–24. https://doi.org/10.1159/000511384
Mori Y, Tamai H, Shingaki N et al (2015) Signal intensity of small hepatocellular carcinoma on apparent diffusion coefficient mapping and outcome after radiofrequency ablation. Hepatol Res 45(1):75–87. https://doi.org/10.1111/hepr.12311
Ma X, Ouyang H, Wang S, Wang M, Zhou C, Zhao X (2019) Histogram analysis of apparent diffusion coefficient predicts response to radiofrequency ablation in hepatocellular carcinoma. Chin J Cancer Research 31(2):366–74. https://doi.org/10.21147/j.issn.1000-9604.2019.02.11
Barat M, Fohlen A, Cassinotto C et al (2017) One-month apparent diffusion coefficient correlates with response to radiofrequency ablation of hepatocellular carcinoma. J Magn Resonance Imag 45(6):1648–1658. https://doi.org/10.1002/jmri.25521
Hu Z, Yu N, Wang H, Li S, Yan J, Zhang G (2020) Pre-radiofrequency ablation MRI imaging features predict the local tumor progression in hepatocellular carcinoma. Medicine (Baltimore) 99(52):23924. https://doi.org/10.1097/md.0000000000023924
Wei H, Yang T, Chen J, Duan T, Jiang H, Song B (2022) Prognostic implications of CT/MRI LI-RADS in hepatocellular carcinoma: State of the art and future directions. Liver Int 42(10):2131–2144. https://doi.org/10.1111/liv.15362
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440. https://doi.org/10.1002/mrm.20508
Yuan ZG, Wang ZY, Xia MY et al (2019) Comparison of diffusion kurtosis imaging versus diffusion weighted imaging in predicting the recurrence of early stage single nodules of hepatocellular carcinoma treated by radiofrequency ablation. Cancer Imaging 19(1):30. https://doi.org/10.1186/s40644-019-0213-9
Lee DH, Lee JM (2018) Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol 19(4):545–559. https://doi.org/10.3348/kjr.2018.19.4.545
Kobe A, Kindler Y, Klotz E et al (2021) Fusion of preinterventional MR imaging with liver perfusion CT after RFA of hepatocellular carcinoma: early quantitative prediction of local recurrence. Invest Radiol 56(3):188–196. https://doi.org/10.1097/rli.0000000000000726
Yoon JH, Lee JM, Klotz E et al (2018) Prediction of local tumor progression after radiofrequency ablation (RFA) of hepatocellular carcinoma by assessment of ablative margin using pre-RFA MRI and post-RFA CT registration. Korean J Radiol 19(6):1053–1065. https://doi.org/10.3348/kjr.2018.19.6.1053
Wang F, Numata K, Nihonmatsu H et al (2020) Intraprocedurally EOB-MRI/US fusion imaging focusing on hepatobiliary phase findings can help to reduce the recurrence of hepatocellular carcinoma after radiofrequency ablation. Int J Hyperthermia 37(1):1149–1158. https://doi.org/10.1080/02656736.2020.1825837
Horvat N, Araujo-Filho JAB, Assuncao-Jr AN et al (2021) Radiomic analysis of MRI to predict sustained complete response after radiofrequency ablation in patients with hepatocellular carcinoma—a pilot study. Clinics. 76:e2888. https://doi.org/10.6061/clinics/2021/e2888
Petukhova-Greenstein A, Zeevi T, Yang J et al (2022) MR imaging biomarkers for the prediction of outcome after radiofrequency ablation of hepatocellular carcinoma: qualitative and quantitative assessments of the liver imaging reporting and data system and radiomic features. J Vasc Interv Radiol 33(7):814–24. https://doi.org/10.1016/j.jvir.2022.04.006
Wen L, Weng S, Yan C et al (2021) A radiomics nomogram for preoperative prediction of early recurrence of small hepatocellular carcinoma after surgical resection or radiofrequency ablation. Front Onco 11:657039. https://doi.org/10.3389/fonc.2021.657039
Lv X, Chen M, Kong C et al (2021) Construction of a novel radiomics nomogram for the prediction of aggressive intrasegmental recurrence of HCC after radiofrequency ablation. Eur J Radiol 144:109955. https://doi.org/10.1016/j.ejrad.2021.109955
Wei J, Jiang H, Gu D et al (2020) Radiomics in liver diseases: current progress and future opportunities. Liver Int 40(9):2050–2063. https://doi.org/10.1111/liv.14555
Funding
This work was funded by the National Natural Science Foundation of China (No. 81971571) and the Science and Technology Support Program of Sichuan Province (No. 2021YFS0021).
Author information
Authors and Affiliations
Contributions
Study concept and design: YZ and HW. Data acquisition: YZ and HW. Data analysis and interpretation: YZ and HW. Writing and editing of the manuscript: YZ and HW. Study supervision: BS. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Wei, H. & Song, B. Magnetic resonance imaging for treatment response evaluation and prognostication of hepatocellular carcinoma after thermal ablation. Insights Imaging 14, 87 (2023). https://doi.org/10.1186/s13244-023-01440-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01440-7