Abstract
Background
The mechanistic effects of gamma transcranial alternating current stimulation (tACS) on hippocampal gamma oscillation activity in Alzheimer’s Disease (AD) remains unclear. This study aimed to clarify beneficial effects of gamma tACS on cognitive functioning in AD and to elucidate effects on hippocampal gamma oscillation activity.
Methods
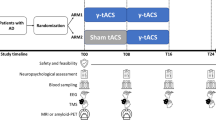
This is a double-blind, randomized controlled single-center trial. Participants with mild AD were randomized to tACS group or sham group, and underwent 30 one-hour sessions of either 40 Hz tACS or sham stimulation over consecutive 15 days. Cognitive functioning, structural magnetic resonance imaging (MRI), and simultaneous electroencephalography–functional MRI (EEG-fMRI) were evaluated at baseline, the end of the intervention and at 3-month follow-up from the randomization.
Results
A total of 46 patients were enrolled (23 in the tACS group, 23 in the sham group). There were no group differences in the change of the primary outcome, 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog) score after intervention (group*time, p = 0.449). For secondary outcomes, compared to the control group, the intervention group showed significant improvement in MMSE (group*time, p = 0.041) and MoCA scores (non-parametric test, p = 0.025), which were not sustained at 3-month follow-up. We found an enhancement of theta-gamma coupling in the hippocampus, which was positively correlated with improvements of MMSE score and delayed recall. Additionally, fMRI revealed increase of the local neural activity in the hippocampus.
Conclusion
Effects on the enhancement of theta-gamma coupling and neural activity within the hippocampus suggest mechanistic models for potential therapeutic mechanisms of tACS.
Trial registration
ClinicalTrials.gov, NCT 03920826; Registration Date: 2019-04-19.
Similar content being viewed by others
Introduction
Disruption of gamma rhythm affects learning-induced neuronal ensembles [1], and specific theta-gamma relationships underpin entorhinal-hippocampal communication and episodic memory [2]. Greater theta-gamma coupling predicts successful encoding [3]. Previous studies have shown alterations in hippocampal theta-gamma cross-frequency coupling prior to Aβ overproduction in Alzheimer’s disease (AD) transgenic mice [4]. Loss of gamma-band synchronization has also been reported in mild cognitive impairment (MCI) and AD dementia [5, 6]. Evidence of AD-related alteration of neural oscillations has been translated into encouraging findings of potential benefit from brain stimulation, especially in the gamma range [7].
Transcranial alternating current stimulation (tACS) entrains endogenous EEG oscillations in a specific frequency, which was suggested to have effects on cognitive improvements in aging population [8]. 40 Hz tACS has gained recent attention as an intervention for AD, and limited randomized, double-blind controlled studies suggest that gamma-tACS improves overall cognitive function and memory performance [9]. Mechanistic explanations for the apparent benefit have included restoration of cholinergic neurotransmission, increased brain perfusion [10], and amyloid and tau clearance [11, 12].
A challenge for tACS is the generation of effects within deep brain structures, especially the hippocampus [13]. In nonhuman primates, conventional two scalp electrode tACS influences the spiking time of single neurons in deep brain structures, including the hippocampus [14]. Recently, our team directly recorded local field potentials (LFP) in the hippocampus in patients with drug-resistant epilepsy with stereoelectroencephalography (SEEG) electrodes and demonstrated that compared to 0 mA, greater than 7 mA tACS were required to produce significant differences in LEP in the hippocampus [15]. Additionally, it was observed that the LFPs increased significantly with higher external currents, especially at 15 mA. Most importantly, no seizure activity was observed in patients during tACS, indicating that 15 mA may offer more effective stimulation compared to lower currents, while remaining safe. These research data suggest that tACS can be proposed for neuromodulation of deep brain structures. However, we do not yet know the nature of changes of oscillations in the hippocampus after tACS and its potential to improve cognitive function in AD.
We conducted this randomized, sham-controlled, clinical trial with the primary objective to determine the effect of 40 Hz tACS on global cognitive functioning of patients with mild dementia due to AD with evidence of amyloid pathology obtained either through CSF or amyloid PET scan. As a secondary objective, we used simultaneous electroencephalography–functional magnetic resonance imaging (EEG-fMRI) to determine the effects of tACS on hippocampal gamma activity.
Materials and methods
Study design
This single-center, double blind, randomized sham-controlled trial (ClinicalTrials.gov, NCT 03920826, Registration Date: 2019-04-19) [16] was conducted to examine the safety and effects of 30 one-hour sessions of 40 Hz tACS on cognitive function and hippocampal neural synchrony in mild AD. Ethical approval was received from Xuanwu Hospital, Capital Medical University (2018-077) in accordance with the Declaration of Helsinki. We conducted this study following both the CONsolidated Standards of Reporting Trials (CONSORT) statement and the CONSORT statement for non-pharmacological interventions. The informed consent from all participants was obtained.
Participants
All participants met NIA-AA core clinical criteria for probable AD dementia [17] and the following additional inclusion criteria: (1) Literate in Han Chinese; (2) aged 45 to 75 years; (3) completed at least 6 years of education; (4) Global score of Clinical Dementia Rating Scale (CDR) 1.0 [18]; (5) positive result for amyloid PET examination or low CSF Abeta42 [19]; (6) stable dose of cholinesterase inhibitors and memantine for > 6 weeks. Exclusion criteria included: (1) current or past history of any neurological disorder other than AD, such as epilepsy, stroke, progressive neurological disease (e.g., multiple sclerosis), poorly controlled migraines or intracranial brain lesions, and history of previous neurosurgery or head trauma that resulted in residual neurological impairment; (2) prescribed medication that might affect cognitive function such as anticonvulsants, antipsychotics, benzodiazepines, muscle relaxants, opiates, stimulants, and tricyclic antidepressants; (3) contraindications for undergoing MRI or receiving tACS, for example metal implants, pacemaker, claustrophobia and incomplete skulls; (4) eczema or sensitive skin; (5) abnormal brain structural MRI scan including hydrocephalus, stroke, structural lesions, significant white matter lesions (Fazekas score = 3–6), could potentially confound the outcome; (6) depression or other psychiatric disorders; (7) clinically significant gastrointestinal, renal, hepatic, respiratory, infectious, endocrine, or cardiovascular diseases, cancer, alcoholism or drug addiction.
Amyloid testing
PiB-PET imaging
PiB was synthesized and radiolabeled with 11 C following the method previously described [20]. Detailed scanning procedures are outlined in our protocol article [16]. A global cortical PiB-PET retention ratio was calculated by taking the median uptake across voxels in key regions of interest (prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus) and normalizing it to the cerebellar gray matter median. A retention ratio greater than 1.25 was considered positive.
CSF Aβ42 levels
CSF Aβ42 levels were measured using commercially available ELISA kits (Fujirebio, Ghent, Belgium), adhering strictly to the manufacturer’s instructions [21]. Results were reported as precise concentrations, calculated using standard curves established daily with six ready-to-use calibrators. Each measurement was validated with two quality control samples and two internal controls. The normal reference intervals for these CSF biomarkers varied by age group: 610–974 pg/mL (21–50 years), 562–1018 pg/mL (51–70 years), and 567–1027 pg/mL (> 70 years). An Aβ42 level below the lower limit for the respective age group was considered positive.
Randomization and masking
Participants were randomly assigned 1:1 to tACS or sham stimulation. An independent statistician generated the randomization sequence and coded the tACS device. The random allocation was performed using the random number table method in SAS software (SAS Institute, Inc., Cary, NC). Trained nurses administered the tACS, opening an opaque, sealed envelope containing each participant’s group allocation code before the first intervention. A single experienced psychological assessor, blinded to the group allocation, conducted all patient cognitive assessments. Participants, caregivers, outcomes assessors, and nurses were all blinded to randomization status. The details are shown in the published protocol and supplemental files (page 1–12, statistical analysis plan).
Procedures
To better observe and manage potential adverse reactions, all participants were admitted to the ward and received interventions during their hospitalization. A NEXALIN ADI transcranial alternating current stimulator was used to give 40 Hz stimulation; peak-to-peak amplitude 15 mA tACS [22]. A 4.45 × 9.53 cm rectangular pad was positioned on the forehead, aligning with Fpz, Fp1, and Fp2 of the international placement system. Meanwhile, two 3.18 × 3.81 cm rectangular pads were affixed behind each ear over the mastoid region. The location of the electrodes and the computational modelling of the electric fields from tACS are shown in the appendix (Figure S1).
Participants received 30 one-hour sessions of tACS or sham tACS across consecutive 15 days (twice a day, intervals of at least 4 h). At baseline, the end of the intervention, and three months after randomization, all participants underwent a cognitive testing battery, structural MRI, and simultaneous EEG-fMRI. We collected EEG and EEG-fMRI data while participants were in a resting state. The study schedule of the trial is shown in Table 1.
Outcomes
The primary outcome was the change in the scores of the 11-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog) [23] from the baseline to the end of intervention. To reduce the potential practice effect, an alternate list of words was used for the Word Recall Task (Item 1) at the end of the intervention, and the original list was used at baseline and at the 3-month follow-up. Secondary outcomes included: the Mini-Mental State Examination (MMSE) [24]; the Montreal Cognitive Assessment (MoCA) [25]; the World Health Organization-University of California-Los Angeles auditory verbal learning test (WHO-UCLA AVLT) [26]; digit span; the trail-making test (TMT) B minus A score; the Boston Naming Test (BNT); the activities of daily living (ADL) scale; the Neuropsychiatric Inventory (NPI) [27]; the Geriatric Depression Scale (GDS) [28]; changes in EEG indices, including gamma frequency power and intensity of theta-gamma coupling in the hippocampus; and changes in the neural activity of the hippocampus.
T1-weighted images of the whole brain were acquired using a sagittal three-dimensional (3D) magnetization prepared rapid gradient echo (MPRAGE) sequence. EEG data were collected simultaneously with fMRI using an MRI-compatible 64-channel (Ag/AgCl) electrode cap. Detailed description of EEG/fMRI data acquisition and data preprocessing was included in the supplementary materials.
In this study, adverse events were assessed after every five sessions. Every participant was asked about the presence and severity of the following symptoms: headache, neck pain, scalp pain, tingling, itching, ringing/buzzing noise, burning sensations, skin redness, sleepiness, trouble concentrating, acute mood change, dizziness, flickering lights, and any other symptoms.
EEG analysis
Details of EEG preprocessing could be found in the supplemental methods. For each EEG channel, spectral power was calculated for gamma band (30–50 Hz). Channels were grouped into four zones (Fig. 1a left; Figure S2a left). The left frontal zone included F1, F3, F5, F7, FC1, FC5, and FT7. The right frontal zone included F2, F4, F6, F8, FC2, FC4, FC6, and FT8. The left posterior zone included P1, P3, P5, P7, PO3, PO5, and PO7. The right posterior zone included P2, P4, P6, P8, PO4, PO6, and PO8. Statistical comparison of spectral power between different sessions was implemented for each zone.
Volume source localization for signals from the hippocampus was implemented using Brainstorm [29]. The head model for each participant was computed by OpenMEEG boundary element method (BEM) [30, 31] based on their T1 image and the corresponding Freesurfer output. Then EEG signals were inversed using dSPM method [32]. Sources in each side of the hippocampus were extracted and averaged using Desikan-Killiany Atlas [33]. Phase amplitude coupling within left and right hippocampus was calculated as mean vector length (MVL) modulation index [34], implemented by PACTools, an EEGLAB plug-in. MVL modulation indices were normalized by non-parametric permutation (n = 1000).
Increase of gamma power in EEG channels from baseline to the end of the intervention. (a) The distribution of the gamma power change in the sham group and the tACS group is shown. Channels are grouped into four regions: left frontal, right frontal, left posterior, and right posterior regions, shown by the dotted lines. (b) The change of gamma power in both tACS group and sham group are presented. Error bars represent the 95% confidence interval
* uncorrected p < 0.05. Those results were not significant after false discovery rate correction
fMRI data analysis
Details of fMRI preprocessing could be found in the supplemental methods. fMRI-based functional connectivity analysis was carried out using CONN toolbox (version 21b) [35]. Preprocessed dataset from fMRIPrep was imported and then denoised. Specifically, noise components from white matter (WM) and cerebrospinal areas (CSF), motion, outlier scans, and session effects were regressed out. Temporal band-pass filtering (0.008–0.09 Hz) was applied. Since it has been shown that the hippocampus actually includes a series of distinct and interacting subregions by previous studies [36, 37], which could be influenced differently by AD [38]. To see whether tACS intervention also impact hippocampus subregions differently, the hippocampus was partitioned into head, body, and tail by FreeSurfer [39]. To measure whether the intervention improved the regional activity in different subregions of the hippocampus, average fractional amplitude of low-frequency fluctuation (fALFF) was calculated in the subregions of the hippocampus. fALFF, as the ratio of low-frequency power to the full-range power, is thought to improve the sensitivity and specificity on detecting changes of regional brain activity [40].
EEG-fMRI data analysis
To study the association between gamma power fluctuation and hippocampus neural activation, the fluctuation of gamma power (30–50 Hz) in scalp EEG channels was calculated using EEGLAB with a window size of two seconds, which corresponds to the sampling rate of fMRI BOLD signal. A regression analysis was carried out with the fluctuation of gamma power as the regressor and the average BOLD signals in the subregions of the hippocampus as the response. The changes of beta values (regression coefficients) between sessions were calculated.
Statistical analyses
The sample size was calculated based on the results of our preliminary experiments, in which ADAS-Cog scores improved after 30 sessions of tACS intervention (the changes of the scores in tACS group vs. control group: mean ± SD: -3.12 ± 2.35 vs. -0.67 ± 2.13). To allow for a maximum dropout rate of 20%, we aimed to recruit and randomize 40 participants to provide 83.9% power to obtain a statistically significant group difference with a two-sided α level of 0.05.
Data were analyzed according to intention-to-treat principles. SAS 9.4 statistical software (SAS Institute, license number: 11202165) was utilized for the analysis. Independent sample t-tests for continuous data and chi-square tests for dichotomous variables (Fisher’s exact tests, if needed) were used to explore the baseline characteristics of our participants. For the neuropsychological assessment data, we first performed a Shapiro-Wilk test to check for normality. For data that followed a normal distribution, we used a mixed-effects model for analysis. Group, time, and group*time interactions were fixed effects, with intercept as the random term. We utilized two distinct linear mixed-effects models. The first model evaluated changes from baseline to the end of the intervention, while the second examined the differences between baseline and the 3-month follow-up. For data that did not follow a normal distribution, we used the Mann-Whitney U non-parametric test on the pre-post intervention difference.
In EEG analysis, the changes of gamma power after intervention in each group were tested by one-sample t-tests. The group comparison between sham and tACS groups in the changes of theta-gamma coupling were tested by two-sample independent t-tests. The correlation between the changes of theta-gamma coupling and the changes of MMSE and delayed recall scores was evaluated using Pearson’s r. In fMRI analysis, the changes of fALFF and the association between BOLD signal and gamma power fluctuation after intervention were tested by one-sample t-tests.
Results
Participants and the changes of cognitive functions
Between September 12, 2019, and December 27, 2021, 112 individuals were screened for inclusion; 66 were excluded (Fig. 2). Table 2 displays the baseline characteristics of participants.
Table 3 presents the changes in outcome measure scores. Analysis of the primary outcome showed that there was no statistically significant improvement in the ADAS-Cog score with tACS at the completion of treatment (group*time, p = 0.449, mixed-effect models) or at 3-month follow-up (group*time, p = 0.739, mixed-effect models). Among the secondary outcomes, compared to the control group, the intervention group showed significant improvement in MMSE (group*time, p = 0.041) and MoCA scores (non-parametric test, p = 0.025) (Table 3). However, this effect was not sustained at the 3-month follow-up. No statistically significant positive results were observed for other neuropsychological tests.
EEG analysis
For EEG analysis, channels were clustered into left frontal, right frontal, left posterior, and right posterior channels (Fig. 1a left). There was significant increase of gamma power (30–50 Hz) in left frontal (t = 2.37, p = 0.032, t-tests), left posterior (t = 2.68, p = 0.017, t-tests), and right posterior (t = 2.13, p = 0.049, t-tests) regions in the tACS group (Fig. 1b). However, those effects did not reach significance after false discovery rate (FDR) correction. No change of gamma power was observed in the sham group (ps > 0.05, t-tests). There were also no significant changes in gamma power from baseline to 3-month follow-up in either group (all ps > 0.05; t-tests, Figure S2).
In the left hippocampus, coupling between 5 and 6 Hz phase and 30–42 Hz amplitude increased statistically significantly from baseline to the end of intervention by comparing the tACS group and the sham group (two-sample t test, ps < 0.05; Fig. 3a). Within this range, we found a statistically significant correlation between the change of theta-gamma coupling in the left hippocampus and the change of MMSE (5.5 –40.5 Hz: r = 0.34, p = 0.049; 5.5 –41.5 Hz: r = 0.37, p = 0.029; Pearson’s r, Fig. 3b left). There was no statistically significant change in theta-gamma coupling in the right hippocampus. However, we found a statistically significant correlation between post-intervention theta-gamma coupling in the right hippocampus and post-intervention delayed recall score in the tACS group (6.5–30 Hz: r = 0.51, p = 0.044; Pearson’s r, Fig. 3b right), but not the in the sham group (6.5–30 Hz: r = -0.16, p = 0.519, Pearson’s r). This correlation was not observed in the baseline session of the tACS group (6.5–30 Hz: r = 0.20, p = 0.462, Pearson’s r). No statistically significant difference in theta-gamma coupling changes was observed between the intervention group and the control group in the hippocampus from baseline to the 3-months follow-up session (all ps > 0.05; Figure S3, t-tests).
EEG source signals in the hippocampus. Due to data quality issue, only a part of the participants was included in the EEG analysis (n = 34). (a) The changes of theta-gamma coupling from baseline to the end of the intervention were contrasted between the tACS group (n = 16) and the sham group (n = 18). The results are displayed for the left and right hippocampus within the range of theta phase (4–8 Hz) and gamma power (30–50 Hz). (b) A statistically significant correlation was observed between the change of theta-gamma coupling at 5.5–41.5 Hz and the change of MMSE (left, n = 34). There was also a statistically significant correlation between the theta-gamma coupling at 6.5–30 Hz and the delayed recall score in the tACS group (n = 16) at the end of intervention (right)
TGC, theta-gamma coupling
fMRI analysis
We calculated fALFF, based on BOLD signal in the left and right hippocampus of the tACS group. Each side of the hippocampus was split into head, body and tail. We found a statistically significant increase of fALFF after intervention in the right hippocampus head (t = 2.20, p = 0.039; t-tests, Fig. 4a). We also regressed BOLD signals in the hippocampus by gamma power fluctuation in the channels with most statistically significant increase of gamma power (F3, F7, PO3, PO7, PO8). We found increased association between BOLD signals in the left and right hippocampus tails and gamma power fluctuation in PO8 from baseline to the end of intervention in the tACS group (left hippocampus tail: t = 2.14, p = 0.049; right hippocampus tail: t = 2.56, p = 0.022; Fig. 4b). No statistically significant differences in fMRI results were seen in the hippocampus from the baseline to 3-month follow-up (Figure S4).
The changes of fMRI outcomes. (a) The changes of fALFF in the left and right hippocampus in the tACS group. (b) The changes of coefficients derived from regression of hippocampus BOLD signals by gamma power fluctuation in PO8 channel in the tACS group. fALFF, fractional amplitude of low-frequency fluctuation
* p < 0.05
Adverse events
All participants underwent intervention while hospitalized, allowing for continuous monitoring by doctors for adverse reactions. This arrangement also allowed participants to promptly report any concerns to doctors. To ensure that no potential side effects went unnoticed, we administered a questionnaire following every five sessions. This helped in tracking side effects and acted as a reminder for patients to be alert to any symptoms. During the course of the study, one patient in the tACS group reported a tingling sensation during the first two sessions, while another in the control group reported transient itching at the first session. No serious adverse events were observed in either group.
Discussion
Earlier studies have shown cognitive improvement following single or multi-session 40 Hz tACS in patients with AD [9]. Our study failed to show effects of 40 Hz tACS on the primary outcome of global cognition as measured by ADAS-Cog. However, effects on the secondary outcome measure of delayed recall supports the hypothesis that tACS may improve more specific cognitive functions, including episodic memory, that can be confirmed in subsequent studies. We found that tACS enhanced theta-gamma coupling in the hippocampus of patients with AD. This increase in gamma oscillation synchrony in the hippocampus was correlated with the improvement of MMSE and delayed recall. Our study also showed that tACS increased local neural activity in the hippocampus as measured by low frequency fluctuations in fMRI, providing additional evidence for effects of tACS on the hippocampus.
tACS has been shown to induce whole-brain effects, with studies suggesting the entrainment of neurons across widespread cortical regions [41]. This implies that even weak stimulation could induce large-scale modulation of neural activity via network resonance [10, 41]. Within our study, using computational modelling, we demonstrated that electric stimulation fields reached widespread brain regions. We also found gamma power was increased in multiple regions, including frontal and parietal-occipital lobes, not limited to the stimulation areas. Thus, tACS is capable of producing changes in whole-brain activity in people with AD. In this study, we did not detect statistically significant improvements in the ADAS-Cog, however we did find statistically significant improvement in MMSE and MoCA score. Although we had a priori power calculation and reached the calculated sample size, it is still possible that the small sample size used in the trial may have obscured benefits that would have been demonstrable with the several hundreds of participants currently used to detect modest benefits in AD treatment trials. Our data indicating good safety and acceptability, together with positive results for some outcomes and consistent change in neurophysiological measures, would support the utility of launching a phase 3 trial, provided it is adequately powered to detect small treatment benefits on overall cognitive functioning and activities of daily living. We believe that there were several factors influencing the response to tACS intervention. For instance, in a previous study, ApoE genotype and baseline cognitive impairment were identified as predictors of response to tACS [42]. We plan to conduct a subgroup analysis in our subsequent study to further identify such predictors of response.
Among secondary outcomes, we found that memory function improved statistically significantly after the intervention. Two previous randomized controlled studies have shown that tACS can improve memory function by stimulating the Pz and precunus, both of which are key nodes in the default model network (DMN) [42, 43]. In addition to using the Rey Auditory Verbal Learning (RAVL) to assess episodic memory, these two studies also used the Face-Naming Association memory task (FNAT) to assess associative memory, where performance has been suggested to depend primarily on the hippocampus. Although these studies did not directly measure neural activity in the hippocampus, they speculated that the improvement in the FNAT after tACS is due to the functional connection between the DMN network and the hippocampus. Thus, although we had aimed to use an ADAS-Cog as an outcome that was comparable with other clinical trials, in future studies we would recommend using a cognitive outcome that is closer to the assumed mechanism of action of the intervention, i.e. memory consolidation. Here, indeed we found a statistically significant effect of tACS on delayed recall, underscoring a potential mechanistic effect of tACS on memory.
Unlike superficial brain areas such as the Pz and precuneus, the hippocampus is challenging to stimulate. Current studies on the effects of tACS on hippocampal neuron activity in AD are still insufficient, although it should be mentioned that one study suggested that tACS stimulation of the temporal lobe region increased blood flow in the hippocampus in AD [10], and a recently published study showed that 40 Hz tACS increased the functional connectivity between hippocampus and inferior parietal lobe [44]. Our prior study using the same tACS device suggested greater than 7 mA tACS were required to produce significant LEP in the hippocampus and a 15 mA electrical stimulation for one hour with this device is safe and did not induce seizure activity [15]. Our study initially used computational modelling to show that electrical current can reach the hippocampus. Then, through EEG-fMRI regression, we demonstrated an increased association between neural activity in the hippocampus and gamma power fluctuation in the intervention group. This suggested that the increased gamma power recorded from scalp channels was at least partly due to enhanced activity of the hippocampus. We analyzed neural activity in the hippocampus using EEG source localization for signals in the hippocampus and found that theta-gamma coupling was enhanced in the hippocampus, which correlated with MMSE improvement and scores for delayed recall. fMRI analyses further showed an increase in local hippocampal BOLD signals. These results indicate that tACS can reach and influence the hippocampus, modulating neuronal activity in this region. In the entorhinal-hippocampal system, theta-gamma coupling is observed specifically in the slow-gamma frequency [45]. As the difficulty of cognitive task is increased, theta-slow gamma coupling is augmented, suggesting that theta-gamma coupling reflects a compensatory mechanism to maintain memory function [46]. Our study suggests that the improvements in cognitive function of patients with AD after tACS are associated with effects on theta-gamma coupling in the hippocampus.
The hippocampus is a critical brain region for cognitive function, particularly episodic memory. However, current researches on the lateralization of hippocampal function remain inconclusive. Regarding verbal memory function, some studies suggest that the left hippocampus is more relevant [47, 48], while others indicate that the volume of the right hippocampus is associated with delayed verbal recall [49]. Our study found that delayed verbal recall improved after intervention and was associated with an increase in theta-gamma coupling in the right hippocampus after intervention, along with an increase in fALFF values in the right hippocampus. The different findings in the left and right hippocampi in our study may be related to the lateralization of hippocampal functions, but it could also be due to the small sample size, leading to less comprehensive results. Therefore, our exploratory study suggests that tACS can alter neuronal activity in the hippocampus, but whether the intervention effects are lateralized requires further research for verification. Additionally, regarding the subregion analysis of the hippocampus, we found significant increase of fALFF in right hippocampus head after intervention. According to previous studies, the anterior hippocampus is involved in the encoding of episodic memory, while the posterior hippocampus participates in the decoding of episodic memory [50, 51]. We suspected that the tACS intervention might enhance memory encoding by activating the hippocampus head. On the other hand, the association between BOLD signals and gamma power fluctuation increased after intervention in the tail of both left and right hippocampus. In other words, gamma-band activity account for more brain activation in the hippocampus tail, which might increase the efficiency of memory retrieval.
Any improvements in cognitive function and changes in hippocampal neuronal activity that we observed post-intervention were not evident at the 3-month follow-up. A recent systematic review of tACS for MCI and dementia [9] indicated that most tACS interventions are single-session, suggesting that tACS can exhibit rapid effects. Published studies suggest that repeated stimulation increases gamma power, but this diminishes upon cessation of stimulation [12]. One study examined the long-term effects of tACS and found that 30 sessions improved MMSE and ADAS-Cog scores, but the ADAS-COG scores returned to baseline at the 12-week follow-up [11].
Limitations
We acknowledge a number of study limitations. The sample size was relatively small. And although we found that the effects of stimulation could reach and affect the hippocampus, we are still not sure if the stimulation locations are optimal for treating AD. Another point to note is that, for safety consideration, the participants we enrolled are relatively younger compared to those in other studies [10, 42, 43]. Currently, there is a lack of research on the effectiveness of tACS intervention in AD across different age groups. However, some studies have explored the impact of age on responsiveness to non-invasive stimulation paradigms delivered to the motor cortex, including tACS [52,53,54]. These studies suggested that increasing age may reduce responsiveness to stimulation. However, the considerable heterogeneity in the findings makes it difficult to draw definitive conclusions. Therefore, further research is needed to analyze the impact of age on the responsiveness of AD patients to tACS interventions. In the present study, some of the EEG-fMRI results did not pass correction for multiple comparisons. Furthermore, we did not identify a substantial group difference between the treatment group and the sham group for the change in gamma power. These null results may be due to the relatively small sample size, which prevented sufficient statistical power. Therefore, we consider our results to be exploratory and preliminary. We hope that our study can provide a foundation for future larger studies to validate our results. Lastly, at the 3-month follow-up, there were 2 vs. 5 participants missing, which may impact the results of the 3-month evaluation. The lasting effects of tACS on AD need further clarification in subsequent studies.
Conclusion
In conclusion, multi-session 40 Hz tACS over the forehead and two mastoid regions is safe and well tolerated and has potential to improve general cognitive function and delayed memory in mild AD. Our results suggest that enhancement of theta-gamma coupling and neural activity of the hippocampus is a potential therapeutic mechanism of tACS.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Fernandez-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G et al. Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 2021, 372(6537).
Wang DX, Schmitt K, Seger S, Davila CE, Lega BC. Cross-regional phase amplitude coupling supports the encoding of episodic memories. Hippocampus. 2021;31(5):481–92.
Lega B, Burke J, Jacobs J, Kahana MJ. Slow-Theta-to-Gamma Phase-Amplitude Coupling in Human Hippocampus supports the formation of New Episodic Memories. Cereb Cortex. 2016;26(1):268–78.
Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot JG, Quirion R, et al. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Abeta overproduction in a mouse model of Alzheimer’s disease. Eur J Neurosci. 2013;37(12):1896–902.
Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2005;26(2):165–71.
Stam CJ, van Cappellen AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer’s Disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19(6):562–74.
Mehak SF, Shivakumar AB, Kumari S, Muralidharan B, Gangadharan G. Theta and gamma oscillatory dynamics in mouse models of Alzheimer’s disease: a path to prospective therapeutic intervention. Neurosci Biobehav Rev. 2022;136:104628.
Grover S, Fayzullina R, Bullard BM, Levina V, Reinhart RMG. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci Transl Med. 2023;15(697):eabo2044.
Manippa V, Palmisano A, Nitsche MA, Filardi M, Vilella D, Logroscino G et al. Cognitive and neuropathophysiological outcomes of Gamma-tACS in Dementia: a systematic review. Neuropsychol Rev 2023.
Sprugnoli G, Munsch F, Cappon D, Paciorek R, Macone J, Connor A, et al. Impact of multisession 40Hz tACS on hippocampal perfusion in patients with Alzheimer’s disease. Alzheimers Res Ther. 2021;13(1):203.
Zhou D, Li A, Li X, Zhuang W, Liang Y, Zheng CY, et al. Effects of 40 hz transcranial alternating current stimulation (tACS) on cognitive functions of patients with Alzheimer’s disease: a randomised, double-blind, sham-controlled clinical trial. J Neurol Neurosurg Psychiatry. 2022;93(5):568–70.
Dhaynaut M, Sprugnoli G, Cappon D, Macone J, Sanchez JS, Normandin MD, et al. Impact of 40 hz transcranial Alternating Current Stimulation on Cerebral Tau Burden in patients with Alzheimer’s Disease: a Case Series. J Alzheimers Dis. 2022;85(4):1667–76.
Louviot S, Tyvaert L, Maillard LG, Colnat-Coulbois S, Dmochowski J, Koessler L. Transcranial Electrical Stimulation generates electric fields in deep human brain structures. Brain Stimul. 2022;15(1):1–12.
Krause MR, Vieira PG, Csorba BA, Pilly PK, Pack CC. Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc Natl Acad Sci U S A. 2019;116(12):5747–55.
Shan Y, Wang H, Yang Y, Wang J, Zhao W, Huang Y et al. Evidence of a large current of transcranial alternating current stimulation directly to deep brain regions. Mol Psychiatry 2023.
Xing Y, Wei P, Wang C, Shan Y, Yu Y, Qiao Y, et al. TRanscranial AlterNating current stimulation FOR patients with mild Alzheimer’s Disease (TRANSFORM-AD study): protocol for a randomized controlled clinical trial. Alzheimers Dement (N Y). 2020;6(1):e12005.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72.
Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62.
Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11 C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–54.
Lei D, Mao C, Li J, Huang X, Sha L, Liu C, et al. CSF biomarkers for early-onset Alzheimer’s disease in Chinese population from PUMCH dementia cohort. Front Neurol. 2022;13:1030019.
Wang H, Wang K, Xue Q, Peng M, Yin L, Gu X, et al. Transcranial alternating current stimulation for treating depression: a randomized controlled trial. Brain. 2022;145(1):83–91.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–64.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Maj M, D’Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch Clin Neuropsychol. 1993;8(2):123–35.
Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–14.
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49.
Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011;2011:879716.
Gramfort A, Papadopoulo T, Olivi E, Clerc M. OpenMEEG: opensource software for quasistatic bioelectromagnetics. Biomed Eng Online. 2010;9:45.
Kybic J, Clerc M, Abboud T, Faugeras O, Keriven R, Papadopoulo T. A common formalism for the integral formulations of the forward EEG problem. IEEE Trans Med Imaging. 2005;24(1):12–28.
Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic Stat Parametric Mapp Neuron. 2000;26(1):55–67.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–80.
Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–8.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41.
Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A. 2009;106(28):11794–9.
Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13(1):164–74.
Zarei M, Beckmann CF, Binnewijzend MA, Schoonheim MM, Oghabian MA, Sanz-Arigita EJ, et al. Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer’s disease. NeuroImage. 2013;66:28–35.
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–37.
Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–41.
Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, et al. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30(34):11476–85.
Benussi A, Cantoni V, Grassi M, Brechet L, Michel CM, Datta A, et al. Increasing Brain Gamma Activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s Disease. Ann Neurol. 2022;92(2):322–34.
Benussi A, Cantoni V, Cotelli MS, Cotelli M, Brattini C, Datta A, et al. Exposure to gamma tACS in Alzheimer’s disease: a randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul. 2021;14(3):531–40.
Jones KT, Gallen CL, Ostrand AE, Rojas JC, Wais P, Rini J, et al. Gamma neuromodulation improves episodic memory and its associated network in amnestic mild cognitive impairment: a pilot study. Neurobiol Aging. 2023;129:72–88.
Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510(7503):143–7.
Tamura M, Spellman TJ, Rosen AM, Gogos JA, Gordon JA. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun. 2017;8(1):2182.
Aslaksen PM, Bystad MK, Orbo MC, Vangberg TR. The relation of hippocampal subfield volumes to verbal episodic memory measured by the California Verbal Learning Test II in healthy adults. Behav Brain Res. 2018;351:131–7.
Ezzati A, Katz MJ, Zammit AR, Lipton ML, Zimmerman ME, Sliwinski MJ, et al. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia. 2016;93(Pt B):380–5.
Aumont E, Bussy A, Bedard MA, Bezgin G, Therriault J, Savard M, et al. Hippocampal subfield associations with memory depend on stimulus modality and retrieval mode. Brain Commun. 2023;5(6):fcad309.
Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17(11):1071–80.
Kim H. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: the HERNET model. Hippocampus. 2015;25(4):500–10.
Shah M, Suresh S, Paddick J, Mellow ML, Rees A, Berryman C, et al. Age-related changes in responsiveness to non-invasive brain stimulation neuroplasticity paradigms: a systematic review with meta-analysis. Clin Neurophysiol. 2024;162:53–67.
Fresnoza S, Christova M, Feil T, Gallasch E, Korner C, Zimmer U, et al. The effects of transcranial alternating current stimulation (tACS) at individual alpha peak frequency (iAPF) on motor cortex excitability in young and elderly adults. Exp Brain Res. 2018;236(10):2573–88.
Guerra A, Asci F, Zampogna A, D’Onofrio V, Berardelli A, Suppa A. The effect of gamma oscillations in boosting primary motor cortex plasticity is greater in young than older adults. Clin Neurophysiol. 2021;132(6):1358–66.
Acknowledgements
The authors would like to thank the participants in this trial and their families and caregivers.
Funding
This project was funded by National Natural Science Foundation of China (82220108009, 81970996, 82371199, 62271331, 62171300, 62301343), National Key Research and Development Program of China (2022YFC3602600, 2023YFC3603203), and STI2030-Major Projects (2021ZD0201801).
Author information
Authors and Affiliations
Contributions
YT, JL, CLL and GGZ designed and administrated this study, and also reviewed the writing. All authors participated in data interpretation. YX, WZ, BJX, XRS, YSY, TW, YXM, and YCQ did the literature search and data collection. LWS, RJT, ZBW, CMW, SZY, PHW, YFY and YSZ made the collection and analysis of EEG and neuroimaging data. YX and KY was responsible for data analysis of cognitive scores. YX and LWS drafted the paper. XZ, JPJ, SJT and RH made key revisions of the paper. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was received from Xuanwu Hospital, Capital Medical University (2018-077). The informed consent from all participants was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tang, Y., Xing, Y., Sun, L. et al. TRanscranial AlterNating current stimulation FOR patients with mild Alzheimer’s Disease (TRANSFORM-AD): a randomized controlled clinical trial. Alz Res Therapy 16, 203 (2024). https://doi.org/10.1186/s13195-024-01570-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01570-0