Abstract
Background
Cardiometabolic multi-morbidity (CMM) is emerging as a global healthcare challenge and a pressing public health concern worldwide. Previous studies have principally focused on identifying risk factors for individual cardiometabolic diseases, but reliable predictors of CMM have not been identified. In the present study, we aimed to characterize the relationship of triglyceride-glucose (TyG) index with the incidence of CMM.
Methods
We enrolled 7,970 participants from the China Health and Retirement Longitudinal Study (CHARLS) and placed them into groups according to quartile of TyG index. The endpoint of interest was CMM, defined as the presence of at least two of the following: stroke, heart disease, and diabetes mellitus. Cox regression models and multivariable-adjusted restricted cubic spline (RCS) curves were used to evaluate the relationship between TyG index and CMM.
Results
In total, 638 (8.01%) incident cases of CMM were recorded among the participants who did not have CMM at baseline (2011) during a median follow-up of 84 months (interquartile range, 20‒87 months). The incidences of CMM for the participants in quartiles (Q) 1–4 of TyG index were 4.22%, 6.12%, 8.78%, and 12.60%, respectively. A fully adjusted Cox model showed that TyG index was closely associated with the incidence of CMM: the hazard ratio (HR) [95% confidence interval (CI)] for each 1.0-unit increment in TyG index for CMM was 1.54 (1.29–1.84); and the HRs (95% CIs) for Q3 and Q4 (Q1 as reference) of the TyG index for CMM were 1.41 (1.05–1.90) and 1.61 (1.18–2.20), respectively. The association of TyG index with the incidence of CMM was present in almost all the subgroups, and persisted in the sensitivity analyses and additional analyses. Multivariable-adjusted RCS analysis revealed a significant dose-response relationship of TyG index with the risk of CMM (overall P < 0.001; non-linear P = 0.129).
Conclusions
We found that a high TyG index is associated with a higher risk of incident CMM. This finding may have significance for clinical practice and facilitate the creation of a personalized prevention strategy that involves monitoring the TyG index.
Graphical Abstract

Similar content being viewed by others
Introduction
Multi-morbidity, which is the simultaneous presence of at least two chronic diseases in an individual, has become an international health challenge and is a global health research priority [1,2,3]. It imposes substantial health and economic burdens on society and individuals, and is associated with a reduction in quality of life, a reduction in life expectancy, and an increase in healthcare expenditure [4, 5]. Cardiometabolic multi-morbidity (CMM), defined as the presence of at least two of stroke, heart disease, and diabetes mellitus (DM), which are also known as cardiometabolic diseases (CMDs) [6,7,8], is considered as one of the most serious and common multi-morbidity profiles [9]. CMM has been reported to be associated with a two-fold higher risk of all-cause mortality than an individual CMD [4, 10]. Moreover, a recent study showed that individuals with CMM are at a 1.89-times higher risk of mental stress than those with no cardiometabolic condition [11]. However, despite the increasing burden of disease and concerns about the effects of these diseases on health, previous studies have focused on individual CMDs, rather than on identifying predictors of CMM [12,13,14].
A great deal of evidence shows that lipid and glucose metabolic disorders play crucial roles in the initiation, progression, and pathogenesis of CMD [15,16,17], and insulin resistance (IR) underpins these relationships. The hyperinsulinemic-euglycemic clamp is regarded as the gold-standard method of assessing IR [18], but it is difficult to perform in clinical practice because of the expense associated and the complexity of the procedure. Fortunately, several more convenient and valid alternative measures of IR have been established, including the TyG index, which is a highly accurate method of diagnosing IR (sensitivity: 96.5%; specificity: 85.0%) [19].
Previous studies have principally focused on the relationships of TyG index with individual CMDs, such as DM [17, 20], prediabetes [21], adverse cardiovascular events [22, 23], and ischemic stroke [24]. However, to date, few studies have focused on the relationship between IR and CMM. More importantly, the evidence for CMM prevention is more concentrated in European and American populations, and there are also inconsistent results of risk factors for CMM between European, American and Asian populations [6, 7, 25]. For example, overall obesity, quantified by body mass index (BMI) is a crucial risk factor for CMM [6, 7], while abdominal obesity is superior to BMI in predicting CMM in Chinese population [25].
To address these knowledge gaps mentioned above, and considering that the huge health and economic burdens of CMM on society and individuals, we collected data from CHARLS, a prospective, nationwide and representative cohort study, and aimed to improve the current status of the primary prevention of CMM through investigating the association between IR and CMM.
Methods
Study design and participants
The study sample comprised participants in the CHARLS (data are available at http://charls.pku.edu.cn/en), which has been described in detail previously [26]. In brief, the CHARLS is a prospective, nationwide cohort study of residents in rural and urban areas of China of ≥ 45 years of age that commenced in 2011 and their spouse [27]. To prepare a representative sample, multi-stage probability sampling of participants in the baseline survey was performed. This survey took place in 2011 and was of individuals resident in 28 provinces on the Chinese mainland [27]. To date, five follow-up surveys have been completed, in 2011, 2013, 2015, 2018, and 2020, but the 2020 data have not been released to the public at the time of writing.
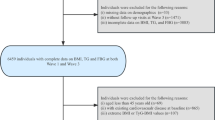
We initially screened 17,708 participants in the CHARLS and selected 7,970 participants, who were placed into four subgroups according to quartile of TyG index at baseline. The other 9,738 individuals were excluded because of missing laboratory measurements at baseline (n = 6,072), the presence of self-reported confirmed CMM (n = 446), age < 45 years or missing data regarding age (n = 422), missing data/unknown status regarding CMM, or death or loss to follow-up (n = 2,595). The enrolled participants were followed up every 1–2 years until 2018. Fig. 1 details the selection criteria.
Collection of study data and measurements made
Extensive baseline information was collected by trained interviewers in accordance with standard procedures, including their age, sex (male or female), total cholesterol (TC) concentration, alcohol consumption and smoking status (current, former and never) [28], low-density lipoprotein (LDL) concentration, and region of residence (south or north). After at least 5 min spent sitting down, the blood pressure of the participants was measured three times by a trained interviewer at 45-second intervals using a digital sphygmomanometer (Omron TM HEM-7200). We used a digital weighing scale (Omron Corporation, HN-286) and a stadiometer (Seca Corporation, 213) to measure body mass and height to within 0.1 kg and 0.1 cm, respectively, while the participants were wearing lightweight clothes and no shoes. Body mass index (kg/m2) was calculated as body mass/height2.
After an overnight fast, three tubes of venous blood were collected from the participants by professional staff, and parameters were measured according to standard procedures. Fasting blood glucose (FBG) and serum lipid parameters were measured using enzymatic colorimetric assays. Blood urea nitrogen (BUN) was measured using an enzymatic UV method involving urease. The serum creatinine and uric acid (UA) concentrations and glycosylated hemoglobin (HbA1c) were measured using the rate-blanked and compensated Jaffe creatinine method, the UA Plus method, and boronate-affinity high-performance liquid chromatography, respectively.
Definitions
North and south region were defined according to the Qinling Mountains-Huaihe River Line [28]. Hypertension was diagnosed based on a self-reported physician diagnosis (a positive response to “Have you been diagnosed with hypertension?”), and/or recent use of an antihypertensive agent (a positive response to “Are you currently taking any antihypertensive drugs to treat or control your blood pressure?”), and/or a systolic blood pressure/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg [29]. Stroke and heart disease were recorded in the presence of a self-reported physician diagnosis (a positive response to “Have you been diagnosed with stroke and/or heart disease?”). DM was defined as follows [30]: FBG ≥ 126 mg/dL, and/or an HbA1c level ≥ 6.5%, and/or a self-reported physician diagnosis (a positive response to “Have you been diagnosed with DM?”), and/or the recent use of a hypoglycemic agent (insulin and/or any hypoglycemic drug). Heart disease and kidney disease were defined as the self-report of physician’s diagnosis (a positive response to “Have you been diagnosed with any heart disease, including angina, congestive heart failure, myocardial infraction, coronary heart disease and any other heart problems ?”) or (a positive response to “Have you been diagnosed with any kidney disease [did not include tumor or cancer]?”), following the previous CHARLS study [31].
Exposure and endpoint
As in the previous study, the TyG index was calculated as ln(TG (mg/dL) × FBG (mg/dL)/2) [32]. The endpoint was CMM, defined as the simultaneous presence of at least two of the following: stroke, heart disease, and DM [6, 9].
Handling of missing data
The distribution of missing data for the included participants is shown in Supplementary file, Table S1. Data from participants with a complete set of data were included in the regression models, but an additional analysis was performed using the multiple imputations method (random forest) to impute the missing data and thereby minimize the potential bias.
Statistical analysis
Statistical analyses were conducted using STATA MP version 17.0 and RStudio 4.2.1. The normal distribution and equality of variance of continuous datasets were evaluated using the Kolmogorov-Smirnov test and Levene test, respectively. All the continuous datasets were normally distributed and are described using mean ± standard deviation; one-way ANOVA was used to identify differences among the groups. Categorical datasets are described as counts and percentages, and the chi-square test was used to identify differences among the groups. The participants were followed from baseline (2011) to the onset of CMM or the most recent survey (2018), whichever came first. The incidence of CMM is stated per 1,000 person-years. Cumulative incidences of CMM were estimated using Kaplan–Meier curves and log-rank test.
Cox regression analyses were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the TyG index for CMM. Three models were constructed: Model 1 was a crude model; Model 2 was adjusted for age, sex, SBP, DBP, BMI, and smoking and alcohol consumption status; and Model 3 was adjusted for these variables, plus marital status, educational level, rural vs. urban residence, heart rate, BUN, serum creatinine, UA, hemoglobin, total cholesterol (TC), LDL, and the presence of stroke, heart disease, or DM. We plotted Schoenfeld residuals against time to evaluate the proportional hazards assumption. In addition, multivariable-adjusted restricted cubic splines (RCSs) were used to assess the level-response association of the TyG index with the risk of incident CMM. All the variables included in the models were evaluated for collinearity, and no clear evidence of multicollinearity was detected (the all-variance inflation factor of the included variables was < 10) (Supplementary file, Figure S1).
Subgroup analyses were performed on data stratified according to the age (< 60 or ≥ 60 years), sex (male or female), BMI (< 24 or ≥ 24 kg/m2), residence area (rural or urban area), and smoking and alcohol consumption status (never, former and current smoker or alcohol consumers). In addition, given the substantial effect of blood pressure on the incidence of CMM, we placed participants into normotension (< 120/80 mmHg), prehypertension (≥ 120/80 but < 140/90 mm Hg), and hypertension (as defined above) groups and assessed the relationship of TyG index with the risk of CMM in each group [33]. Several sensitivity analyses were also performed to ensure that the findings were robust. In brief, we sequentially excluded participants who had experienced only stroke (n = 110), only had DM (n = 364), or only had heart disease (n = 752), and simultaneously excluded those mentioned above (experienced stroke, or DM, or heart disease (n = 1,226)). To fully explain the observed association, E-value analysis was performed to quantify the exposure-confounder and confounder-outcome relationships [34]. Moreover, an additional analysis was performed to exclude the potential influence of kidney diseases on the observed association. P < 0.05 was defined as indicating statistical significance.
Results
Figure 1 shows the selection criteria used in the present study. Data for 7,970 participants were analyzed according to the quartile of the TyG index: Q1 (≤ 8.22, n = 1,992), Q2 (8.22–8.59, n = 1,993), Q3 (8.59–9.03, n = 1,993), and Q4 (> 9.03, n = 1,992). The characteristics of the participants who were included and excluded are shown in Supplementary file, Table S2. The age, TyG index, DBP, BMI, and region of residence were similar for these two groups, although there were some significant P-values. The excluded individuals had higher SBP (130.66 ± 22.14 mmHg vs. 128.53 ± 20.92 mmHg) and prevalence of DM (7.4% vs. 4.6%) and hypertension (60.5% vs. 46.9%) than the included individuals (all P < 0.001).
Baseline characteristics of the participants
The mean age of the included participants was 58.29 ± 8.76 years, and 54.3% were female. Their baseline characteristics, according to the quartile of the TyG index, are shown in Table 1. The mean TyG index was 8.67 ± 0.66, and Supplementary file, Figure S2 shows the frequency distribution of the TyG index in detail. In general, compared with participants in Q1, those in Q2–Q4 were more likely to be women; less likely to live in a rural area (all P < 0.05); less likely to have high SBP, DBP, BMI, hemoglobin, FBG, HbA1c, TC, TG, LDL, and UA; more likely to have hypertension and/or DM; and more likely to have low BUN and high-density lipoprotein concentrations (all P < 0.001). The proportions of never smokers and never alcohol consumers were also higher in Q2–Q4 than in Q1. There were no significant differences with respect to marital status or educational level among the quartiles. Supplementary file, Table S3 shows the baseline data for the participants, classified according to whether or not they subsequently developed CMM.
Relationship of TyG index with the incidence of CMM
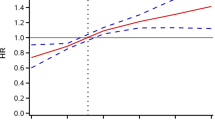
In total, 638 (8.01%) incident cases of CMM were documented in the participants. The incidences of CMM were 4.22%, 6.12%, 8.78%, and 12.60% in Q1–Q4, respectively, during a median 84 months of follow-up (interquartile range, 20–87 months). The cumulative incidence of CMM were gradually increased from Q1 to Q4 (Fig. 2). Table 2 shows the incidence and HR with 95% CI of TyG index for CMM. Each 1.0-unit increase in the TyG index was associated with a higher risk of incident CMM in the crude Cox model (HR: 1.76, 95% CI: 1.60–1.94). This relationship was slightly weaker, but remained significant (HR: 1.54, 95% CI: 1.29–1.84) after multivariate adjustment (Model 3) for age, sex, blood pressure, BMI, smoking and alcohol consumption status, marital status, educational status, rural vs. urban residence, heart rate, BUN, serum creatinine, UA, hemoglobin, TC, LDL, and the presence of stroke, heart disease, and DM. Consistent with these results, Model 3 showed that the fully adjusted HRs (95% CIs) of participants in Q3 and Q4 of the TyG index for the development of CMM were 1.41 (1.05–1.90) and 1.61 (1.18–2.20), respectively, vs. those in Q1. Moreover, multivariable-adjusted RCS analysis showed a significant dose-response relationship of TyG index with the risk of CMM (overall P < 0.001; non-linear P = 0.129) (Fig. 3).
Restricted cubic spline curve for CMM by TyG index after covariate adjustment. Heavy central line represents the estimated adjusted hazard ratio, with shaded ribbons denoting 95% confidence interval. The vertical dotted line indicates the threshold value of TyG index at 8.59. The horizontal dotted line represents the hazard ratio of 1.0. The model is adjusted for age, sex, SBP, DBP, BMI, alcohol consumption and smoking status, marital status, education, rural residence, heart rate, BUN, serum creatinine, UA, hemoglobin, TC, LDL, stroke, heart disease, and DM
The relationship between TyG index and the incidence of CMM, expressed according to blood pressure status, is shown in Table 3. In the participants with prehypertension, the higher quartiles of TyG index were positively associated with the risk of incident CMM (Q3: HR: 1.66, 95% CI: 1.15–5.73, P = 0.021; Q4: HR: 2.47, 95% CI: 1.08–5.66, P = 0.033) in Model 3, relative to Q1. Consistent with this, each 1.0-unit increment in the TyG index was associated with a 91% increase in the incidence of CMM (HR: 1.91, 95% CI: 1.26–2.90, P = 0.002). In addition, increases in TyG index were significantly associated with increases in the incidence of CMM in participants with hypertension (HR: 1.37, 95% CI: 1.10–1.71, P = 0.005), but there were no significant differences among the quartiles. Moreover, participants with normotension who were in the higher quartiles of TyG index did not differ from those in Q1 with respect to the risk of CMM.
Subgroup analyses
The relationship of TyG index with the incidence of CMM was further assessed in subgroup analyses. Notably, there was an interaction between age and incident CMM (P for interaction = 0.045), but both of the subgroups showed close associations with CMM (< 60 years vs. ≥60 years, HR: 1.67, 95% CI 1.29–2.17 and HR: 1.45, 95% CI: 1.13–1.86, respectively). However, the interactions of sex, BMI, residence, alcohol consumption status, smoking status, and the TyG index with the risk of CMM were not significant, and the association was abolished in the former alcohol consumer (HR: 1.08, 95% CI: 0.61–1.93, P = 0.787) and former smoker (HR: 1.54, 95% CI: 0.85–2.78, P = 0.155) subgroups (Fig. 4).
Sensitivity analyses
To ensure that these findings were robust, we performed several sensitivity analyses. First, we excluded participants who had DM at baseline (n = 364), and the association of TyG index with CMM persisted (Q3 vs. Q1: HR: 1.50, 95% CI: 1.10–2.06; Q4 vs. Q1: HR: 1.79, 95% CI: 1.28–2.51; for each 1.0-unit increase, HR: 1.72, 95% CI: 1.40–2.11) (Supplementary file, Table S4). We next excluded participants who had experienced stroke at baseline (n = 110), and found no substantial change in the relationship (Q3 vs. Q1: HR: 1.41, 95% CI: 1.03–1.91, P = 0.030; Q4 vs. Q1: HR: 1.61, 95% CI: 1.17–2.22, P = 0.004; for each 1.0-unit increase, HR: 1.55, 95% CI: 1.29–1.85, P < 0.001) (Supplementary file, Table S5). We also found a similar relationship when we excluded participants with heart disease at baseline (n = 752): TyG index correlated with the risk of CMM (HR: 1.49, 95% CI: 1.22–1.82), and the fully adjusted HRs (95% CI) for Q2–Q4 of TyG index for CMM were 1.15 (0.81–1.65), 1.16 (0.82–1.64), and 1.42 (0.99–2.04), respectively, vs. Q1 (Supplementary file, Table S6). Finally, we excluded participants who had experienced stroke or had DM or heart disease at baseline (n = 1,226), and the results were similar to those of the primary analysis (Q4 vs. Q1: HR: 1.62, 95% CI: 1.07–2.44, P = 0.022; for each 1.0-unit increase, HR: 1.73, 95% CI: 1.35–2.22, P < 0.001) (Table 4).
Additional analysis
As shown in Supplementary file, Figure S3, we calculated an HR and lower confidence limit of 2.45 and 1.90, respectively, in the E-value analysis for the risk of CMM. The results of the additional analysis were consistent with those of the primary analysis, even after the exclusion of participants with kidney disease (Supplementary file, Table S7) or following the use of multiple imputation to deal with missing data (Supplementary file, Table S8).
Discussion
In the present cohort study of 7,970 members of the general population in China, we have shown that the risk of incident CMM increases as the TyG index increases, and in particular in individuals with prehypertension, even after adjustment for potential confounders, and this relationship remained in sensitivity and subgroup analyses. To our knowledge, this is the first study to show that the TyG index is a predictor of CMM, independent of age, sex, and BMI. Most importantly, we have identified a novel means of aiding the prevention of CMM, which may have far-reaching beneficial effects on public health and in clinical practice.
CMM, defined as the coexistence of at least two of stroke, DM, and heart disease [6,7,8], is a common form of multi-morbidity that has been shown to be associated with a higher risk of mortality than each CMD on its own [4]. However, little is known regarding the risk factors for CMM. In recent years, studies aimed at identifying potential risk factors for CMM have been attracting attention. A study of 8,270 participants in the Whitehall II cohort study showed that an unhealthy lifestyle is associated with a 209% higher risk of CMM [7], but accurately quantifying the risk of CMM is very difficult.
A study of data from the UK Biobank showed that exposure to air pollution plays a major role in both the initiation and progression of CMM [35], which is consistent with the findings of another study conducted in China [36]. However, it is very difficult for people to avoid breathing in polluted air. More recently, a study conducted in China explored the relationship of obesity with the risk of CMM [25], but its cross-sectional design meant that causal links could not be confirmed. More importantly, the results obtained concerning the predictive value of BMI for CMM have not been consistent: a pooled analysis of 16 cohort studies conducted in Europe and the United States showed that the risk of CMM increases as BMI increases, and this relationship remained in all the subgroup and sensitivity analyses [6], whereas data collected in China showed that BMI is not an ideal predictor of CMM [25]. These contradictory findings suggest that BMI or obesity might not be a useful predictor of CMM in all populations.
In contrast, a large body of evidence demonstrates the predictive value of IR for incident CMM [37, 38]. IR, which is defined as a failure of insulin to have its physiological effects in target tissues, has been shown not only to contribute the development of atherosclerosis and coronary artery disease in individuals with or without DM, but also to possibly represent a novel and promising predictor of adverse cardiovascular events [37, 39, 40]. Therefore, the early recognition of IR should have important clinical implications, permitting the institution of timely measures aimed at the primary and secondary prevention of cardiovascular events through the risk stratification of patients. However, to date, the relationship between IR and CMM have not been well elucidated.
As mentioned above, the TyG index, which is a novel and validated index of IR [19], is closely associated with individual CMDs. In a retrospective observational study of 5.6 million individuals, Hong et al. found that those in Q4 of the TyG index were at a 26% higher risk of stroke (HR: 1.26, 95% CI: 1.23–1.29), a 31% higher risk of myocardial infarction (HR: 1.31, 95% CI 1.28–1.35), and a 28% higher risk of both (HR: 1.28, 95% CI: 1.26–1.30) [41], which is consistent with the results of the present study. Mounting evidence suggests that the TyG index closely correlates with the prevalence and burden of cerebrovascular disease, and has been proposed to be a useful biomarker for stroke or intracerebral hemorrhage [42, 43]. Data from a study of 12,326 Asian participants showed that the TyG index might be a useful predictor of heart disease [44]. In addition, Zou et al. showed that the TyG index is associated with the prevalence of adverse cardio-cerebrovascular events in women (odds ratio (OR): 1.68, 95% CI: 1.12–2.54), but not in men (OR: 0.95, 95% CI: 0.74–1.21); however, this study involved only a 29.8-month follow-up period [45], which may have biased the findings toward the null hypothesis because there may not have been sufficient time for adverse events to occur in response to high baseline TyG index. More importantly, previous studies reported that TyG index had a better sensitivity and specificity in predicting IR using the hyperglycemic clamp test compared with Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) [46, 47]. Multiple studies have shown IR is a strong predictor of CMD [17, 20, 23]. Elevated HOMA-IR has a significant association with risk of incident cardiovascular events, ischemic stroke and diabetes [48, 49]. We also observed a similar results that higher insulin resistance determined by the TyG index was significantly associated with higher risk of future CMM events. However, a recent study found that the HOMA-IR, was not associated with cardiovascular events in patients with diabetes and ACS who do not receive insulin treatment [50]. Compared with the HOMA-IR, the TyG index does not require the concentration of insulin and may be available all of the patients treated with insulin.
In the present study, a high TyG index was found to be associated with a higher incidence of CMM, independent of conventional risk factors, including TC, age, sex, and LDL. Moreover, we identified a linear correlation of the TyG index with the incidence of CMM. This is in contrast to the findings of a published 10-year follow-up study of 5,014 participants that showed that a high TyG index is not associated with a higher risk of cerebrovascular disease (HR: 1.45, 95% CI: 0.83–2.54) [51]. Similarly, Cho et al. found that individuals with DM in the higher quartiles of TyG index were not more likely to have ischemic heart disease than those in Q1 (all P > 0.05) [52]. These inconsistencies in findings may be explained by heterogeneity in the study participants, the sample sizes, the durations of follow-up, and the study designs [51, 52]. In addition, the endpoint in our study is defined as CMM, a composite of DM, stroke and heart disease, which may partly explain the discrepancy with previous studies [51, 52]. More importantly, we enrolled participants from CHARLS, a prospective, nationwide cohort study, rather than including inpatients or outpatients who usually have more underlying diseases, which significantly differs from the two studies mentioned above [51, 52]. Moreover, several recent systematic review and meta-analysis suggested that elevated TyG index was significantly associated with higher risk of hypertension, arterial stiffness, CVD and heart failure [53,54,55,56], which is similar with our findings.
Previous studies have principally evaluated the utility of the TyG index for patients developing only one CMD. Here, we have explored the potential for the use of the TyG index to predict the risk of incident CMM. Our findings remained significant even after adjustment for conventional confounders, such as SBP, DBP, BMI, and the prevalence of a single CMD. In the subgroup analyses, the interactions were not significant, except with respect to age and the risk of CMM, but both of the two subgroups were closely associated with the incidence of CMM, which suggests that the findings of the present study may be applicable to the general population.
In addition, individuals with prehypertension and values of the TyG index in the higher quartiles were more likely to develop CMM than those in Q1, although the relationship was blunted when the participants were categorized according to whether they had normotension or hypertension, suggesting that individuals with prehypertension are at a higher risk of CMM and that physicians should pay more attention to these individuals in clinical practice. Similarly, the results of previous studies have suggested that individuals with prehypertension are at a higher risk of adverse cardiovascular events [57, 58], which may be because of the lack of attention paid to prehypertension and delays to appropriate interventions because the current international guidelines do not provide a consensus regarding the treatment of this group of patients [29, 59]. Therefore, further prospective intervention studies are urgently needed to assess the effects of prehypertension on the subsequent risk of CMM and to determine the optimal time for interventions to be made. Finally, the additional and sensitivity analyses showed that the associations identified in the primary analysis were robust. Notably, the E-value analysis suggested that the effects of unmeasured confounding factors were minor, further confirming that the present findings are reliable.
The mechanisms underpinning the association of TyG index with CMM have not been characterized, but may involve the following. First, IR is involved in the pathogenesis of atherosclerosis: high circulating insulin concentrations cause a reduction in the production of nitric oxide via the activation of serum and glucocorticoid kinase 1, and the lower nitric oxide concentration, in turn, leads to matrix protein deposition and fibrosis [60]. Second, the lipid and glucose metabolic disorders that are induced by IR may cause the overproduction of reactive oxide species through the activation of signaling pathways, including the protein kinase C pathway and the nuclear factor (NF)κB pathway, which may incite CMM [36]. Third, IR causes fluid retention and high blood pressure via the ectopic synthesis of angiotensinogen [61] and the consequent inappropriate activation of the RAAS [62]. Finally, insulin promotes thrombosis and platelet aggregation by impairing fibrinolysis, secondary to an increase in the circulating concentration of plasminogen activator inhibitor 1 [63].
The prospective design and nationwide nature of the present cohort study, as well as its large sample size, permitted us to identify potential risk factors for CMM in a robust fashion. The present study is the first to evaluate the predictive value of the TyG index for CMM using a prospective design and in a nationwide cohort with long-term follow-up. We have addressed the knowledge gap regarding the relationship between IR and CMM, which should aid the prevention of CMM by permitting better risk stratification of patients. However, this study had several limitations. First, although many potential confounding factors have been adjusted for, the possibility of residual confounding cannot be completely eliminated. However, the E-value analysis indicated that there was only minor unmeasured confounding and the observed association remained in our subgroup and sensitivity analyses. Second, CMM was defined using a self-reported physician diagnosis, which may have led to information bias. Therefore, large-scale, randomized controlled trials are urgently needed to confirm the present findings. Third, we only enrolled individuals aged ≥ 45 years in the study, which may limit the generalizability of the results. Finally, because it was an observational study, we only included data from China and we excluded a large number of participants, although this is common in cohort studies of this kind, suggesting some selection bias is inevitable. Therefore, although the association remained in all the subgroup and sensitivity analyses, the findings should be interpreted and generalized with caution. Further studies of people of other ethnicities and in other countries should thus be performed to test the generalizability of the findings.
Conclusions
In the present study, we have shown that a high TyG index is positively associated with a higher risk of incident CMM. Therefore, physicians may be able to reduce the risk of CMM in their patients by keeping their TyG indexes low. Because the TyG index is a readily available surrogate index of IR, early and close monitoring could be used to identify patients at high risk of CMM, thereby significantly improving their prognosis, which may exert far-reaching significance on public health.
Data Availability
The data supporting the findings of this study are available the CHARLS website (http://charls.pku.edu.cn/en).
Abbreviations
- IR:
-
insulin resistance
- BUN:
-
blood urea nitrogen
- CI:
-
confidence interval
- CMD:
-
cardiometabolic diseases
- CMM:
-
cardiometabolic multimorbidity
- DBP:
-
diastolic blood pressure
- OR:
-
odds ratio
- HbA1c:
-
glycosylated hemoglobin
- DM:
-
diabetes mellitus
- FBG:
-
Fasting blood glucose
- HR:
-
hazard ratio
- Q:
-
quartile
- RCS:
-
restricted cubic splines
- SBP:
-
systolic blood pressure
- TyG:
-
triglyceride-glucose
- BMI:
-
body mass index
- UA:
-
uric acid
References
McKee M, Dunnell K, Anderson M, Brayne C, Charlesworth A, Johnston-Webber C, et al. The changing health needs of the UK population. Lancet (London England). 2021;397(10288):1979–91.
Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ (Clinical Research ed). 2012;345:e5205.
Han Y, Hu Y, Yu C, Guo Y, Pei P, Yang L, et al. Lifestyle, cardiometabolic Disease, and multimorbidity in a prospective Chinese study. Eur Heart J. 2021;42(34):3374–84.
Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of Cardiometabolic Multimorbidity with Mortality. JAMA. 2015;314(1):52–60.
Lu Y, Li G, Ferrari P, Freisling H, Qiao Y, Wu L, et al. Associations of handgrip strength with morbidity and all-cause mortality of cardiometabolic multimorbidity. BMC Med. 2022;20(1):191.
Kivimäki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2(6):e277–e85.
Singh-Manoux A, Fayosse A, Sabia S, Tabak A, Shipley M, Dugravot A, et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: a cohort study. PLoS Med. 2018;15(5):e1002571.
Freisling H, Viallon V, Lennon H, Bagnardi V, Ricci C, Butterworth AS, et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic Diseases: a multinational cohort study. BMC Med. 2020;18(1):5.
Busija L, Lim K, Szoeke C, Sanders KM, McCabe MP. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur J Epidemiol. 2019;34(11):1025–53.
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes Mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41.
Sakakibara BM, Obembe AO, Eng JJ. The prevalence of cardiometabolic multimorbidity and its association with physical activity, diet, and stress in Canada: evidence from a population-based cross-sectional study. BMC Public Health. 2019;19(1):1361.
Jia Y, Guo D, Sun L, Shi M, Zhang K, Yang P, et al. Self-reported daytime napping, daytime sleepiness, and other sleep phenotypes in the development of cardiometabolic Diseases: a mendelian randomization study. Eur J Prev Cardiol. 2022;29(15):1982–91.
Sattar N, Gill JMR, Alazawi W. Improving prevention strategies for cardiometabolic Disease. Nat Med. 2020;26(3):320–5.
Zhang Y, Zhuang P, Wu F, He W, Mao L, Jia W, et al. Cooking oil/fat consumption and deaths from cardiometabolic Diseases and other causes: prospective analysis of 521,120 individuals. BMC Med. 2021;19(1):92.
Huang R, Lin Y, Ye X, Zhong X, Xie P, Li M, et al. Triglyceride-glucose index in the development of Heart Failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol. 2022;29(11):1531–41.
Sajdeya O, Beran A, Mhanna M, Alharbi A, Burmeister C, Abuhelwa Z, et al. Triglyceride glucose index for the prediction of subclinical Atherosclerosis and arterial stiffness: a Meta-analysis of 37,780 individuals. Curr Probl Cardiol. 2022;47(12):101390.
Park B, Lee HS, Lee Y-J. Triglyceride glucose (TyG) index as a predictor of incident type 2 Diabetes among nonobese adults: a 12-year longitudinal study of the Korean genome and epidemiology study cohort. Transl Res. 2021;228:42–51.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294(1):E15–E26.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–51.
Xuan X, Hamaguchi M, Cao Q, Okamura T, Hashimoto Y, Obora A, et al. U-shaped association between the triglyceride-glucose index and the risk of incident Diabetes in people with normal glycemic level: a population-base longitudinal cohort study. Clin Nutr. 2021;40(4):1555–61.
Wen J, Wang A, Liu G, Wang M, Zuo Y, Li W, et al. Elevated triglyceride-glucose (TyG) index predicts incidence of Prediabetes: a prospective cohort study in China. Lipids Health Dis. 2020;19(1):226.
Xu L, Wu M, Chen S, Yang Y, Wang Y, Wu S, et al. Triglyceride-glucose index associates with incident Heart Failure: a cohort study. Diabetes Metab. 2022;48(6):101365.
Su W-Y, Chen S-C, Huang Y-T, Huang J-C, Wu P-Y, Hsu W-H et al. Comparison of the effects of Fasting glucose, Hemoglobin A1c, and triglyceride-glucose Index on Cardiovascular events in type 2 Diabetes Mellitus. Nutrients. 2019, 11(11).
Zhao Y, Sun H, Zhang W, Xi Y, Shi X, Yang Y, et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic Stroke: the rural Chinese cohort study. Diabetes Metab. 2021;47(4):101246.
Lu Y, Liu S, Qiao Y, Li G, Wu Y, Ke C. Waist-to-height ratio, waist circumference, body mass index, waist divided by height0.5 and the risk of cardiometabolic multimorbidity: a national longitudinal cohort study. Nutr Metab Cardiovasc Dis. 2021;31(9):2644–51.
Gong J, Wang G, Wang Y, Chen X, Chen Y, Meng Q, et al. Nowcasting and forecasting the care needs of the older population in China: analysis of data from the China Health and Retirement Longitudinal Study (CHARLS). Lancet Public Health. 2022;7(12):e1005–e13.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–8.
Lin L, Wang HH, Liu Y, Lu C, Chen W, Guo VY. Indoor solid fuel use for heating and cooking with blood pressure and Hypertension: a cross-sectional study among middle-aged and older adults in China. Indoor Air. 2021;31(6):2158–66.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial Hypertension. Eur Heart J. 2018;39(33):3021–104.
Yu J, Yi Q, Chen G, Hou L, Liu Q, Xu Y, et al. The visceral adiposity index and risk of type 2 Diabetes Mellitus in China: a national cohort analysis. Diabetes Metab Res Rev. 2022;38(3):e3507.
Zheng X, Han L, Shen S. Hypertension, remnant cholesterol and Cardiovascular Disease: evidence from the China health and retirement longitudinal study. J Hypertens. 2022;40(11):2292–8.
Wu TD, Fawzy A, Brigham E, McCormack MC, Rosas I, Villareal DT, et al. Association of Triglyceride-Glucose Index and Lung Health: a Population-based study. Chest. 2021;160(3):1026–34.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The Seventh Report of the Joint National Committee on Prevention, detection, evaluation, and treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72.
Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in Observational studies. JAMA. 2019;321(6):602–3.
Luo H, Zhang Q, Yu K, Meng X, Kan H, Chen R. Long-term exposure to ambient air pollution is a risk factor for trajectory of cardiometabolic multimorbidity: a prospective study in the UK Biobank. EBioMedicine. 2022;84:104282.
Chen W, Wang X, Chen J, You C, Ma L, Zhang W, et al. Household air pollution, adherence to a healthy lifestyle, and risk of cardiometabolic multimorbidity: results from the China health and retirement longitudinal study. Sci Total Environ. 2023;855:158896.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in Cardiovascular Disease development. Nat Reviews Endocrinol. 2014;10(5):293–302.
Zhao Q, Zhang T-Y, Cheng Y-J, Ma Y, Xu Y-K, Yang J-Q, et al. Triglyceride-glucose index as a surrogate marker of insulin resistance for Predicting Cardiovascular outcomes in nondiabetic patients with Non-ST-Segment elevation Acute Coronary Syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–94.
Nishimura M, Tokoro T, Nishida M, Hashimoto T, Kobayashi H, Yamazaki S, et al. Association of insulin resistance with de novo coronary stenosis after percutaneous coronary artery intervention in hemodialysis patients. Nephron Clin Pract. 2008;109(1):c9–17.
Uetani T, Amano T, Harada K, Kitagawa K, Kunimura A, Shimbo Y, et al. Impact of insulin resistance on post-procedural myocardial injury and clinical outcomes in patients who underwent elective coronary interventions with drug-eluting stents. JACC Cardiovasc Interv. 2012;5(11):1159–67.
Hong S, Han K, Park C-Y. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic Cardiovascular Disease: a population-based study. BMC Med. 2020;18(1):361.
Mac Grory B, Yaghi S, Cordonnier C, Sposato LA, Romano JG, Chaturvedi S. Advances in recurrent Stroke Prevention: Focus on Antithrombotic therapies. Circul Res. 2022;130(8):1075–94.
Hao Z, Chen Y, Wright N, Qin H, Turnbull I, Guo Y, et al. Natural history of silent lacunar infarction: 10-year follow-up of a community-based prospective study of 0.5 million Chinese adults. Lancet Reg Health West Pac. 2021;17:100309.
Won K-B, Park EJ, Han D, Lee JH, Choi S-Y, Chun EJ, et al. Triglyceride glucose index is an Independent predictor for the progression of coronary artery calcification in the absence of heavy coronary artery calcification at baseline. Cardiovasc Diabetol. 2020;19(1):34.
Zou S, Xu Y. Association of the triglyceride-glucose index and major adverse cardiac and cerebrovascular events in female patients undergoing percutaneous coronary intervention with drug-eluting stents: a retrospective study. Diabetes Res Clin Pract. 2021;181:109073.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100.
Jin A, Wang S, Li J, Wang M, Lin J, Li H, et al. Mediation of systemic inflammation on insulin resistance and prognosis of nondiabetic patients with ischemic Stroke. Stroke. 2023;54(3):759–69.
Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without Diabetes: meta-analysis. PLoS ONE. 2012;7(12):e52036.
Stähli BE, Nozza A, Schrieks IC, Buse JB, Malmberg K, Mellbin L, et al. Homeostasis Model Assessment of Insulin Resistance and survival in patients with Diabetes and Acute Coronary Syndrome. J Clin Endocrinol Metab. 2018;103(7):2522–33.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Cho Y-R, Ann SH, Won K-B, Park G-M, Kim Y-G, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery Disease according to the presence of Diabetes. Sci Rep. 2019;9(1):6129.
Xu AR, Jin Q, Shen Z, Zhang J, Fu Q. Association between the risk of Hypertension and triglyceride glucose index in Chinese regions: a systematic review and dose-response meta-analysis of a regional update. Front Cardiovasc Med. 2023;10:1242035.
Liu F, Ling Q, Xie S, Xu Y, Liu M, Hu Q, et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol. 2023;22(1):111.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of Cardiovascular Diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, et al. Triglyceride-glucose index and Heart Failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22(1):244.
Han M, Li Q, Liu L, Zhang D, Ren Y, Zhao Y, et al. Prehypertension and risk of Cardiovascular Diseases: a meta-analysis of 47 cohort studies. J Hypertens. 2019;37(12):2325–32.
Huang Y, Cai X, Liu C, Zhu D, Hua J, Hu Y et al. Prehypertension and the risk of coronary Heart Disease in Asian and western populations: a meta-analysis. J Am Heart Assoc. 2015, 4(2).
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH et al. /ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018, 71(19):e127-e248.
Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res. 2022;118(1):130–40.
Gao Q, Lin Y, Xu R, Luo F, Chen R, Li P, et al. Positive association of triglyceride-glucose index with new-onset Hypertension among adults: a national cohort study in China. Cardiovasc Diabetol. 2023;22(1):58.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced Heart Disease. Diabetologia. 2018;61(1):21–8.
Brazionis L, Rowley K, Jenkins A, Itsiopoulos C, O’Dea K. Plasminogen activator inhibitor-1 activity in type 2 Diabetes: a different relationship with coronary Heart Disease and diabetic retinopathy. Atertio Thromb Vasc Biol. 2008;28(4):786–91.
Acknowledgements
The present study used data from the CHARLS. The authors thank CHARLS research team and individuals included in the study. And the authors thank Mark Cleasby, PhD from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Funding
This study was supported by grants from the National Key Research and Development Program of China (2016YFC1300100), Nonprofit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019XK320057), CAMS Innovation Fund for Medical Science (CIFMS, 2022-I2M-C&T-A-010).
Author information
Authors and Affiliations
Contributions
Zenglei Zhang, Xu Meng and Xianliang Zhou designed this study. Zenglei Zhang performed the statistical analysis. Zenglei Zhang wrote the first draft of the manuscript. Lin Zhao and Yiting Lu wrote some sections of the manuscript. Xu Meng and Xianliang Zhou revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
CHARLS was approved by the Institutional Review Board of Peking University (approval number: IRB00001052-11015 for household survey and IRB00001052-11014 for blood sample), and all participants provided written consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Z., Zhao, L., Lu, Y. et al. Relationship of triglyceride-glucose index with cardiometabolic multi-morbidity in China: evidence from a national survey. Diabetol Metab Syndr 15, 226 (2023). https://doi.org/10.1186/s13098-023-01205-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-023-01205-8