Abstract
Background
This study aims to provide baseline data on the resistance status to insecticides, the frequency of mechanisms involved and the impact of the association with the synergist piperonyl butoxide (PBO) on resistant Anopheles gambiae (s.l.) populations in two regions of northern Benin, prior to an indoor residual spraying campaign and introduction of next generation long-lasting insecticidal nets (LLINs) incorporating PBO.
Methods
Adult Anopheles gambiae (s.l.) originating from larvae collected in two study regions (Alibori within the Kandi-Gogounou-Segbana districts and Donga within the Djougou-Copargo-Ouake districts) were tested with impregnated papers (bendiocarb 0.1%, pirimiphos-methyl 0.25%, permethrin 0.75% and deltamethrin 0.05%). The synergist PBO was used to check for the involvement of detoxification enzymes in pyrethroid resistant populations. Molecular analyses were performed for the identification of species within the Anopheles gambiae (s.l.) complex and kdr L1014F and G119S Ace-1 mutations. Biochemical assays assessed the activity of detoxification enzymes.
Results
Anopheles gambiae (s.l.) was resistant to pyrethroids, with a mortality range of 25–83% with deltamethrin and 6–55% with permethrin. A significant increase in mortality was observed after pre-exposure to PBO for both deltamethrin (63–99%) and permethrin (56–99%). With bendiocarb, An. gambiae (s.l.) were susceptible in Kandi (99% mortality), with possible resistance (92–95%) recorded in Djougou, Copargo, Gogounou, Ouake and Segbana. All study populations were fully susceptible to pirimiphos-methyl. The frequencies of resistant mutations varied according to species and sites: 0.67–0.88 for L1014F kdr and 0–0.06 for G119S Ace-1. Three study locations (Djougou, Gogounou and Kandi) showed high oxidase activity and four sites (Djougou, Ouake, Copargo and Kandi) showed elevated esterase activity.
Conclusions
This study confirms resistance to pyrethroids and suggests emerging bendiocarb resistance in An. gambiae (s.l.) populations in northern Benin. However, recovery of susceptibility to pyrethroids after PBO exposure, and susceptibility to organophosphates in the An. gambiae (s.l.) populations indicate that next generation LLINs incorporating PBO synergist combined with an indoor residual spraying (IRS) campaign with organophosphate insecticides may be regarded as alternative control tools.
Similar content being viewed by others
Background
Vector control is an essential component in malaria prevention strategies [1]. In Africa, it relies primarily on two effective and complementary tools: long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [2,3,4]. Several studies have demonstrated the effectiveness of both tools in reducing the incidence of malaria [5, 6] morbidity and mortality in Africa [7,8,9,10]. In Benin, malaria vector control relies mainly on the mass distribution of LLINs, and on IRS operations. From 2008 to 2015, IRS with bendiocarb (a carbamate) in southern Benin and with pirimiphos-methyl (an organophosphate) in the northern region, showed a significant reduction in malaria transmission [11, 12]. Although LLINs and IRS have been shown to be effective, they have performed below expectations in some settings, including several locations in Benin [13,14,15]. One of the reasons is the emergence and expansion of resistance of Anopheles vectors to insecticides, especially pyrethroids [16,17,18,19,20,21,22,23] and more recently bendiocarb [24,25,26]. The main insecticide resistance mechanisms involve an increase in the activity of detoxification enzymes (oxidases, esterases and glutathione-S-transferases) [18, 19, 27, 28] and the kdr L1014F and G119S Ace-1 target site mutations frequently found in An. gambiae (s.l.) populations [16, 29,30,31]. Studies suggest that the use of the same classes of insecticides in public health as well as in agriculture, especially in cotton cultivation, may have led to the increase in the allelic frequencies of the kdr L1014F and G119S Ace-1 in Benin [22, 23, 25, 26, 32, 33]. In Benin, the IRS program implemented in 2017 targeted all houses in the regions of Alibori and Donga with pirimiphos-methyl. In the same year, the Benin national malaria control program (NMCP), supported by USAID, Global Fund and the WHO, undertook large-scale distribution of Yorkool LLINs impregnated with deltamethrin. In preparation for the implementation of these two control campaigns, the present study was initiated to collect data on the resistance of vectors to insecticides in the two targeted regions. These baseline data inform selection of insecticide candidates for IRS and help to define strategies for effective insecticide resistance management in the study area.
Methods
Study regions and mosquito sampling sites
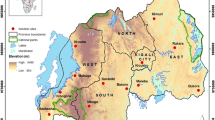
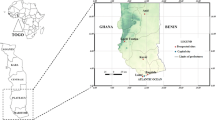
The study was conducted during the rainy season (June to October 2016) in six districts of northern Benin. These six districts are grouped into two healthcare facility’s catchment areas: the Kandi-Gogounou-Segbana health zone (KGS) located in the Alibori region and the Djougou-Copargo-Ouake health zone (DCO) in the Donga region. The general census of the population and housing carried out in May 2013 revealed estimated populations of 867,463 and 543,130 inhabitants in Alibori and Donga, respectively [34]. These two regions are located in a dry savanna area and in a dry and wet savanna area, respectively (Fig. 1). The Alibori region is crossed by several rivers and water dams and the soil is sandy. In the Donga region, the soil is clay.
Study areas
Kandi-Gogounou-Segbana (KGS) health zone
This area covers approximately 12,943 km2 and is the largest health zone in Benin. It is composed of three districts: Kandi (11°07' to 29.32'N, 2°56' to 9.57'E), Gogounou (10°33' to 10°57'N, 2°15' to 3°15'E) and Segbana (10°32' to 11°23'N, 3°08' to 3°50'E) (Fig. 1). It is recognized as the cotton cultivation area of northern Benin, with large quantities of insecticides used to control cotton pests [35]. Its climate is Sudanese with two seasons a year: a single rainy season from May to October and a dry season from November to April. The mean temperature and relative humidity are about 28 °C and 70%, respectively. Rainfall varies between 700–1200 mm with heavy rainfall recorded between July and September [36].
Djougou-Copargo-Ouake (DCO) health zone
This health zone covers an area of 5465 km2 and is composed of three districts: Djougou (09°42' to 10°1'N, 01°40' to 55°4'E), Copargo (09°50' to 19°3'N, 01°32' to 39°5”E) and Ouake (09°40' to 45.3'N, 01°22' to 51°7'E) (Fig. 1). In this zone, Djougou is the district where cotton cultivation is highly developed with a high use of insecticide [35]. It has a Sudano-Guinean climate with two seasons. The rainy season extends over 6 months (from mid-April to mid-October). The average rainfall is between 1200–1300 mm. The mean temperature is around 27 °C. The main crops are yam, cereal and cotton [37].
Mosquito collections
Larvae of Anopheles mosquitoes were collected in breeding sites using the dipping technique. Larvae and pupae were collected from various breeding sites (e.g. rain water collection, irrigation channels, river beds, wells, etc.), so that the mosquitoes tested were fully representative of the vector population in the area.
Insufficient numbers of larvae were collected at Segbana and Ouake to perform all of the susceptibility bioassay tests. Species were identified using a morphological key [38].
Insecticide susceptibility tests
Susceptibility tests were performed using the WHO tube bioassay test [39]. The following insecticides and synergist were tested: deltamethrin (0.05%), permethrin (0.75%), deltamethrin (0.05%) + PBO (4%), permethrin (0.75%) + PBO (4%), bendiocarb (0.1%), and pirimiphos-methyl (0.25%).
The PBO synergist was used to evaluate the involvement of detoxification enzymes (oxidases and esterases) [40] in the phenotypic resistance of the populations of An. gambiae (s.l.) of each district.
About 100 adult female mosquitoes were exposed to each insecticide, tested in 4 replicates each of c.25 mosquitoes. In addition, 50 mosquitoes served as controls in 2 replicates of c.25 mosquitoes. Knockdown was recorded at 10, 15, 20, 30, 40, 50 and 60 min. All tests were conducted at 25 °C and 80% humidity.
Mortality after 24 h was determined and interpreted according to the WHO protocol [39]. At the end of the tests, live and dead specimens from each district were used for species identification and determination of resistance mechanisms (kdr L1014F, kdr L1014S and G119S Ace-1) using PCR methods.
According to the site, 72–270 individuals randomly selected from live and dead mosquitoes from susceptibility tests were analyzed according to the protocol of Santolamazza et al. [41] to determine species within the An. gambiae (s.l.) complex. The same mosquitoes were genotyped for the kdr L1014F, kdr L1014S and G119S Ace-1 mutations, according to the protocols of Martinez-Torres et al. [29], Ranson et al. [30] and Weill et al. [42], respectively.
Biochemical analyses
Thirty females of An. gambiae (s.l.) from each district of the KGS and DCO health zones, aged 2–5 days and which were not previously used for any insecticidal test, were used for biochemical analyses. Biochemical assays were performed to compare the level of activities of mixed function oxidases (MFOs), non-specific esterases (α and β-esterases) and glutathione S-transferases (GSTs) [43] of the different field mosquito populations to the Kisumu susceptible strain. All mosquitoes were tested according to the protocol described by Hemingway et al. [44]. Oxidase activity was assessed with the heme-peroxidase test which allowed detection of the increase in the quantity of heme. Alpha-Naphthol acetate (αNaph) (Sigma N-1000, Saint Louis, Missouri, USA) and Beta-Naphthol acetate (βNaph) (Sigma N-185507) were used to evaluate the non-specific esterase activity. GST activity was determined by measuring in time the formation of the Glutathione-S-CDNB at 340 nm after a catalysis reaction between the 1-chloro-2,4-dinitrobenzene (CDNB) and reduced glutathione (GSH).
Data analysis
Any mosquito population with a mortality rate between 98–100% was considered susceptible. When mortality was between 90–97%, the population was suspected of resistance. Below a 90% mortality rate, the population was considered resistant. The mortality rates of populations of An. gambiae (s.l.) were compared using a Chi-square test of comparison of proportions. The allelic frequencies of kdr L1014F and G119S Ace-1 were calculated as follows: F(R) = [2n.RR+ n.RS]/[2(n.RR+ n.RS+ n.SS)] [45] (n. is the number of mosquitoes of a given genotype), to assess their variability across populations. A linear regression with variance analysis was used to assess the variation of enzymatic activity in each locality. Mann-Whitney U-test was used to compare enzyme activity between field- and laboratory-susceptible mosquitoes (Kisumu). Statistical analyses were performed with software R 3.3.2 [46].
Results
Mortality rates of An. gambiae (s.l.)
With pirimiphos-methyl, the mortality rates observed in all tested populations were 100%, thus showing full susceptibility (Fig. 2a).
The mortality rate observed with bendiocarb in Kandi was 98.88% (Fig. 2b), which shows susceptibility of this mosquito population. In the other five districts, possible resistance was observed with mortality rates varying from 92.63% in Ouake to 95.23% in Djougou (Fig. 2b).
All populations of An. gambiae (s.l.) from the surveyed districts, were found to be resistant to permethrin with mortality rates ranging from 6.06% in Djougou to 55.1% in Copargo) (Fig. 3a). In Djougou, Kandi, Gogounou and Copargo, the mortality rates increased from 6.06, 9.09, 44.21 and 55.1%, respectively, with permethrin alone to 93.93% (χ2 = 149.41, df = 1, P < 0.0001), 57.57% (χ2 = 50.205, df = 1, P < 0.0001), 82.52% (χ2 = 53.269, df = 1, P < 0.0001) and 99.02% (χ2 = 53.269, df = 1, P < 0.0001) with permethrin + PBO (Fig. 3a).
All populations tested were resistant to deltamethrin with mortality rates ranging from 25.27% (Djougou) to 83.14% (Gogounou) (Fig. 3b). With pre-exposure to PBO, an increase in susceptibility to deltamethrin was noted. At Copargo, Gogounou, Djougou, Kandi and Segbana, mortality rates increased from 79.2, 83.14, 25.27, 41.41 and 60%, respectively, with deltamethrin alone to 99.06% (χ2 = 19.613, df = 1, P = 0.0009), 98.03% (χ2 = 11.23, df = 1, P = 0.0008), 93.20% (χ2 = 109.04, df = 1, P < 0.0001), 63.10% (χ2 = 8.675, df = 1, P = 0.0322) and 85.71% (χ2 = 15.967, df = 1, P = 0.0064) with deltamethrin + PBO (Fig. 3b).
Distribution of sibling species of the Anopheles gambiae complex by site and in dead and live mosquitoes
Out of the 1163 specimens of An. gambiae (s.l.) analyzed by PCR in the two investigated health zones, 55.46% were An. gambiae and 44.54% An. coluzzii. Overall, An. coluzzii was predominant in Kandi and Ségbana with a mean of 61.6% in the KGS health zone as compared to An. gambiae (38.4%). In the DCO health zone, An. gambiae was in majority in all three districts (Djougou, Copargo and Ouaké) with a mean of 68.92% compared to An. coluzzii (31.08%) (Table 1).
Overall, in Kandi, Gogounou and Segbana, mortality occurred mostly in An. coluzzii as compared to An. gambiae (χ2 = 13.357, df = 1, P = 0.0003 and χ2 = 13.837, df = 1, P = 0.0002, for bendiocarb and deltamethrin, respectively) in the KGS health zone (Table 2). By contrast, in the DCO health zone, mortality occurred similarly in An. gambiae and An. coluzzii (χ2 = 1.456, df = 1, P = 0.227 for bendiocarb; χ2 = 0.482, df = 1, P = 0.487 for permethrin; and χ2 = 0.0359, df = 1, P = 0.849 for deltamethrin) (Table 2).
Distribution of L1014F kdr and G119S Ace-1 mutations in An. gambiae and An. coluzzii
Tables 3 and 4 show the distribution of the frequency of the kdr L1014F and G119S Ace-1 mutations in An. gambiae and An. coluzzii in the six surveyed districts. Overall, the mean frequency of the kdr L1014F gene at all districts was 0.77. These frequencies were higher in An. gambiae than in An. coluzzii at all sites with a significant difference between the frequencies of the two sibling species at Kandi and Gogounou (Table 3). The kdr L1014S resistant allele was not detected in our samples.
The G119S Ace-1 mutation was identified in all districts at very low frequency (between 1–6%) (Table 4). It varied from 2 to 6% in An. gambiae and from 0 to 2% in An. coluzzii (Table 4) with a significant difference between both species when all sites were combined.
Expression of oxidases, esterases and GSTs in An. gambiae (s.l.)
Figures 4 and 5 display the mean levels of enzymatic activities in field mosquito populations and the Kisumu reference susceptible strain. In all investigated districts, at least one class of detoxification enzyme revealed elevated activity relative to the Kisumu strain. Oxidase activity was significantly elevated in the districts of Djougou (Mann-Whitney U-test, U = 48.50, P < 0.0001), Gogounou (U = 149.5, P < 0.0001) and Kandi (U = 280.5, P < 0.0001) compared to the Kisumu strain (Fig. 4a). The highest glutathione-S-transferase (GST) activities were observed in the Copargo and Gogounou populations with a significant difference compared to the Kisumu strain (U = 312, P = 0.0009 and U = 151.1, P < 0.0001, respectively) (Fig. 4b).
Mono-oxygenase (a) and glutathione-S-transferase (b) activities in field populations of Anopheles gambiae (s.l.). *Population with a significantly higher enzyme activity as compared to the Kisumu reference susceptible strain. The red horizontal lines indicates the mean level of enzymatic activity. Abbreviations: MFO, Mixed function oxidases; GST, glutathione S-transferase
The activity of α esterases was higher in the populations of Djougou (U = 369, P = 0.009), Ouake (U = 190, P = 0.0014) and Kandi (U = 322, P = 0.0005) compared to the Kisumu strain (Fig. 5a). Significantly elevated β esterase activities were observed in Djougou (U = 265.5, P = 0.0001), Ouake (U = 157, P = 0.0002), Copargo (U = 357, P = 0.0055) and Kandi (U = 144, P < 0.0001) compared to the Kisumu strain (Fig. 5b).
Discussion
Monitoring is an integral part of any resistance management strategy which allows informed decisions about the choice of insecticides [47]. The present study shows confirmed resistance of malaria vectors to deltamethrin and permethrin (pyrethroids), increased susceptibility to pyrethroids through the use of PBO, decreased susceptibility to bendiocarb in some districts and full susceptibility to pirimiphos-methyl.
The predominance of An. coluzzii in Alibori could be due to the presence of rivers and water dams that create numerous permanent and semi-permanent larval habitats conducive to the emergence of this mosquito species. In addition, the sandy soil of the region may cause a fast infiltration of water after rainfall, which would favor the formation of only very few temporary breeding sites. By contrast, the clay soil in Donga retains water after rainfall and, as a result, several temporary larval habitats could be formed, thus allowing the development of An. gambiae which was in majority in this region.
In the present study, levels of resistance to permethrin and deltamethrin observed after recording the 24-h mortality rates varied between districts. Pyrethroid resistance in An. gambiae (s.l.) observed in the present study confirms findings of previous studies carried out in Benin [22, 23, 25]. The lowest mortality rates to pyrethroids were observed in the districts of Kandi, Djougou and Segbana. This could be due to the strong selection pressure exerted by the large-scale cotton production [35]. The wide distribution and high level of malaria vectors’ resistance to pyrethroids might be due to the expansion of agriculture [48, 49] and the mass use of pyrethroid-treated mosquito nets, distributed at the national level over past years [50, 51]. As mortality occurred mostly in the predominant species (An. coluzzii) in the KGS health zone but was similar in each species in the DCO health zone, no conclusion can be drawn on a higher susceptibility to insecticides between the species.
Overall, An. gambiae (s.l.) was resistant to pyrethroids and displayed high frequencies of the kdr L1014F mutation in all surveyed districts. The kdr L1014F frequency was higher in An. gambiae than in An. coluzzii in most localities, which confirms the recent findings of Gnanguenon et al. [26] and Yahouédo et al. [52] in some sites located on the north-south axis of Benin. Several previous studies have also shown that frequencies of kdr L1014F are higher in An. gambiae in west and central Africa compared to An. coluzzii [41, 53], except for some urban and peri-urban coastal areas [54]. In addition to kdr L1014F that could compromise the effectiveness of vector control tools such as LLINs and IRS [55], the involvement of mono-oxygenases in pyrethroids resistance in our study sites has also been noted since the level of vector resistance to deltamethrin and permethrin was significantly reduced by the use of PBO. These oxidases are involved in the detoxification of pyrethroids in Anopheles gambiae (s.l.) [56, 57]. Moreover, our biochemical data have revealed their overexpression in An. gambiae (s.l.) in Djougou, Gogounou and Kandi. This result is similar to that obtained by Djouaka et al. [27] in natural populations of An. funestus (s.l.) in Pahou and confirms the works of Djègbè et al. [22] and Aizoun et al. [24] at Kandi and Malanville, respectively, two sites of northern Benin near our study area. The simultaneous presence of kdr L1014F and elevated oxidase activity could confer higher resistance in mosquitoes. In these conditions, the use of PBO LLINs (Permanet 3.0 nets and Olyset Plus mosquito nets) associated with a pirimiphos-methyl-based IRS could be implemented for effective malaria vector control in Alibori and Donga, two regions selected to receive both interventions. Indeed, a recent study carried out by Protopopoff et al. [58] in Tanzania reported a better performance of the strategy combining PBO LLINs and IRS with pirimiphos-methyl on malaria transmission as compared to standard LLINs, in pyrethroids resistance areas. The high activity of glutathione-S-transferases in the wild populations of An. gambiae (s.l.) in the districts of Copargo and Gogounou could play a minor role in the resistance to pyrethroids due to the oxidative stress [59]. Other studies attribute this overexpression of GSTs to the resistance of An. gambiae (s.l.) to DDT [19]. In such case, in the presence of kdr L1014F, GSTs could increase phenotypic resistance to pyrethroids and DDT and broaden the spectrum of resistance to independent compounds [60].
The possible resistance to bendiocarb (carbamate) and the low frequencies of the G119S Ace-1 observed in our study sites was also previously reported by Djenontin et al. [61]. This start of resistance to bendiocarb and the observed presence of some heterozygous (RS) individual mosquitoes for the G119S Ace-1R mutation is worrying given several studies have shown that this insecticide represents a potential alternative to pyrethroids for the management of resistance [62, 63]. The highest frequencies of G119S Ace-1R were found in An. gambiae (2–6%) and the lowest in An. coluzzii (0–2%). These results corroborate those obtained by Aikpon et al. [25] and Gnanguenon et al. [26] in the Atacora and Kandi districts of Benin, respectively. However, even though the G119S Ace-1 mutation is often incriminated in vectors resistant to carbamates and organophosphates, it does not fully explain the observed possible resistance to bendiocarb, because some susceptible homozygous individuals survive after exposure to this carbamate [54]. Susceptibility of An. gambiae (s.l.) to pirimiphos-methyl observed in our study area confirms the findings of Asidi et al. [64] which showed that the presence of G119S Ace-1 does not confer a systematic resistance to organophosphates.
Conclusions
With high pyrethroid resistance, overexpression of some metabolic enzymes (MFO, GST) and the high kdr L1014F allelic frequencies observed in An. gambiae (s.l.) in the KGS and DCO health zones, IRS with pirimiphos-methyl - for which full susceptibility was detected - is recommended for control. Furthermore, the increased susceptibility level of vectors to pyrethroids after pre-exposure to PBO suggests that implementation of PBO-treated LLINs (Permanet 3.0 and OlysetPlus) could be a productive strategy to replace conventional LLINs in the two targeted regions.
Abbreviations
- IRS:
-
indoor residual spraying
- USAID:
-
United States agency for international development
- WHO:
-
world health organization
- LLIN:
-
long lasting insecticidal nets
- NMCP:
-
national malaria control program
- MFO:
-
mixed function oxidases
- GST:
-
glutathione S-transferase
- PBO:
-
piperonyl butoxide
References
WHO. Global Plan for Insecticide Resistance Management. Geneva: World Health Organization; 2012.
Chavasse DC, Yap HH, World Health Organization. Division of Control of Tropical Diseases. Chemical methods for the control of vectors and pests of public health importance. Geneva: World Health Organization; 1997.
Curtis CF, Mnzava AE, Misra S, Rowland M. Malaria control: bednets or spraying? Summary of the presentations and the discussion. Trans R Soc Trop Med Hyg. 1999;93:460.
Okumu FO, Moore SJ. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J. 2011;10:208.
Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363.
Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657.
Alonso PL, Lindsay SW, Armstrong JR, Conteh M, Hill AG, David PH, et al. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–02.
Alonso PL, Lindsay SW, Armstrong Schellenberg JR, Keita K, Gomez P, Shenton FC, et al. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. The impact of the interventions on mortality and morbidity from malaria. Trans R Soc Trop Med Hyg. 1993;2:37–44.
D’Alessandro U, Olaleye BO, McGuire W, Langerock P, Bennett S, Aikins MK, et al. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. Lancet. 1995;345:479–83.
Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH, et al. Impact of permethrin impregnated bednets on child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial. Trop Med Int Health. 1996;1:147–54.
Akogbéto M, Padonou GG, Bankole HS, Gazard DK, Gbedjissi GL. Dramatic decrease in malaria transmission after large-scale indoor residual spraying with bendiocarb in Benin, an area of high resistance of Anopheles gambiae to pyrethroids. Am J Trop Med Hyg. 2011;85:586–93.
Akogbéto MC, Aikpon R, Azondekon R, Padonou G, Osse R, Agossa FR, et al. Six years of experience in entomological surveillance of indoor residual spraying against malaria transmission in Benin: lessons learned, challenges and outlooks. Malar J. 2015;14:242.
N’Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area. Benin. Emerg Infect Dis. 2007;13:199–06.
Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis. 2008;8:387–9.
Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8.
Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, et al. Current distribution of pyrethroid resistance gene (kdr) in Anopheles gambiae complex from West Africa and further evidence for reproductive isolation of Mopti form. Parassitologia. 1999;41:319–22.
Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–9.
Etang J, Manga L, Chandre F, Guillet P, Fondjo E, Mimpfoundi R, et al. Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera:Culicidae) in the Republic of Cameroon. J Med Entomol. 2003;40:491–7.
Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin. West Africa. Acta Trop. 2007;101:207–16.
Yadouleton A, Asidi A, Djouaka R, Braïma J, Agossou C, Akogbeto M. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103.
Djogbenou L, Pasteur N, Bio-Bangana S, Baldet T, Irish SR, Akogbeto M, et al. Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114:116–22.
Djegbe I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261.
Sovi A, Djegbe I, Soumanou L, Tokponnon F, Gnanguenon V, Azondekon R, et al. Microdistribution of the resistance of malaria vectors to deltamethrin in the region of Plateau (southeastern Benin) in preparation for an assessment of the impact of resistance on the effectiveness of long-lasting insecticidal nets (LLINs). BMC Infect Dis. 2014;14:103.
Aïzoun N, Aïkpon R, Gnanguenon V, Oussou O, Agossa F, Padonou GG, et al. Status of organophosphate and carbamate resistance in Anopheles gambiae sensu lato from the south and north Benin, West Africa. Parasit Vectors. 2013;6:274.
Aïkpon R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, et al. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192.
Gnanguenon V, Agossa FR, Badirou K, Govoetchan R, Anagonou R, Oke-Agbo F, et al. Malaria vectors resistance to insecticides in Benin: current trends and mechanisms involved. Parasit Vectors. 2015;8:223.
Djouaka R, Irving H, Tukur Z, Wondji CS. Exploring mechanisms of multiple insecticide resistance in a population of the malaria vector Anopheles funestus in Benin. PLoS One. 2011;6:e27760.
Mitchell SN, Stevenson BJ, Müller P, Wilding CS, Egyir-Yawson A, Field SG, et al. Identification and validation of a gene causing cross resistance between insecticide classes in Anopheles gambiae from Ghana. Proc Natl Acad Sci USA. 2012;109:6147–52.
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroids knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84.
Ranson H, Jensen B, Vulule J, Wang X, Hemingway J, Collins F. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–7.
Awolola TS, Oyewole IO, Amajoh CN, Idowu ET, Ajayi MB, Oduola A, et al. Distribution of the molecular forms of Anopheles gambiae and pyrethroid knock down resistance gene in Nigeria. Acta Trop. 2005;95:204–5.
Yadouleton A, Martin T, Padonou G, Chandre F, Asidi A, Djogbenou L, et al. Cotton pest management practices and the selection of pyrethroids resistance in Anopheles gambiae population in northern Benin. Parasit Vectors. 2011;4:60.
Djogbenou L, Pasteur N, Akogbeto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25:256–67.
INSAE. RGPH4 : Que retenir des effectifs de population en 2013 au Bénin? https://www.insae-bj.org/images/docs/insae-statistiques/demographiques/population/Resultats%20definitifs%20RGPH4.pdf. Accessed 21 Oct 2018.
Ton P. La production du coton au Bénin. 2004. http://www.slire.net/download/1889/la_production_du_coton_au_b_nin.pdf. Accessed 13 Oct 2018.
Ministère de la santé du Bénin. Annuaire des statistiques sanitaires 2013, zone sanitaire de Kandi-Gogounou-Segbana; 2014.
Ministère de la santé du Bénin. Annuaire des statistiques sanitaires 2014 des départements de l’Atacora et de la Donga; 2015.
Gillies MT, Coetzee MA. Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region). Pub South Afri Inst Med Res. 1987;55:141–3.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2013.
Khot AC, Bingham G, Field LM, Moores GD. A novel assay reveals the blockade of esterases by piperonyl butoxide. Pest Manag Sci. 2008;64:1139–42.
Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 2008;7:163.
Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol. 2004;13:1–7.
Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Hemingway J, Hawkes N, Prapanthadara L, Jayawardenal KGI, Ranson H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos Trans R Soc Lond B Biol Sci. 1998;353:1695–9.
Philip N. Support de cours de génétique des populations. Collège National des Enseignants et Praticiens de Génétique Médicale, Département de génétique médicale, Marseille, 2010–2011, page 6. http://campus.cerimes.fr/genetique-medicale/enseignement/genetique_2/site/html/cours.pdf. Accessed 22 August 2018.
R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011.
WHO. The technical basis for coordinated action against insecticide resistance. Preserving the effectiveness of modern malaria vector control, meeting report, 4–6 May 2010. Geneva: World Health Organization; 2011.
Curtis CF, Maxwell CA, Finch RJ, Njunwa KJ. A comparison of use of pyrethroids either for house spraying or for bed net treatment against malaria vectors. Trop Med Int Health. 1998;3:619–31.
Diabate A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–22.
Czeher C, Labbo R, Arzika I, Duchemin J-B. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189.
Protopopoff N, Van Bortel W, Marcotty T, Van Herp M, Maes P, Baza D, et al. Spatial targeted vector control is able to reduce malaria prevalence in the highlands of Burundi. Am J Trop Med Hyg. 2008;79:12–8.
Yahouédo GA, Cornelie S, Djégbé I, Ahlonsou J, Aboubakar S, Soares C, et al. Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large-scale implementation of vector control interventions. Parasit Vectors. 2016;9:385.
Chandre F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, et al. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull World Health Organ. 1999b;77:231–4.
Tia E, Akogbeto M, Koffi A, Toure M, Adja AM, Moussa K, et al. Situation de la résistance d’Anopheles gambiae s.s. aux pyréthrinoïdes et aux DTT dans cinq écosystèmes agricoles de Côte d’Ivoire. Bull Soc Pathol Exot. 2006;99:278–82.
Chouaïbou M, Etang J, Brévault T, Nwane P, Hinzoumbé CK, Mimpfoundi R, et al. Dynamics of insecticide resistance in the malaria vector Anopheles gambiae s.l. from an area of extensive cotton cultivation in northern Cameroon. Trop Med Int Health. 2008;4:476–86.
Miller TA. Mechanism of resistance to pyrethroid insecticides. Parasitol Today. 1988;4:S8–12.
Berge JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos Trans R Soc Lond B Biol Sci. 1998;353:1701–5.
Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–88.
Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653–65.
Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defense agents confer pyrethroids resistance in Nilaparvata lugens. Biochem J. 2001;357:65–72.
Djènontin A, Bio-Bangana S, Moiroux N, Henry M-C, Bousari O, Chabi J, et al. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): a pre-intervention study. Parasit Vectors. 2010;3:83.
Guillet P, N’Guessan R, Darriet F, Traore-Lamizana M, Chandre F, Carnevale P. Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med Vet Entomol. 2001;15:105–12.
Djènontin A, Chabi J, Baldet T, Irish S, Pennetier C, Hougard J-M, et al. Managing insecticide resistance in malaria vectors by combining carbamate-treated plastic wall sheeting and pyrethroid-treated bed nets. Malar J. 2009;8:233.
Asidi AN, N’Guessan R, Koffi AA, Curtis CF, Hougard JM, Chandre F, et al. Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J. 2005;4:25.
Acknowledgements
We are grateful to the President’s Malaria Initiative which financially supported this study. We thank Bruno Akinro for statistical analysis. We would also like to acknowledge Monica Patton, Peter Thomas and Raymond Beach of CDC who provided technical support to the study and critically revised the manuscript.
Funding
This study was financially supported by the US President’s Malaria Initiative (PMI) through the United States Agency for International Development (USAID) Africa Indoor Residual Spraying Project (AIRS).
Availability of data and materials
The data supporting the conclusions of this article are included within the article. The raw data used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
ASS, LI, FA, RA and MCA conceived the study. ASS, LI, FA and MCA participated in the design of the study. ASS, IA, SA, FA and AAS collected entomological data. ASS, SA, FA and AAS carried out bioassays and laboratory analysis. ASS and MCA drafted the manuscript. FA, AS, RA, LI, FD and MCA critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Salako, A.S., Ahogni, I., Aïkpon, R. et al. Insecticide resistance status, frequency of L1014F Kdr and G119S Ace-1 mutations, and expression of detoxification enzymes in Anopheles gambiae (s.l.) in two regions of northern Benin in preparation for indoor residual spraying. Parasites Vectors 11, 618 (2018). https://doi.org/10.1186/s13071-018-3180-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3180-2