Abstract
Background
The Aedes aegypti mosquito is the primary vector for dengue, chikungunya, yellow fever and Zika viruses worldwide. The first record of Ae. aegypti in southwestern Saudi Arabia was in 1956. However, the first outbreak and cases of dengue fever were reported in 1994, and cases have increased in recent years. Vector control for Ae. aegypti mainly uses pyrethroid insecticides in outdoor and indoor space spraying. The constant use of pyrethroids has exerted intense selection pressure for developing target-site mutations in the voltage-gated sodium channel (vgsc) gene in Ae. Aegypti against pyrethroids—mutations that have led to knockdown resistance (kdr).
Methods
Aedes aegypti field populations from five regions (Jazan, Sahil, Makkah, Jeddah and Madinah) of southwestern Saudi Arabia were genotyped for known kdr mutations in domains IIS6 and IIIS6 of the vgsc gene using polymerase chain reaction (PCR) amplification and sequencing. We estimated the frequency of kdr mutations and genotypes from Saudi Arabia as well as from other countries, Thailand, Myanmar (Southeast Asia) and Uganda (East Africa). We constructed haplotype networks to infer the evolutionary relationships of these gene regions.
Results
The three known kdr mutations, S989P, V1016G (IIS6) and F1534C (IIIS6), were detected in all five regions of Saudi Arabia. Interestingly, the triple homozygous wild genotype was reported for the first time in two individuals from the highlands of the Jazan region and one from the Al-Quoz, Sahil region. Overall, nine genotypes comprising four haplotypes were observed in southwestern Saudi Arabia. The median-joining haplotype networks of eight populations from Saudi Arabia, Southeast Asia and East Africa for both the IIS6 and IIIS6 domains revealed that haplotype diversity was highest in Uganda and in the Jazan and Sahil regions of Saudi Arabia, whereas haplotype diversity was low in the Jeddah, Makkah and Madinah regions. Median-joining haplotype networks of both domains indicated selection acting on the kdr-mutation containing haplotypes in Saudi Arabia.
Conclusions
The presence of wild type haplotypes without any of the three kdr mutations, i.e. that are fully susceptible, in Saudi Arabia indicates that further consideration should be given to insecticide resistance management strategies that could restore pyrethroid sensitivity to the populations of Ae. aegypti in Saudi Arabia as part of an integrative vector control strategy.
Graphical Abstract

Similar content being viewed by others
Background
The mosquito Aedes aegypti is responsible for transmitting life-threatening infectious arboviruses, including dengue, chikungunya, yellow fever and Zika [1]. This mosquito has spread from Africa to other tropical and subtropical regions of the world, resulting in a significant increase in their burden of vector-borne diseases [2, 3]. Aedes aegypti was reported in the Arabian Peninsula, including Saudi Arabia, in 1956 [4]. The form of Ae. aegypti in Saudi Arabia has been genetically characterised as the domestic form that spread out of Africa [5, 6]. However, the first dengue fever outbreak was reported in the 1990s [7], and in more recent years, the dengue incidence rate increased to 21.71 per 100,000 persons/year in 2013, and then dropped to 11.59 in 2019 [8, 9]. Dengue fever cases are concentrated in the country’s southwest regions, i.e. Jazan, Sahil, Jeddah, Makkah and Madinah [9,10,11]. There was one confirmed case of chikungunya in 2011 [12] and another in 2021 [13], but no reported cases to date of Zika virus.
Although new vector control strategies are in development (i.e. genetic manipulation and Wolbachia-based population control), controlling Ae. aegypti using insecticides remains the primary method applied, especially during an outbreak [14]. In Saudi Arabia, insecticide usage for vector control has been documented since 1948 due to endemic malaria [15, 16]. Various insecticides have been used in the country: dichlorodiphenyltrichloroethane (DDT) was used from 1948 to 1954 and from then, other insecticides were used instead [16]. The first report of pyrethroid resistance was in 2011 in Makkah [17], followed by Jeddah [18] and Jazan [19]. Pyrethroids have been used mainly against Ae. aegypti both publicly by the government [18, 19] and privately by house owners [20]. The widespread use of pyrethroids worldwide has been due to their lower environmental effects, low mammalian toxicity and, most importantly, their fast action on the target pest [21]. To date, pyrethroids are the only recommended insecticides for indoor application [22]. With the overuse of insecticides, mosquitoes, particularly Ae. aegypti, have developed resistance worldwide to multiple insecticides, making them extremely difficult to control [14, 23].
DDT and pyrethroid insecticides are neurotoxins that deliver toxic effects by binding to the voltage-gated sodium channel protein (VGSC). The binding of insecticides to the sodium channel causes a change in its gating properties and keeps it open for a long time, which eventually causes death [24]. The VGSC-encoding gene (vgsc) is large in insects, with a single copy encoding for a protein (about 2050 amino acids) [24]. In Ae. aegypti, the vgsc comprises 37 exons with a length of approximately 480 kb (VectorBase). Several non-synonymous mutations in the vgsc that cause knockdown resistance (kdr) are associated with pyrethroid and DDT resistance [23,24,25]. These mutations (S989P, V1016G and F1534C) were reported in the Middle East and Southeast Asia [25]. Kdr mutations at amino acid 1016 have been reported from different geographical locations; for example, V1016G in Southeast Asia since 2009 [26,27,28,29,30,31,32,33], Ghana [34] and Saudi Arabia [17, 35]. The mutation S989P is thought not to cause any resistance alone but when co-occurring with V1016G confers resistance against permethrin and deltamethrin [17]. The F1534C mutation is found in Asian, American and African populations of Ae. aegypti [36, 37].
Frequencies of vgsc genotypes involving the three kdr mutations (S989P, V1016G and F1534C) vary among locations. To date, eight and 12 genotypes have been reported from Saudi Arabia and Myanmar, respectively, while Thailand has reported only three [30, 35, 38]. The genotype that is homozygous for mutations S989P and V1016G but is wild type at F1534C has a high resistance level to both type I (i.e. permethrin) and type II (i.e. deltamethrin) pyrethroids [35]. In contrast, the genotype (S989P, V1016G wild type with F1534C homozygous mutant, i.e. SS + VV + CC) confers low resistance to type I pyrethroids [17, 39, 40]. Additionally, intermediate (in type I and II pyrethroids) and nearly susceptible levels of resistance (in type II pyrethroids) were observed in the fully heterozygous genotype SP + VG + FC and the partially heterozygous genotype SP + VG + CC [39]. The genotype that is only heterozygous for a kdr mutation at position 1534 (SS + VV + FC) had a slightly elevated resistance level compared with the wild type (in type I pyrethroids) [39]. In a previous study in Saudi Arabia (2017), the resistance status of genotypes PP + GG + FC and SP + VG + CC was not completely ascertained, but they were perhaps susceptible (in type II pyrethroids) [17]. Finally, the triple homozygous mutant genotype PP + GG + CC confers a much higher resistance level when expressed in Xenopus oocytes [40]. However, this genotype is rarely observed in natural populations of Ae. aegypti [30, 35].
For effective vector control campaigns, it is essential to detect kdr mutations and characterise their frequencies and distributions. Further, understanding the evolution of kdr mutations may help us in managing insecticide resistance. Within this context, this study aimed to (i) detect and investigate the frequency of kdr mutations in five dengue fever-endemic regions of Saudi Arabia, (ii) characterise the genotypes and haplotypes within Saudi Arabia and assess whether they are shared with other populations worldwide, and (iii) construct putative evolutionary relationships amongst sequences to infer the dispersal and origin of kdr haplotypes in Saudi Arabia.
Methods
Study area
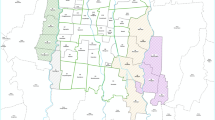
Saudi Arabia, which lies in Western-South Asia, is the largest country in the Arabian Peninsula, occupying a total land area of about 2,000,000 km2 and a total population of about 35 million [41]. Geographically, Saudi Arabia is divided into three distinct zones: the rain-fed highlands of the western and southwestern regions (Sarawat Mountains), the arid and extra-arid lands of the interior (Najd) and the coastal plain along the Red Sea in western Saudi Arabia (known as the Tihamah) that includes the east of the plain represented by the Hejaz and the Asir mountain range. Five different regions of Saudi Arabia, namely, Jazan, Sahil, Jeddah, Makkah and Madinah were selected for this study (Fig. 1 and Additional file 1: Table S1).
The Jazan region is located in the southwestern part of the country (16°53’N, 42°34’E) alongside the Red Sea and shares a border with Yemen to the south (120 km), where it is known for endemic vector-borne diseases such as dengue fever and malaria [42]. Jeddah and Makkah (21°32′N, 39°10′E and 21°25′N 39°49′E) are about 700 km north of Jazan. The Jeddah region (with a population of 3.4 million) is considered to be the area with the second-largest economy in Saudi Arabia, after the capital city (Riyadh), which makes it attractive for migrant workers from all over the world [43]. Furthermore, Jeddah has the largest and busiest port (Jeddah Islamic Port) in the Middle East and North Africa [44]. Every year, over two million people gather from across the world, including South Americans, Africans, Europeans and Asians, in Makkah (arriving through the international airport in Jeddah) to practise Islamic pilgrimages (Hajj and Umrah) [45]. The Madinah region (with a population of 2.2 million) is located in the western part of Saudi Arabia (25°00’N 39°30’E). The capital city, Medina, considered the second-holiest city in Islam, attracts more than seven million annual visitors from all over the world [46]. In addition, samples were also collected from five areas (Al-Shuqaiq, Al-Qahma, Al-Quoz, Al-Qunfudhah and Al-Lith) located along the coastal inhabited area. This area (referred to as the Sahil region in this study) stretches ~ 400 km along the Red Sea coast, linking Jazan with Makkah and Jeddah.
Mosquito sampling and taxonomic identification
Aedes aegypti larvae and eggs were collected between 2019 and 2021 from the five selected regions of Saudi Arabia. All larvae were maintained at 28 ± 2 °C with relative humidity of 75 ± 10%, as previously described for adult emergence [47]. The emerged adults were identified morphologically to species level using a mosquito taxonomic key [48]. For comparison, samples were also included from Southeast Asia (Thailand and Myanmar) and Africa (Uganda). The Chiang Mai, Thailand samples were collected in 2017–2018, and the samples from the three regions in Myanmar were collected in 2004–2005 (Additional file 1: Table S2) [49]. Ugandan Ae. aegypti DNA from 2012 and samples from 2020 were used to enable comparison with the ancestral African variation [50].
DNA extraction and polymerase chain reaction (PCR) amplification
Specimens of Ae. aegypti from Saudi Arabia were identified and preserved in tubes with silica gel. Genomic DNA was extracted using the DNeasy Blood and Tissue Kit (QIAGEN Sciences, Germantown, MD, USA). Overall, genomic DNA from a total of 267 adult mosquitoes from Saudi Arabia, Thailand, Myanmar and Uganda was used in this study.
The protocol for the PCR amplification was adapted from Yanola et al. [29]. Two regions in the vgsc of the Ae. aegypti Saudi strain were amplified using PCR primers: domain IIS6 in VGSC IIP–IIS6 F: GGTGGAACTTCACCGACTTC and R: GGACGCAATCTGGCTTGTTA; and domain IIIS6 in VGSC IIIS4–IIIS6 F: GCTGTCGCACGAGATCATT and R: GTTGAACCCGATGAACAACA. The first primer pair targeted a region containing amino acid positions 989 and 1016, and the second primer pair targeted the region containing position 1534. The PCR reactions were carried out using 5 μl of 5× Q5 reaction buffer, 0.5 μl of 10 mM dNTPs, 1.25 μl (10 μM) of forward and reverse primers, 0.25 μl of 5Q high-fidelity DNA polymerase (New England Laboratory, UK), 2 μl of the DNA template and molecular-grade water to a final reaction volume of 25 μl. Thermal cycling conditions were an initial denaturation at 98 °C for 30 s and 35 cycles as follows: 98 °C for 10 s, 71 °C for 25 s, 72 °C for 25 s) and a 2-min final extension step at 72 °C. The second region was amplified under the same cycling conditions, except that the annealing temperature was 68 °C for 25 s. For the African samples, it was necessary to lower the annealing temperature to 68 °C for successful amplification of domain IIS6, which we suspected was due to sequence variation within the primer-binding regions. Agarose gel electrophoresis (1% agarose gel run for 45–50 min at 100 V) was used to check the quantity and size of the DNA fragments produced. The PCR purification kit (New England Biolabs) was used for amplicon purification prior to Sanger sequencing.
Sequencing
PCR products were sequenced using the amplification primers (Eurofins Genomics, Germany). Sequence data quality was checked and edited using Geneious Prime software (version 2020.0.3, Biomatters Ltd.) [51]. The resulting sequences were aligned and compared with the Ae. aegypti reference sequence from VectorBase (AaegL5.0 Transcript: AAEL023266; Liverpool strain). Following convention, kdr mutations are numbered with reference to the homologous house fly Musca domestica vgsc. Due to the 233-base-pair (bp) intron in domain IIS6, between the S989P and V1016G mutations, some PCR products were cloned when sequences could not be read from direct sequencing of the PCR product (see the method in Additional file 1: Text S1).
Genetic diversity analysis
Haplotypes were inferred from the sequenced genotypes using DnaSP (v.6.12.03) [52]. The Population Analysis with Reticulate Trees (PopArt) software (v.4.8.5.) was used to construct median-joining (MJ) haplotype networks to display the putative evolutionary relationships amongst the kdr sequences [53]. The method of MJ haplotype network construction was developed to combine features of two algorithms: the heuristic algorithm (Farris's maximum-parsimony), which is used to raise new vertices sequentially known as median vectors, and Kruskal's, which favours short connections to find minimum spanning trees [54]. Haplotype diversity was estimated using DnaSP (v.6.12.03) [52].
Results
Detection of kdr mutations in the vgsc
Sequencing of domains IIS6 and IIIS6 of vgsc in 226 individual specimens of Ae. aegypti in Saudi Arabia revealed the presence of three kdr mutations: S989P and V1016G (domain IIS6) and F1534C (domain IIIS6) due to the substitutions of TCC → CCC, GTA → GGA and TTC → TGC, respectively. The S989P and V1016G mutations are close to each other (~ 311 bp) in domain IIS6 on the sequence alignment and are inferred to be in complete linkage disequilibrium (LD). These mutations varied in frequency among regions (Fig. 1 and Table 1). For S989P and V1016G, the frequency was highest (0.64) in Jeddah and lowest in Sahil (0.28) and Jazan (0.29). For S989P and V1016G, the frequency was low in Myanmar (0.23) and completely absent from Uganda. The frequency of F1534C ranged between 0.56 in Madinah and 0.62 in Sahil (Fig. 1 and Table 1). This mutation was also found in Thailand and Myanmar but was absent from Uganda.
Overall, the exon regions of domains IIS6 and IIIS6 were conserved (consistent with worldwide populations of Ae. aegypti [36]). No other known kdr mutations (i.e. G923V, L982W, I1011M/V, L1014F/S and V1016I) were observed. Mutation F1534S was observed in one individual of the Thailand collection. Four synonymous mutations were found with a relatively high frequency in Saudi Arabia. The first mutation was L971L (TTG → TTA) in domain IIS6 in Saudi Arabia (n = 75) and Southeast Asia (n = 29) (Additional file 1: Table S3). L971L was only found on haplotypes carrying kdr mutations S989P and V1016G (see haplotype network and genetic diversity section for details). Furthermore, the samples that were heterozygous for positions 989 and 1016 (domain IIS6) indicate that mutations S989P and V1016G are associated with a different intron length from the wild type. The other three synonymous mutations were in domain IIIS6 (F1518F, E1591E and G1567G), but were only observed in Jazan and Sahil. Numerous low-frequency non-synonymous substitutions are present in domains IIS6 and IIIS6 in Jazan, Sahil and Uganda, the details of which are shown in Additional file 1: Table S3.
Genotypes and haplotype frequencies
A total of nine genotypes involving the three kdr mutations S989P, V1016G and F1534C were detected in Saudi Arabia (Fig. 2). These nine genotypes were inferred to comprise four haplotypes with respect to these kdr mutations (Fig. 3). Haplotype 1 (H1) is the wild type carrying no known kdr mutations; haplotype 2 (H2) carries the F1534C mutation in domain IIIS6; haplotype 3 (H3) is the opposite of H2 and carries S989P and V1016G mutations in domain IIS6; finally, haplotype 4 (H4) is a triple mutant with these kdr mutations in both domains IIS6 and IIIS6 (Fig. 3). The triple heterozygote genotype (SP + VG + FC) could theoretically be composed of H1 with H4 or H2 with H3 (Fig. 3). Based on negative linkage disequilibrium observed previously in Saudi Arabia, where mutations S989P and V1016G occur on an alternative haplotypic background to F1534C [17], the triple heterozygote genotypes (SP + VG + FC) observed here are most likely composed exclusively, or almost exclusively, of H2 with H3. On this basis, we estimate haplotype totals of H1 (25), H2 (215), H3 (135), and H4 (11) in our Saudi Arabian samples.
Genotype frequencies of kdr mutations S989P + V1016G and F1534C in Aedes aegypti from five regions in Saudi Arabia compared with samples from Southeast Asia (Thailand and Myanmar). The bar with one asterisk (*) is the triple homozygous mutant (PP, GG and CC), and the bar with two asterisks (**) is the triple homozygous wild type (SS, VV and FF)
Kdr genotypes in Aedes aegypti observed from five regions in Saudi Arabia and their possible constituent haplotypes. SS, VV and FF are homozygous wild types for S989P, V1016G and F1534C. PP, GG and GG are homozygous mutants for S989P, V1016G and F1534C. SP, VG and FC are heterozygotes for S989P, V1016G and F1534C. H1 triple wild type (SVF), H2 wild types in 989 + 1016 and mutant at 1534 (SVC), H3 mutant at 989 + 1016 and wild type at 1534 (PGF), H4 triple mutants (PGC)
The triple heterozygote genotype (SP + VG + FC) and the genotype containing the homozygous F1534C mutation (SS + VV + CC) were the most frequent genotypes overall in the Saudi Arabia regions sampled (Fig. 2). These genotypes were also commonly observed in Thailand but not in Myanmar, where only one individual had the triple heterozygous genotype (Fig. 2). In this study, a triple homozygous wild genotype (SS + VV + FF) was observed in Jazan (n = 2) and Sahil (n = 1). This genotype was common in the Myanmar samples (n = 14) collected from 2004 to 2005. The genotype that was homozygous for all three kdr mutations (PP + GG + CC) was also observed in this study, but only in Jazan (n = 1).
Haplotype network and genetic diversity
Sequence alignment showed large indels (insertions and deletions) in the intron of domain IIS6 (233 bp) and several in the IIIS6 region (only Jazan and Sahil, along with Uganda). The variation in introns of domain IIS6, particularly between exon 20 (which carries S989P) and exon 21 (carrying V1016G), was particularly large. Indels were frequent in the heterozygotes of S989P + V1016G. In addition, two types of introns in domain IIS6 were observed that differed by ~ 16 bp (corresponding to clade A and B introns), with clade A associated with the mutations in domain IIS6 (S989P + V1016G) (Fig. 4).
Median-joining haplotype network for domain IIS6 of vgsc for Saudi Arabia, Uganda, Thailand and Myanmar populations of Aedes aegypti. The coloured circle represents the haplotype and the population. Haplotypes are connected according to their similarity, and hatch marks between haplotypes show the number of mutations. H haplotype, LVP Liverpool strain
A total of 275 and 468 haplotype sequences of domains IIS6 and IIIS6, respectively, from this study, were aligned and used to construct networks representing the evolutionary relationships amongst the haplotypes (sequence alignments of domain IIS6 and IIIS6 showing polymorphism amongst the haplotypes are shown in Additional file 1: Fig. S3). The networks shown in Figs. 4 and 5 include only the sequences generated in this study using 456 bp and 459 bp from domains IIS6 and IIIS6, respectively. For both domains, haplotypes carrying the resistance mutations occurred at high frequency and were geographically widespread. In each haplotype network, there was also a high-frequency, geographically widespread haplotype that was susceptible (i.e. carrying no kdr mutations for that domain). We infer this high frequency due to its carrying kdr mutation(s) on the alternative domain to which it is physically linked. In both the IIS6 and IIIS6 networks, these high-frequency haplotypes were the only ones present in the Makkah, Jeddah and Madinah regions of Saudi Arabia, and Thailand, consistent with their high levels of kdr mutations. Conversely, there are many low-frequency haplotypes from the Jazan and Sahil regions of Saudi Arabia in both networks, where they intermingle with a large number of diverse haplotypes from Uganda, as well as some haplotypes from Myanmar. This is reflected in the high haplotype diversity for Jazan, Sahil and Uganda for both domains IIS6 and IIIS6 (Table 2).
Median-joining haplotype network analysis for domain IIIS6 of vgsc for Saudi Arabia, Uganda, Thailand and Myanmar populations of Aedes aegypti. The coloured circle represents the haplotype and population. Haplotypes are connected according to their similarity, and hatch marks between haplotypes show the number of mutations. H haplotype, LVP Liverpool strain
Our sequence data were also combined with haplotypes from 40 populations retrieved from GenBank to generate networks [36] (Additional file 1: Figs. S1, S2). This utilised a total of 483 and 590 homologous sequences that were 173 and 314 bp in length for domains IIS6 and IIIS6, respectively. Overall, there were 57 haplotypes (haplotype diversity: 0.758) in domain IIS6 and 45 haplotypes (haplotype diversity: 0.657) in domain IIIS6 (Additional file 1: Figs. S1, S2).
Discussion
Dengue fever is endemic in the five geographical regions of southwestern Saudi Arabia studied here, namely, Jazan, Sahil, Jeddah, Makkah and Madinah [55]. This study concurrently characterised kdr mutations in these dengue-endemic regions of Saudi Arabia by sequencing domains IIS6 and IIIS6 of vgsc and comparing them with the variation found in samples from Thailand, Myanmar and Uganda. The kdr mutations S989P, V1016G and F1534C have previously been reported from Makkah and Jeddah [18, 35, 56] and from a laboratory strain of Ae. aegypti from Jazan [57]. This is the first report of kdr mutations S989P, V1016G and F1534C from natural populations in Sahil, Madinah and Jazan.
The higher frequency of the S989P and V1016G kdr mutations in the cities of Jeddah, Makkah and Madinah compared with the more rural areas of Sahil and Jazan indicates differences in insecticide application and selection pressures between urban and rural areas. Given the high levels of resistance to both type I and type II pyrethroids due to the genotype S989P + V1016G [25], we suggest that this has resulted from higher levels of insecticide usage, of both thermal and ultra-low-volume fogging, and household sprays (Additional file 1: Table S4), in urban relative to rural areas. Other studies have similarly found higher levels of resistance in urban compared with rural areas, i.e. in Indonesia [58], Malaysia [59] and Tunisia [60].
In all studied areas of Saudi Arabia, the F1534C mutation was also present at high frequency. As F1534C is almost always located on a different haplotypic background from S989P + V1016G, the highest frequency possible of the triple heterozygous genotype is ~ 0.5, resulting from underlying allele frequencies of ~ 0.5 for both S989P + V1016G and F1534C. The triple heterozygous kdr genotype (SP + VG + FC) is at high frequency throughout Saudi Arabia, reaching the highest possible frequency in Jeddah and Makkah. This concurs with a recent report on the high frequency of the triple heterozygote genotype in Jeddah [35]. Notably, very similar allele frequencies for these kdr mutations and the triple heterozygote genotype have also been observed in Chiang Mai, Thailand [38], with their temporal stability suggested as being maintained by balancing selection [61]. Given that the double homozygous S989P + V1016G genotype, i.e. PP + GG + FF, has high levels of resistance to both type I and type II pyrethroids [25], balancing selection is most likely due to increased fitness of the triple heterozygous genotype (SP + VG + FC) in the absence of insecticidal selection pressure, as was demonstrated by Saingamsook et al. [62].
As previously seen in Saudi Arabia and elsewhere [33, 36, 56, 63], we observed that kdr mutations S989P and V1016G in our study were linked to the clade A intron type. The haplotype network for our study populations, as well as other worldwide populations (Fig. 4, Additional file 1: Table S3; Fig. S1), indicates that these kdr mutations occurred on the haplotypic background carrying not only the clade A intron type but also the synonymous mutation L971L, indicating a single evolutionary origin of these kdr mutations, as previously inferred [36].
The F1534C kdr mutation has previously been inferred to have at least two independent evolutionary origins [36]. Our haplotype network for domain IIIS6, which contains this mutation, shows extensive reticulation in the network involving primarily synonymous mutations from Uganda and Saudi Arabia but also F1534C (Fig. 5 and Additional file 1: Fig. S2). The reticulation indicates either recombination or homoplasy, i.e. repeated mutations at the same site, but the latter seems more likely given the limited length of this region. We cannot exclude the possibility of F1534C arising more than once, but it is possible to explain all the data with a single evolutionary origin of the widespread haplotype containing F1534C coupled with repeated mutations at synonymous sites.
We found in this study one individual from Thailand with a non-synonymous substitution to 1534S. This could have arisen from the susceptible background, i.e., by mutation F1534S or from the background carrying the kdr mutation, i.e., by mutation C1534S. F1534S has been reported as a kdr mutation in Ae. albopictus collected from the United States and China [64, 65] and its resistance status in Ae. aegypti should be tested.
Interestingly, this study reported one individual with a triple homozygous mutant genotype (PP + GG + CC) from the Jazan region, and the triply mutant haplotypes can also be inferred to be present in Jazan, Jeddah, Makkah and Sahil (from Figs. 2 and 3). This genotype has previously been reported at low frequency in Jeddah [35, 56], Myanmar [30] and Laos [66]. Given the large intron (> 44.5 kb) between domains IIS6 and domain IIIS6, the triple mutant haplotype carrying all kdr mutations could likely have arisen due to recombination. This genotype provided an extremely high resistance level to pyrethroid insecticides when artificially introduced into Xenopus oocytes [40]. It may therefore be favoured by insecticide pressure, but its low frequency in natural populations suggests a high fitness cost in the absence of insecticide application. A major concern is that secondary mutations may arise that mitigate this fitness cost, which could then result in extremely high levels of pyrethroid resistance in Saudi Arabia. Continuous surveillance for this triply mutated haplotype, the triple homozygous mutant genotype and potential fitness-restoring mutations is a high priority.
A high frequency (~ 58%) of fully susceptible wild type genotypes (SS + VV + FF) was detected in this study in samples from Myanmar. They were collected in 2004–2005 when insecticide usage was likely much lower. This fully susceptible genotype was not reported in a more recent study in Myanmar; however, the susceptible haplotype is still present [27, 30]. Previous studies have reported the completely susceptible wild type genotype in the Indo-Pacific, including Taiwan [33], Queensland, Australia [67] and Malaysia [68]. For the first time, this study reported the presence of the triple homozygote wild type genotype in two individuals from the highlands of Jazan and the Al-Quoz governorate of Sahil, where it is most likely that insecticides have been less intensively applied than in the large cities. However, we can also infer the presence of the wild type haplotypes (S + V + F) from Jeddah and Makkah (from Figs. 2 and 3). In Saudi Arabia, the susceptible haplotype was observed only in Jeddah previously [18]. This indicates the possibility of restoring susceptibility to pyrethroids through a well-designed public health pest management strategy in Saudi Arabia.
Finally, for the first time, this study reported exceptionally high levels of genetic diversity in the Sahil and Jazan populations at wild type alleles that are comparable to African levels of genetic diversity. This implies a direct contribution of African ancestry to Saudi Arabian populations, which we will explore in detail elsewhere (Mashlawi et al. in preparation).
Conclusion
This study has brought new insight into the population genetics of kdr in Ae. aegypti populations from Saudi Arabia. The presence of the triple homozygous wild type haplotypes in the studied populations calls for further consideration of insecticide resistance management strategies that could restore sensitivity to pyrethroids in Ae. aegypti in Saudi Arabia. Two predominant genotypes were observed (SP + VG + FC and SS + VV + CC) in Ae. aegypti across five regions of Saudi Arabia indicating ongoing selection for resistance to pyrethroids. The genotyping results of specimens from Jazan, Sahil, Jeddah, Makkah and Madinah can be used as baseline information for a potential future trial of the Wolbachia-infected Ae. aegypti method for dengue control due to the requirement of homogeneity of kdr genotypes between field and released strains [35]. Long-term high levels of Ae. aegypti resistance to pyrethroids should not be considered inevitable. For example, although pyrethroid insecticides have been applied in Queensland, Australia since the 1990s in response to dengue fever outbreaks, a strategy of integrated vector control methods has delayed the emergence of resistance and as of 2017, there has been no evidence of kdr mutations [67]. An additional success story of integrated vector management is the case of Singapore, in which the integration of effective tools and approaches, improved surveillance, and community engagement were keys to success [69]. Integrated vector control using multiple approaches and alternatives to insecticides should be considered in Saudi Arabia. The presence of wild type haplotypes in Saudi Arabia carrying no kdr mutations offers the possibility to restore susceptibility that could allow improved efficacy of insecticides as part of an integrated vector control strategy.
Availability of data and materials
The sequences haplotype result for IIS6 and IIIS6 in vgsc, 498 bp and 567 bp, respectively, are available from NCBI GenBank (Accession for domain IIS6: ON651284-ON651417 and ON651284-ON651364 for domain IIIS6) and Dryad Dataset at https://datadryad.org/stash/share/wDIWDnXcz-KFbGRmvrMhcsqBZiB37u2YF_Qa3lToFx8
Abbreviations
- kdr :
-
Knockdown resistance
- VGSC:
-
Voltage-gated sodium channel
- MJ:
-
Median-joining
References
WHO. Dengue and severe dengue. Geneva: World Health Organization, 2022. http://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed 22 June 2022.
Powell JR, Gloria-Soria A, Kotsakiozi P. Recent history of Aedes aegypti: vector genomics and epidemiology records. Bioscience. 2018;68:854–60.
Kraemer MUG, Reiner RC Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63.
Mattingly PF, Knight KL. The mosquitoes of Arabia. I Bull Br Mus Nat Hist. 1956;4:91–141.
Khater EIM, Baig F, Kamal HA, Powell JR, Saleh AA. Molecular phylogenetics and population genetics of the dengue vector Aedes aegypti from the Arabian Peninsula. J Med Entomol. 2021;58:2161–76.
Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, et al. Global genetic diversity of Aedes aegypti. Mol Ecol. 2016;25:5377–95.
Fakeeh M, Zaki AM. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994–1999. Am J Trop Med Hyg. 2001;65:764–7.
Alhaeli A, Bahkali S, Ali A, Househ MS, El-Metwally AA. The epidemiology of Dengue fever in Saudi Arabia: a systematic review. J Infect Public Health. 2016;9:117–24.
Ministry of Health. Statistical Year Book 2019. Chapter III: Public Health. Riyadh: Ministry of Health, 2019. https://www.moh.gov.sa/en/Ministry/Statistics/book/Pages/default.aspx. Accessed 24 June 2022.
Fakeeh M, Zaki AM. Dengue in Jaddah. Saudi Arabia, 1994–2002. Dengue Bull. 2003;27:13–8.
Al-Azraqi TA, El Mekki AA, Mahfouz AA. Seroprevalence of dengue virus infection in Aseer and Jizan regions. Southwestern Saudi Arabia Trans R Soc. 2013;107:368–71.
Hussain R, Alomar I, Memish ZA. Chikungunya virus: emergence of an arthritic arbovirus in Jeddah. Saudi Arabia East Mediterr Health J. 2013;19:506–8.
Hakami AR, Alshamrani AA, Alqahtani M, Alraey Y, Alhefzi RA, Alasmari S, et al. Detection of chikungunya virus in the Southern region. Saudi Arabia Virol J. 2021;18:190.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLOS Negl Trop Dis. 2017;11:e0005625.
Al-Seghayer S, Kenawy M, Ali O. Malaria in the Kingdom of Saudi Arabia epidemiology and control. Sci J King Faisal University. 1999;1:6–20.
Coleman M, Al-Zahrani MH, Coleman M, Hemingway J, Omar A, Stanton MC, et al. A country on the verge of malaria elimination–the kingdom of Saudi Arabia. PLoS ONE. 2014;9:e105980.
Dieng H, Hassan AA, Satho T, Miake F, Salmah MRC, AbuBakar S. Insecticide susceptibility of the dengue vector Aedes aegypti (Diptera: Culicidae) in Makkah City, Saudi Arabia. Asian Pac J Trop Dis. 2011;1:94–9.
Al-Nazawi AM, Aqili J, Alzahrani M, McCall PJ, Weetman D. Combined target site (kdr) mutations play a primary role in highly pyrethroid resistant phenotypes of Aedes aegypti from Saudi Arabia. Parasit Vectors. 2017;10:161.
Alsheikh A, Mohammed W, Noureldin E, Daffalla O, Shrwani K, Hobani Y, et al. Resistance status of Aedes aegypti to insecticides in the Jazan region of Saudi Arabia. Biosci, Biotech Res Asia. 2016;13:155–62.
Al-Raddadi R, Alwafi O, Shabouni O, Akbar N, Alkhalawi M, Ibrahim A, et al. Seroprevalence of dengue fever and the associated sociodemographic, clinical, and environmental factors in Makkah, Madinah, Jeddah, and Jizan. Kingdom of Saudi Arabia Acta Trop. 2019;189:54–64.
Davies T, Field L, Usherwood P, Williamson M. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 2007;59:151–62.
WHO. WHO recommended insecticides for space spraying against mosquitoes. Geneva: World Health Organization, 2016. https://www.who.int/whopes/resources/who_cds_whopes_gcdpp_2003.5/en/. Accessed 20 June 2022.
Ranson H, Burhani J, Lumjuan N, Black WCIV. Insecticide resistance in dengue vectors. TDR/WHO; 2010.
Scott JG. Life and death at the voltage-sensitive sodium channel: Evolution in response to insecticide use. Annu Rev Entomol. 2019;64:243–57.
Chen M, Du Y, Nomura Y, Zhorov BS, Dong K. Chronology of sodium channel mutations associated with pyrethroid resistance in Aedes aegypti. Arch Insect Biochem Physiol. 2020;104:e21686.
Chang C, Shen WK, Wang TT, Lin YH, Hsu EL, Dai SM. A novel amino acid substitution in a voltage-gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect Biochem Mol Biol. 2009;39:272–8. https://doi.org/10.1016/j.ibmb.2009.01.001.
Naw H, Võ TC, Lê HG, Kang J-M, Mya YY, Myint MK, et al. Knockdown resistance mutations in the voltage-gated sodium channel of Aedes aegypti (Diptera: Culicidae) in Myanmar. Insects. 2022;13:322.
Srisawat R, Komalamisra N, Eshita Y, Zheng M, Ono K, Itoh TQ, et al. Point mutations in domain II of the voltage-gated sodium channel gene in deltamethrin-resistant Aedes aegypti (Diptera: Culicidae). Appl Entomol Zool. 2010;45:275–82.
Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara LA. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop Med Int Health. 2011;16:501–9.
Kawada H, Oo SZM, Thaung S, Kawashima E, Maung YNM, Thu HM, et al. Co-occurrence of point mutations in the voltage-gated sodium channel of pyrethroid-resistant Aedes aegypti populations in Myanmar. PLOS Negl Trop Dis. 2014;8:e3032.
Ishak IH, Jaal Z, Ranson H, Wondji CS. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors. 2015;8:181.
Wuliandari J, Lee S, White V, Tantowijoyo W, Hoffmann A, Endersby-Harshman N. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta. Indonesia Insects. 2015;6:658–85.
Chung HH, Cheng IC, Chen YC, Lin C, Tomita T, Teng HJ. Voltage-gated sodium channel intron polymorphism and four mutations comprise six haplotypes in an Aedes aegypti population in Taiwan. PLoS Negl Trop Dis. 2019;13:e0007291.
Kawada H, Higa Y, Futami K, Muranami Y, Kawashima E, Osei JH, et al. Discovery of point mutations in the voltage-gated sodium channel from African Aedes aegypti populations: potential phylogenetic reasons for gene introgression. PLOS Negl Trop Dis. 2016;10:e0004780.
Endersby-Harshman NM, Ali A, Alhumrani B, Alkuriji MA, Al-Fageeh MB, Al-Malik A, et al. Voltage-sensitive sodium channel (Vssc) mutations associated with pyrethroid insecticide resistance in Aedes aegypti (L.) from two districts of Jeddah, kingdom of Saudi Arabia: baseline information for a Wolbachia release program. Parasit Vectors. 2021;14:361.
Cosme LV, Gloria-Soria A, Caccone A, Powell JR, Martins AJ. Evolution of kdr haplotypes in worldwide populations of Aedes aegypti: Independent origins of the F1534C kdr mutation. PLoS Negl Trop Dis. 2020;14:e0008219.
Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black Iv WC. Coevolution of the Ile 1,016 and Cys1,534 mutations in the voltage gated sodium channel gene of Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2015;9:e0004263.
Stenhouse SA, Plernsub S, Yanola J, Lumjuan N, Dantrakool A, Choochote W, et al. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasit Vectors. 2013;6:253.
Plernsub S, Saingamsook J, Yanola J, Lumjuan N, Tippawangkosol P, Sukontason K, et al. Additive effect of knockdown resistance mutations, S989P, V1016G and F1534C, in a heterozygous genotype conferring pyrethroid resistance in Aedes aegypti in Thailand. Parasit Vectors. 2016;9:417.
Hirata K, Komagata O, Itokawa K, Yamamoto A, Tomita T, Kasai S. A single crossing-over event in voltage-sensitive Na+ channel genes may cause critical failure of dengue mosquito control by insecticides. PLoS Negl Trop Dis. 2014;8:e3085.
General Authority for Statistics (GASTAT). Population by gender, age groups and nationality (Saudi/Non-Saudi). Saudi Census. 2019. http://www.stats.gov.sa. Accessed on 25 June 2022.
Humphrey JM, Cleton NB, Reusken CBEM, Glesby MJ, Koopmans MPG, Abu-Raddad LJ. Dengue in the middle East and North Africa: a systematic review. PLOS Negl Trop Dis. 2016;10:e0005194.
Abdulaal WA. Large urban developments as the new driver for land development in Jeddah. Habitat Int. 2012;36:36–46.
Waters ME: Jeddah Islamic Port (2017). Access Year 2018.
Zayed H, C GPD, Zowalaty MEE. Potential routes of spread of Zika virus to the Middle East, North Africa and Asia: action must be taken. Future Virol. 2017;12:159–62.
General Authority for Statistics (GASTAT). The sixteenth services guide 2017- Al-Madinah Al-Monawarah Region. 2017. https://www.stats.gov.sa/sites/default/files/al-madinah_al-monawarah_region_en.pdf. Accessed on 6 Aug 2022.
Mashlawi AM, Jordan HR, Crippen LT, Tomberlin JK. Impact of mycolactone produced by Mycobacterium ulcerans on life-history traits of Aedes aegypti (L) and resulting habitat selection for oviposition. Trop Biomed. 2020;37:973–85.
Rueda LM. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa. 2004;589:1–60.
Hlaing T, Tun-Lin W, Somboon P, Socheat D, Setha T, Min S, et al. Spatial genetic structure of Aedes aegypti mosquitoes in mainland Southeast Asia. Evol Appl. 2010;3:319–39.
Bennett KL, Shija F, Linton YM, Misinzo G, Kaddumukasa M, Djouaka R, et al. Historical environmental change in Africa drives divergence and admixture of Aedes aegypti mosquitoes: a precursor to successful worldwide colonization? Mol Ecol. 2016;25:4337–54.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–2. https://doi.org/10.1093/bioinformatics/btp187.
Leigh JW, Bryant D. POPART: full-feature software for haplotype network construction. Methods Ecol Evol. 2015;6:1110–6.
Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37–48.
Ministry of Health. Statistical Year Book 2016. Riyadh: Ministry of Health, 2016. https://www.moh.gov.sa/en/Ministry/Statistics/book/Documents/Statistical-Yearbook-1437H.pdf. Accessed 20 June 2022.
Fang Y, Tambo E, Xue JB, Zhang Y, Zhou XN, Khater EIM. Molecular analysis of targeted insecticide resistance gene mutations in field-caught mosquitos of medical importance from Saudi Arabia. J Med Entomol. 2021;58:1839–48.
Dafalla O, Alsheikh A, Mohammed W, Shrwani K, Alsheikh F, Hobani Y, et al. Knockdown resistance mutations contributing to pyrethroid resistance in Aedes aegypti population. Saudi Arabia East Mediterr Health J. 2019;25:905–13.
Satoto TBT, Satrisno H, Lazuardi L, Diptyanusa A, Purwaningsih R, et al. Insecticide resistance in Aedes aegypti: An impact from human urbanization? Plos One. 2019;14:e0218079.
Rohani A, Chu W, Saadiyah I, Lee H, Phang S. Insecticide resistance status of Aedes albopictus and Aedes aegypti collected from urban and rural areas in major towns of Malaysia. Trop Biomed. 2001;18:29–39.
Tabbabi A, Daaboub J, Ben Cheikh R, Laamari A, Feriani M, Boubaker C, et al. The potential role of urbanization in the resistance to organophosphate insecticide in Culex pipiens pipiens from Tunisia. Afr Health Sci. 2019;19:1368–75.
Plernsub S, Saingamsook J, Yanola J, Lumjuan N, Tippawangkosol P, Walton C, et al. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta Trop. 2016;162:125–32.
Saingamsook J, Yanola J, Lumjuan N, Walton C, Somboon P. Investigation of relative development and reproductivity fitness cost in three insecticide-resistant strains of Aedes aegypti from Thailand. Insects. 2019;10:265.
Martins AJ, Lins RM, Linss JG, Peixoto AA, Valle D. Voltage-gated sodium channel polymorphism and metabolic resistance in pyrethroid-resistant Aedes aegypti from Brazil. Am J Trop Med Hyg. 2009;81:108–15.
Abernathy HA, Hollingsworth BD, Giandomenico DA, Moser KA, Juliano JJ, Bowman NM, et al. Prevalence of knock-down resistance F1534S mutations in Aedes albopictus (skuse) (Diptera: Culicidae) in North Carolina. J Med Entomol. 2022;10:1093–2054.
Xu J, Bonizzoni M, Zhong D, Zhou G, Cai S, Li Y, et al. Multi-country survey revealed prevalent and novel F1534S mutation in voltage-gated sodium channel (VGSC) Gene in Aedes albopictus. PLoS Negl Trop Dis. 2016;10:e0004696.
Marcombe S, Fustec B, Cattel J, Chonephetsarath S, Thammavong P, Phommavanh N, et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl Trop Dis. 2019;13:e0007852.
Endersby-Harshman NM, Wuliandari JR, Harshman LG, Frohn V, Johnson BJ, Ritchie SA, et al. Pyrethroid susceptibility has been maintained in the dengue vector, Aedes aegypti (Diptera: Culicidae), in Queensland. Australia J Med Entomol. 2017;54:1649–58.
Ahmad NA, Endersby-Harshman NM, Mohd Mazni NR, Mohd Zabari NZA, Amran SNS, Ridhuan Ghazali MK, et al. Characterization of sodium channel mutations in the dengue vector mosquitoes Aedes aegypti and Aedes albopictus within the context of ongoing Wolbachia releases in Kuala Lumpur. Malaysia Insects. 2020;11:529.
Sim S, Ng LC, Lindsay SW, Wilson AL. A greener vision for vector control: The example of the Singapore dengue control programme. PLOS Negl Trop Dis. 2020;14:e0008428.
Acknowledgements
We are grateful to Dr Luciano Cosme and Dr Ademir Martins for helpful discussions. We also thank the Office of Research Administration, Chiang Mai University, Thailand, for supporting the mosquito collection in Thailand and Jazan University, Saudi Arabia for their support.
Funding
Jazan University, Saudi Arabia and the Saudi Arabia Cultural Bureau in London (Ph.D. scholarship for A.M.) and the Research England QR GCRF allocation to the University of Manchester.
Author information
Authors and Affiliations
Contributions
AM and CW designed the study and wrote the paper. AM, HA, HMA, JS and CW analyzed and interpreted the data. JM, JS, MD and HMA provided research services for the study. AA, EN and MK provided samples. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Localities/Districts, region, number of sites, coordinates and elevation (m = meter above the sea level) of collections of Aedes aegypti in Saudi Arabia. Table S2 Countries, regions, coordinates and collection year of Aedes aegypti in Southeast Asia. Table S3. Four synonymous mutations observed in domains IIS6 and IIIS6 of Aedes aegypti in Saudi Arabia and Southeast Asia. Table S4. A summary of vector control strategies and insecticide spraying regimes in major cities of Saudi Arabia, Thailand, Myanmar and Uganda. Text S1. DNA cloning protocol. Figure S1. Median-joining haplotypes network analysis for domain IIS6 of vgsc in 40 populations of Aedes aegypti worldwide. The original haplotype networks are with notes. Circles with colour represent the haplotype and population. Haplotypes are connected according to their similarity, and hatch marks between haplotypes show the base-pair mutations. S = susceptible; R = resistant. Figure S2. Median-joining haplotypes network analysis for domain IIIS6 of vgsc in 27 populations of Aedes aegypti worldwide. The original haplotype networks are with notes. Circles with colour represent the haplotype and population. Haplotypes are connected according to their similarity, and hatch marks between haplotypes show the base-pair mutations. Figure S3. The SNP sites identified in each domain to infer the haplotype network. A is for domain IIS6 and B is for domain IIIS6. Red squares indicate the non-synonymous mutations (kdr) in each domain. These are haplotype networks amongst our sequences.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mashlawi, A.M., Al-Nazawi, A.M., Noureldin, E.M. et al. Molecular analysis of knockdown resistance (kdr) mutations in the voltage-gated sodium channel gene of Aedes aegypti populations from Saudi Arabia. Parasites Vectors 15, 375 (2022). https://doi.org/10.1186/s13071-022-05525-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-022-05525-y