Abstract
Background
Pyogenic Liver Abscesses (PLA) are the most common type of visceral abscess. They generally develop in a context of biliary disease or hematogenous seeding, but a complete diagnostic work-up is always required in order not to miss other important causes, including above all malignancies of the gastro-intestinal tract.
Case presentation
Herein, we report a particular case of a 80 years-old immunocompetent woman hospitalized for sepsis. At the end of the diagnostic process, Streptococcus constellatus (Sc) was identified as the cause of sepsis, multiple PLA were found together with a previous unknown ileal malignancy. We speculated about a possible correlation among these three entities (i.e. sepsis from Sc, PLA and tumors).
Conclusions
Detection of Sc in blood should raise red flags in clinicians as aggressive clinical presentation are possible.
Similar content being viewed by others
Background
Sc is usually part of the normal flora of mouth and gastro-intestinal tract, but aggressive presentations are possible. They mainly consist in abscesses formation and this can occur in association with malignancy [1].
Case presentation
An 80-year-old Caucasian woman, with a medical history of hypertensive heart disease, reached the emergency room (ER) because of a fever lasting 3 days occurring after dental treatment, and no other relevant symptoms.
She also reported two hospitalizations over the previous year: one for anaemia and unexplained deep vein thrombosis of the right leg; the other one for bowel sub-occlusion. During these previous admissions, an abdominal CT scan detected the presence of a jejunal mass, described as a mixed (solid-liquid) roundish lesion (54 × 48 mm). The surgeon did not prescribe any additional diagnostic investigation of the mass.
At the admission to the ER, the patient was febrile (38,5 °C). No remarkable signs were observable at physical examination and chest X-ray was negative. Blood test showed leucocytosis (23.6 × 109/L) with 84.4% neutrophils and increase of both CRP (88.2 mg/L, normal values – nv − 0.0–5.0) and procalcitonin (41.2 ng/ml, nv < 0.5). After blood sample was drawn for blood cultures, an empirical antibiotic therapy with piperacillin-tazobactam was initiated.
Thereafter, the patient was transferred to our Internal Medicine Unit.
On the 3rd day an hypoxemic respiratory failure occurred, despite no inflammation nor infective process was observable on CT scan. It was interpreted as the first manifestation of a probable incipient Multiple Organ Dysfunction Syndrome (MODS). Blood tests only slightly improved (14.3 × 109/L leucocytes with 76% neutrophils, CRP 111.9 mg/L, and procalcitonin 1.8 ng/mL).
On day 6th blood cultures showed the growth of Sc.
Strains of Sc were identified in the blood by matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry (MALDI-TOF) using the VITEK® MS system (bioMérieux).
The pathogen showed the following antibiotic resistance profile:
Sensitivity to piperacillin-tazobactam was not part of the routine test of our Laboratory. After the lack of a significant improvement in clinical or laboratory parameters, teicoplanin was added to therapy and, after four days of treatment, produced a relevant benefit on respiratory signs and oxygen supplementation demand, as well as on laboratory test (further decrease of WBC to 11.3 × 109/L leucocytes with 67% neutrophils, CRP 17.5 mg/L, and normalization of procalcitonin).
Meanwhile, we searched for septic foci. Urine culture was negative. A CT scan of facial bones did not show any periodontal inflammation or pulpal infection, ruling out the oral cavity as the source of the bacteremia. Unfortunately, oral cavity swabbing was not performed, thus precluding the possibility to ascertain whether the same Sc was also part of the “normal flora” of our patient.
Echocardiography excluded an infective endocarditis. An abdominal US revealed the presence of multiple hepatic lesions (Fig. 1).
Abdominal US of the liver showing, in SVIII, a roundish, poorly demarcated lesion of 54.8 × 42.2 mm in size. It shows a dishomogeneous appearance as it mainly consists of a hypoechoic area, suggestive for colliquation, with some internal echoes. The differential diagnosis of a focal liver lesion like this is wide, including primitive or metastatic lesions, and clinical correlation is essential to guide the diagnostic process. Second-line methods such as contrast-enhanced ultrasonography (CEUS), computed tomography (CT) or magnetic resonance (MR) are imperative in these cases and help to correctly determine the nature of these lesions
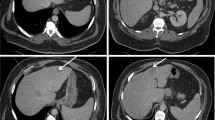
These lesions appeared as three multiloculated cystic lesions surrounded by pseudocapsules at contrast-enhanced CT scan, suggesting the diagnosis of pyogenic liver abscesses; they were in SIII-IV (4 cm), SV (4 cm) and SV-VIII (8 cm) (Fig. 2).
Though the significant peripheral arterialization indicated they were “pure” abscesses, we also considered whether they hid underlying hepatic metastatic lesions eventually infected by Sc. Therefore, we looked for possible primary neoplastic lesion.
Contrast-enhanced abdominal CT showing the presence, in the liver, of three roundish lesions (SIII-SIV, SV and SV-SVIII) with a cystic morphology. They appear as peripherally enhancing e centrally hypoattenuating lesions; we can also see they are surrounded by an inner ring and they are provided of multiple septa. Due to the peculiar pattern of enhancement, these lesions were considered to be consistent primarily with pyogenic liver abscesses
Breast examination did not reveal the presence of a palpable mass, and the CT done on day 3rd failed to identify any potential previous unknown thoracic cancer.
A total colonscopy showed a normal mucosa.
Lastly, we focused on the jejunal mass detected at the previous hospitalization. It was no longer observable as a roundish lesion at CT scan; now it appeared as an eccentric thickening (20 × 10 mm) of the same intestinal loop with some inner cystic areas. Whether the lesion was an abscessed intestinal loop, or a group of inflammed diverticula, or the less common Meckel’s diverticulum, the patient underwent a surgical excision of the jejunal mass. Pathology identified the lesion as a Gastrointestinal Stromal Tumour (GIST). Due to its location, size (30 mm) and mitotic index (25/20 HPF), it was considered as high-risk lesion and a chemotherapy with imatinib was then prescribed.
Discussion and conclusions
The S. anginosus group (SGA) consists of three species of Gram positive, catalase negative cocci, named S. anginosus (Sa), S. intermedius (Si) and S. constellatus (Sc). They are nonmotile, facultative anaerobes which can exhibit different haemolytic patterns [2].
Although in general they are part of normal oral and gastrointestinal (GI) flora [3], they can acquire the capacity to give bacteremia and form abscesses. This could be due to the production of both enzymes such as hyaluronidase, which drives the haematogenous spreading in the organism, and of pyrogenic exotoxins such as intermedilysin, which could play a role in the abscesses formation [4].
Sc has been increasingly reported as potential determinant of suppurative infections in a variety of sites [5,6,7] which seem to be related to the primary location of the bacteria and the route of migration. So, liver abscesses are often associated to pathological processes of GI tract (according to venous drainage of this area), which, however, are not necessarily of malignant nature [8, 9]. Blood transmission could also explain other aggressive manifestations related to Sc, for example intracranial infectious aneurysms following a pulpitis caused by Sc, which was later responsible for an infection in the cavernous sinus [10].
Infection of a metastatic lesion also represent a cause of liver abscesses. Though we ruled out the presence of most common primary colon cancer, eventually we diagnosed a GIST in the jejunum, which may have represented the source of Sc bacteremia and liver abscesses.
Only few cases of such an association - Sc bacteremia, PLA and GIST - have been reported in the literature [11,12,13,14,15].
More frequently, literature highlighted a relation between SGA and other digestive tract tumours, as esophageal [16], gastric [17, 18] and colorectal cancer [19,20,21,22], these last associated to PLA.
In particular, Sasaki H et al. [16, 18] reported cloning of Sa DNA fragments from surgical specimens of esophageal and gastric cancers, but not from lung, cervix and kidney ones. Zhou CB et al. [17] found an association between Sc as well as Sa and gastric cancer and suggested that fecal Sc and Sa (detected using quantitative PCR) may represent non-invasive accurate signatures for precancerous and early stages of this kind of tumour.
The relationship between Sc, PLA and GIST as well as SGA, PLA and colorectal cancer seems similar to the well-described association between these two last entities and Streptococcus bovis [23] or – as more recent studies have suggested – Klebsiella pneumoniae [24] and even other members of the intestinal microbiota. Some authors consider such relations not causative. They think neoplastic growth can disrupt the integrity of gastro-intestinal barrier, creating a pathway for bacterial translocation and systemic spreading [24].
At the end of our diagnostic work-up, we found a correlation between Sc, invasive infections and the presence of a malignancy. Although not yet validate in literature, this relationship should prompt physicians to screen for upper and lower GI neoplasms in patients presenting with sepsis or pyogenic infections caused by Sc, as it could facilitate an appropriate and timely treatment.
Further investigation is needed in the future in order to better understand a possible etiopathogenetic role of Sc in carcinogenic process or as a potential marker of a GI tumor.
Data Availability
Not applicable.
Abbreviations
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- ER:
-
Emergency Room
- GI:
-
Gastro-intestinal
- GIST:
-
Gastro-intestinal stromal tumor
- PLA:
-
Pyogenic Liver Abscess
- Sc:
-
Streptococcus constellatus
- SGA:
-
Streptococcus anginosus group
- US:
-
Ultrasound
References
Whiley RA, Beighton D, Winstanley TG, Fraser HY, Hardie JM, Streptococcus intermedius. S treptococcus constellatus and Streptococcus anginosus (the Streptococcus mileri group): association with different body sites and clinical infections. J Clin Microbiol 1992; 30: 243-4.
Ruoff KL. Streptococcus anginosus (Streptococcus milleri): the unrecognized pathogen. Clin Microbiol Rev. 1988;1:102–8.
Mejaro B, Edwardsson S. Streptococcus milleri (Guthof); an indigenous organism of the human oral cavity. Arch Oral Biol. 1975;20:757.
Nagamune H, Whiley RA, Goto T, Inai Y, Maeda T, Hardie JM, Kourai H. Distribution of the intermedilysin gene among the anginosus group Streptococci and correlation between intermedilysin production and deep-seated Infection with Streptococcus intermedius. J Clin Microbiol. 2000;38:220.
Clarridge JE, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (Streptococcus Milleri Group) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32(10):1511–5.
Brunink EM, Hoogervorst LA, Steentjes K, et al. Osteomyelitis caused by Streptococcus intermedius in immunocompetent adults — a case report and systematic literature review. Eur J Clin Microbiol Infect Dis. 2023;42:1055–61. https://doi.org/10.1007/s10096-023-04640-7
Chamat-Hedemand S, Dahl A, Hassager C, et al. Streptococcal infective endocarditis: clinical features and outcomes according to species. Infection. 2023;51:869–79. https://doi.org/10.1007/s15010-022-01929-1
Akuzawa N, Hatori T, Kitahara Y, Kurabayashi M. Multiple liver abscesses and bacteremia caused by Streptococcus constellatus infection: a case report. Clin Case Rep. 2017;5(1):69–74.
Rossi G, Nguyen Y, Lafont E, et al. Large retrospective study analysing predictive factors of primary treatment failure, recurrence and death in pyogenic liver abscesses. Infection. 2022;50:1205–15.
Zhang L, Liu P, Li Y, et al. Rapid development of an intracranial infectious aneurysm secondary to Streptococcus constellatus infection. Intensive Care Med. 2023;49:593–4.
Matsutani T, Onda M, Miyashita M, Hao K, Yokoyama S, et al. Liver abscesses associated with stromal tumour of the stomach in a young woman. Eur J Gastroenterol Hepatol. 2001;13:1485–148.
Kim BH, Lee JH, Baik du S, Yun SW, Kim JH, et al. A case of malignant gastrointestinal stromal tumor of ileum with liver abscess. Korean J Gastroenterol. 2007;50:393–7.
Kumar A, Artifon E, Siegel C, Halwan B. Gastrointestinal stromal tumor in association with pyogenic liver abscess. Gastroenterol Hepatol. 2008;4:818–9.
Kwon Y, Dang ND, Elmunzer BJ. Gastrointestinal stromal tumor complicated by Streptococcus milleri bacteremia and liver abscess. Eur J Gastroenterol Hepatol. 2009;21(7):824–6.
Gorelik M, Sabates B, Elkbuli A, Dunne T. Ileal GIST presenting with bacteremia and liver abscess: a case report and review of literature. Int J Surg Case Rep. 2018;42:261–5. https://doi.org/10.1016/j.ijscr.2017.12.033. Epub 2017 Dec 27. PMID: 29324374; PMCID: PMC5985260.
Sasaki H, Ishizuka T, Muto M, Nezu M, Nakanishi Y, et al. Presence of Streptococcus anginosus DNA in esophageal cancer, dysplasia of esophagus, and gastric cancer. Cancer Res. 1998;58(14):2991–5.
Zhou CB, Pan SY, Jin P, Deng JW, Xue JH, Ma XY, et al. Fecal signatures of Streptococcus anginosus and Streptococcus constellatus for noninvasive screening and early warning of gastric cancer. Gastroenterology. 2022;162(7):1933–47.
Sasaki H, Hiroyasu I, Ishizuka T, Kogoma Y, Sugimura T, Terada M. Presence of Streptococcus DNA sequence in surgical specimens of gastric cancer. Jpn J Cancer Res. 1995;86:791–4.
Rawla P, Vellipuram AR, Bandaru SS, Raj JP. Colon carcinoma presenting as Streptococcus anginosus bacteremia and liver abscess. Gastroenterol Res. 2017;10(6):376–9.
Millichap JJ, McKendrick AI, Drelichman VS. Streptococcus intermedius liver abscesses and colon cancer: a case report. West Indian Med J. 2005;54(5):341–2.
Masood U, Sharma A, Lowe D, Khan R, Manocha D. Colorectal cancer associated with Streptococcus anginosus bacteremia and liver abscesses. Gastroenterology. 2016;10:769–74.
Tzur T, Liberman S, Felzenstein I, Cohen R, Rivkind AI, Almogy G. Liver abscesses caused by Streptococcus milleri: an uncommon presenting sign of silent colonic cancer. Isr Med Assoc J. 2003;5(3):206–7.
Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17:2970.
Kao WY, Hwang CY, Chang YT, et al. Cancer risk in patients with pyogenic liver abscess: a nationwide cohort study. Aliment Pharmacol Ther. 2012;36:467.
Acknowledgements
S. Chessa, E. Belfiori and G. Mandis gratefully acknowledge the Autonomous Region of Sardinia (RAS) for the financial support of their specialist medical training in Internal Medicine.
Funding
The authors did not receive support from any organization for the submitted work. The authors have no relevant financial or non-financial interests to disclose that are relevant to the content of this article.
Author information
Authors and Affiliations
Contributions
Cared for the patient, extracted the clinical and lab data: S.C., E.B., G.M., E.U., G.M., A.S.Drafting of manuscript: S.C., E.B., G.M.Critical revisions: A.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Patient provided informed consent. The patient agreed her data – properly anonymized – to be used for scientific purposes.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chessa, S., Belfiori, E., Mandis, G. et al. Bacteremia from streptococcus constellatus revealing a gastrointestinal stromal tumor. Gut Pathog 16, 1 (2024). https://doi.org/10.1186/s13099-023-00593-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-023-00593-6