Abstract
Background
Teratomas are a common type of germ cell tumor. However, only a few reports on their genomic constitution have been published. The study of teratomas may provide a better understanding of their stepwise differentiation processes and molecular bases, which could prove useful for the development of tissue-engineering technologies.
Methods
In the present study, we analyzed the copy number aberrations of nine ovarian mature cystic teratomas using array comparative genomic hybridization in an attempt to reveal their genomic aberrations.
Results
The many chromosomal aberrations observed on array comparative genomic hybridization analysis reveal the complex genetics of this tumor. Amplifications and deletions of large DNA fragments were observed in some samples, while amplifications of EVX2 and HOXD9-HOXD13 on 2q31.1, NDUFV1 on 11q13.2, and RPL10, SNORA70, DNASE1L1, TAZ, ATP6AP1, and GDI1 on Xq28 were found in all nine mature cystic teratomas.
Conclusions
Our results indicated that amplifications of these genes may play an important etiological role in teratoma formation. Moreover, amplifications of EVX2 and HOXD9-HOXD13 on 2q31.1, found on array comparative genomic hybridization, may help to explain the characteristics of teratomas in chondrogenesis and osteogenesis.
Summary
Nine ovarian mature cystic teratomas were analyzed by aCGH. The amplifications of EVX2 and HOXD9-HOXD13 on 2q31.1 can be used to explain the characteristics of teratomas in chondrogenesis and osteogenesis.
Similar content being viewed by others
Background
Ovarian germ cell tumors account for 15–20% of all ovarian malignancies and the incidence of malignant ovarian germ cell tumors is 2–6% [1]. The vast majority are teratomas [2]. There are several types of teratomas: mature cystic, immature, and monodermal. Among them, 97% are cystic mature teratomas, which are also called dermoid cysts [3]. They occur in females at almost any age, but most commonly between the ages of 20 and 30 [4]. The genetic and environmental conditions that confer teratoma development remain poorly understood [5]. Further understanding of teratoma polyderm differentiation promises to provide insight into disorders of ovarian and germ cell lineages, such as ovarian tumor formation and infertility [5].
In light of the ethical issues surrounding the use of human stem cells, teratomas are being looked at as an alternative for research studies, as they lack the potential to grow into functional human beings. Formation of three somatic germ layers within teratomas is considered the best indicator of the pluripotency of human embryonic stem cell lines [6, 7]. A further understanding of this process should aid in the development of safer human embryonic stem cell therapies and elucidation of the principles of teratoma formation [8]. In addition, teratomas represent an alternative development model, as developmental processes cannot be investigated in intact mammalian embryos [9]. Teratomas exhibit arrangements of different tissue types that in many ways recapitulate organogenesis within the embryo [10]. Therefore, studying teratomas could result in a better understanding of their stepwise developmental processes and molecular bases, as well as useful information for the development of tissue-engineering technologies [11].
Recently, array comparative genomic hybridization (aCGH) technology has been applied to increase our understanding of oncogensis in a variety of cancer types with some success [12]. However, few studies have been conducted on the developmental role of genomic constitution in mature cystic teratomas of the ovary. There have only been a limted number of reports on ovarian germ cell tumors [13, 14]. The purpose of this study is to clarify the key mechanism for differentiation during teratoma formation. We analyzed the copy number aberrations in nine mature cystic teratomas of the ovary from Taiwanese patients, via the application of aCGH technology, in an attempt to understand the neoplastic or developmental nature and molecular pathogenesis of this tumor.
Materials and methods
Clinical samples
The Institutional Review Board of Chung Shan Medical University Hospital approved all procedures and informed consent was obtained from all subjects prior to collecting their genetic material for the study (reference number CS2-15060). Nine 20–40 year-old patients with ovarian teratoma were enrolled (Tera-1 to -9), including one with bilateral mature cystic teratomas of the ovaries (Tera-9, right and left (data not shown)) [15, 16]. Data of left Tera-9 is not shown due to similarities with right Tera-9. The baseline characteristics of these mature cystic teratomas of the ovary have been described in detail elsewhere [17]. These tumors were considered to be mature without immature components after examination of multiple sections. There was no evidence of malignancy.
Isolation of DNA from blood
Genomic DNA was extracted from paraffin-embedded sections of the teratomas with QIAamp Tissue Kit (Qiagen GmbH., Hilden, Germany) according to the manufacturer’s instructions. DNA was taken from solid nodule within the inner site of the tumor and finally dissolved in 100 µl of TE buffer (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). DNA concentration of each sample was measured using the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Array comparative genomic hybridization (aCGH) analysis
Samples were screened on 60-K oligonucleotide aCGH at 0.5-Mb resolution, with SurePrint G3 ISCA V2 CGH Microarray Kit (Agilent Technologies, California, USA). Sample and reference genomic DNAs (Promega female) were labelled by random priming using Genomic DNA Enzymatic Labeling Kit (Agilent Technologies, California, USA), and purified according to the manufacturer’s protocol. Labelled sample DNA (400 ng) was co-precipitated with equal volumes of labelled reference DNA (Promega, pooled female) and 2.5 µg/µl human COT-1 DNA. The samples were hybridized to the microarray at 65 °C for 40 h. Scanning and image acquisition were carried out using Agilent microarray scanner D (Agilent Technologies, California, USA). Data analysis was performed using Feature Extraction (FE) software v10.5 (Agilent Technologies, California, USA). Copy number was determined by a conservative log2 ratio threshold. Copy number aberrations from segmented data were based on human genome build GRCh37/UCSC hg19. Amp refers to amplification (gain ≥ 0.15) and Del refers to deletion (loss ≤ − 0.15). Profile deviations consisting of 10 or more neighbouring oligonucleotides were considered genomic aberrations.
Immunohistochemistry
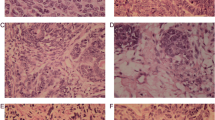
Sections from 4-micrometer-thick, formalin-fixed, paraffin-embedded mature cystic teratomas of the ovary were used for immunohistochemical analysis. Immunohistochemistry for NDUFV1 was performed using a rabbit antibody after antigen affinity purification (1:150 dilution; Proteintech Group, Rosemont, IL, USA). Antigen retrieval was performed with citrate buffer pH 6.0 at 95 °C for 30 min. Tissue staining was carried out with a DAB substrate chromogen solution. Slides were counterstained with hematoxylin, dehydrated, and mounted. Tumor sections with increase in NDUFV1 protein expression were visualized via light microscopy.
Results
Array comparative genomic hybridization (aCGH) analysis
All mature cystic teratomas of the ovary had a normal 46, XX karyotype. We performed analysis twice for Tera-5 and Tera-6 and thrice for Tera-2 to achieve better derivative log ratio spreads (DLRs). DLRs for Tera-1 to Tera-9 were 0.506, 0.220, 0.689, 0.114, 0.200, 0.244, 0.203, 0.352, and 0.245, respectively.
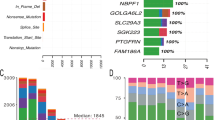
Unequal DNA copy numbers were noted in many chromosomal aberrations in all nine samples on aCGH analysis (Fig. 1). Common chromosomal aberrations and their extents are listed in Tables 1 and 2. Amplifications of EVX2, HOXD13, HOXD12, HOXD11, HOXD10, and HOXD9 on a 41.6 kb segment of 2q31.1 (average log2 ratio = 0.303, Fig. 2), NDUFV1 on a 2.8 kb segment of 11q13.2 (average log2 ratio = 0.685, Fig. 3), and RPL10, SNORA70, DNASE1L1, TAZ, ATP6AP1, and GDI1 on a 44.5 kb segment of chromosome Xq28 (average log2 ratio = 0.336, Fig. 4) were identified in all teratomas. Average log2 ratios for Tera-1 to Tera-9 were 0.276, 0.239, 0.676, 0.414, 0.176, 0.333, 0.160, 0.216, and 0.238, respectively, on the 41.6 kb segment of 2q31.1 (Fig. 2). In addition, average log2 ratios for Tera-1 to Tera-9 were 1.071, 0.445, 1.251, 0.919, 0.710, 0.382, 0.415, 0.552, and 0.416, respectively, on the 2.8 kb segment of 11q13.2 (Figs. 3) and 0.356, 0.166, 0.432, 0.614, 0.359, 0.318, 0.238, 0.236, and 0.309, respectively, on the 44.5 kb segment of chromosome Xq28 (Fig. 4).
Array comparative genomic hybridization (aCGH) analysis: Gene view (Amp/Del) of aCGH analysis shows gene amplifications on chromosome Xq28, Tera-1 to Tera-9 (A-I). Upper panel: RPL10, SNORA70, DNASE1L1, and TAZ; lower panel: ATP6AP1, GDI1 and FAM50A. X axis: Log2 Ratio; Y axis: Xq28 (153.616-153.682 MB)
Amplifications of EGR2 on 10q21.3 (average log2 ratio = 0.700) and a 499 bp segment of chromosome 14q32.2 (average log2 ratio = 0.563), GNASAS and GNAS on 20q13.32 (average log2 ratio = 0.397), BCYRN1 and ZMYM3 on Xq13.1 (average log2 ratio = 0.477), and FLNA and EMD on Xq28 (average log2 ratio = 0.339) were noted in most (8/9) teratomas (Tables 1 and 2). Chromosomal aberrations on aCGH analysis common to all nine teratomas are also listed in Tables 1 and 2.
Chromosomal aberrations on aCGH analysis common to four to six teratomas are listed in Suplemental Table 1. No specific aberration was noted in two bilateral mature cystic teratomas, right and left Tera-9 (data not shown).
Immunohistochemistry
Eight or more points had positive immunoreactive score. Semi-quantitative assessment of NDUFV1 staining by pathologists yielded negative scores of 2 × 2 = 4 (2 in intensity, 2 in proportion of the positive cells: 10–50%) for the stroma of normal ovary (Fig. 5A) and positive scores of 3 × 2 = 12 (3 in intensity, 4 in proportion of the positive cells: >80%) for the parenchyma of mature cystic teratoma of the ovary, Tera-2 (Fig. 5B).
Discussion
Only two previous studies have used comparative genomic hybridization technique for detecting chromosomal aberrations in ovaian teratoma and none have used aCGH analysis. One study reported a case of mature ovarian teratoma with clonal chromosomal alterations including monosomies of chromosomes 6, 14, 16, and 21; trisomies of chromosomes 14 and 21; and deletions of Xq, 5p, 16p, and 17p [18]. Similar to trisomies of chromosomes 14, amplifications of a 499 bp segment of chromosome 14q32.2 (average log2 ratio = 0.563) were noted in most (8/9) teratomas in this study (Tables 1 and 2). Another study on benign cystic teratoma reported del(3q), add(4q), del(12q), and del(16p) in one case and del(6p), add(13q), and del(16p) in another case with bilateral tumors [19]. No such findings were noted in this study.
On aCGH analysis, comprised of 60,000 oligonucleotide probes at a genome-wide resolution of approximately 0.1 Mb, we found previously undescribed aberrations. In all cases, multiple unbalanced chromosomal aberrations were detected. It is possible to identify candidate genes for factors controlling spontaneous ovarian teratocarcinogenesis through aCGH analysis of ovarian teratomas. In an early study, Schneider et al. reported losses of 1p, 4q, and 6q and gains of 1q and 20q in 51 childhood germ cell tumors with comparative genomic hybridization [20]. Chromosomal gain of 12p is characteristic of germ cell tumors in adult patients [21]. However, only amplifications of CLCNKA and CLCNKB on 1p36.13 were noted in most (6/9) teratomas in this study (Supplemental Table 1). These results suggested that the basic characteristics of ovarian benign teratomas differ from those of germ cell tumors. Amiel et al. reported only two of nine cases with unbalanced chromosomal aberrations [19]. Deletion of 16p was observed in two cases and deletion of 6p was observed in one case [19]. None of these unbalanced chromosomal aberrations were observed in our ovarian mature cystic teratomas. This may be due to differences in ethnicity or reference gDNAs, or to higher resolution aCGH.
MYC is a well-characterized human oncogene and common reprogramming factor for generating induced pluripotent stem cells [22]. Amplification of MYCN on 2p24.3 was noted in five teratomas in this study (Suplemental Table 1, shown gray in Fig. 6). Amplifications on chromosomes 1, 8, 12, 17 and X are the most common karyotypic abnormalities detected in pluripotent stem cells [22]. Amplifications on chromosomes 1p36.13, 8p11.1, 17q21.31, 17q24.3, Xp11.22, Xp11.23, Xp22.33, Xq13.1, Xq22.1, and Xq28 were noted in most teratomas in this study (Suplemental Table 1).
Word cloud artwork illustrates the important genes in ovarian mature cystic teratomas identified on aCGH. Amplifications of EVX2 and HOXD9-HOXD13 on 2q31.1 (red), NDUFV1 on 11q13.2 (pink), and RPL10, SNORA70, DNASE1L1, TAZ, ATP6AP1, and GDI1 on Xq28 (light blue) were found in all nine mature cystic teratomas. The chromosomal aberrations on aCGH analysis are present in eight (green), seven (dark blue), six (black), and five (gray) mature cystic teratomas of the ovary, respectively
In addition, amplification of NDUFV1 on a 2.8 kb segment of 11q13.2 was identified in all nine mature cystic teratomas (Fig. 3; Table 1, shown pink in Fig. 6). NDUFV1 gene encodes a 51-kDa subunit of mitochondrial complex I, (NADH dehydrogenase [23] flavoprotein 1) NDUFV1, also known as UQOR1 [23]. Schuelke et al. (1999) detected mutations in the NDUFV1 gene in patients with isolated complex I deficiency, nuclear type 4 [24]. NDUFV1 is a putative developmental/neuropsychiatric susceptibility gene [25] and NDUFV1 transcript has been shown to be overexpressed in blastocysts derived from superovulated heifers [26]. NDUFV1 RNA, but not protein, expression has been detected in ovarian stroma cells in the Human Protein Atlas (www.proteinatlas.org) for normal and cancer tissues based on antibody proteomics [27]. The results of this study regarding absent (negative) NDUFV1 protein expression in the stroma of normal ovary (Fig. 5A) are consistent with those of a previous study [27]. Increased intact (positive) NDUFV1 protein expression of mature cystic teratomas of the ovary (Fig. 5B) indicated that NDUFV1 is a factor in induced differentiation.
In addition, amplifications of RPL10, SNORA70, DNASE1L1, TAZ, ATP6AP1, and GDI1 on a 44.5 kb segment of Xq28 were identified in all nine mature cystic teratomas in this study (Fig. 4; Table 1, shown blue in Fig. 6). Vandewalle et al. (2009) observed 0.3-Mb copy number gain at chromosome Xq28 that included 18 annotated genes, of which RPL10, ATP6AP1, and GDI1 are expressed in the brain [28]. GDI1 encodes GDP dissociation inhibitor 1, which plays an essential role in vesicle transport by slowing the rate of dissociation of GDP in the GDP-GTP exchange reaction of members of the rab family [29]. Vandewalle et al. (2009) considered GDI1 the most likely candidate gene in the chromosome Xq28 duplication syndrome region [28]. Mutations in GDI1 are responsible for X-linked non-specific mental retardation [30]. Dorus et al. (2004) demonstrated that GDI displays significantly higher rates of protein evolution in primates than in rodents and suggested that it plays a role in nervous system development [31]. RPL10 (ribosomal protein L10 gene) encodes RPL10 protein, which is a constituent of the large subunit (60 S) of the ribosome [32]. Missense mutations in RPL10 suggest susceptibility to X-linked autism 5 [33] and X-linked syndromic intellectual developmental disorder 35 [34]. De Keersmaecker et al. (2013) identified somatic mutations in RPL10 in pediatric T-cell acute lymphoblastic leukemia [35]. RPL10 has also been suggested to drive oncogenic processes in the ovaries [36, 37]. ATP6AP1 encodes the accessory S1 subunit (Ac45) of the enzyme V-type proton ATPase [38]. Jansen et al. (2016) identified hemizygous missense mutations in the ATP6AP1 gene in males with immunodeficiency-47, also known as congenital disorder of glycosylation (CDG2S) [39]. This protein may also play a role in early development because mouse Atp6ap1 knockout embryonic stem cells do not give rise to viable embryos [40]. SNORA70 (small nucleolar RNA, H/ACA Box 70), which resides (or is embedded) in the fifth intron of RPL10, is a non-coding RNA (ncRNA) molecule which functions in the biogenesis (modification) of other small nuclear RNAs (snRNAs) [41]. SNORA70 has oncogenic function in osteosarcomas [42]. DNASE1L1 (deoxyribonuclease 1 Like 1) encodes a member of the deoxyribonuclease family and the protein shows high sequence similarity with lysosomal DNase I [43, 44]. DNA degradation is critical to healthy organism development and survival [45]. Its association with Pompe disease is controversial and with DNase1L1 is unclear [45]. TAZ encodes tafazzin, a transacylase essential for cardiolipin formation and central to the etiology of Barth syndrome, also known as 3-methyglutaconic aciduria type II [46]. Tafazzin promotes the tumorigenicity of cervical cancer cells and inhibits apoptosis [47]. Significant expression of tafazzin (TAZ) protein has been observed in colon cancer cells [48]. Further research is necessary to better understand the roles of these genes in the key mechanism for inducing differentiation during teratoma development.
The most interesting findings of this study are amplifications of EVX2, HOXD13, HOXD12, HOXD11, HOXD10, and HOXD9 on a 41.6 kb segment of 2q31.1 in all nine mature cystic teratomas (Fig. 2, Table 1, shown red in Fig. 6). The HOXD9-HOXD13 genes belong to a member of the HOXD cluster of homeobox genes that encode transcription factors involved in limb development [49] by the confined expression of SHH (Sonic hedgehog) at the posterior margin of developing early limb buds [50]. EVX2 gene encodes a homeobox transcription factor that is related to the protein encoded by the Drosophila even-skipped (eve) gene, a member of the pair-rule class of segmentation genes [51]. EVX2 and HOXD9-HOXD13 are essential for proper development of the appendicular skeletons [52]. A 117 kb microdeletion at the 5’ end of the HOXD gene cluster, which includes EVX2 and HOXD9-HOXD13 genes, causes synpolydactyly [53]. HOXD10 and HOXD13 gene expressions have been found to be altered in primary breast cancers with respect to normal breast tissue, switching from off to on [54]. HOXD9 promotes epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma [55] and colorectal carcinoma [56]. HOXD10 is suppressed in colon adenocarcinoma cells [57]. Colon carcinomas show an overexpression of HOXD10 which distinguishes colonic stem cells from colon carcinoma cells [58]. Woo et al. (2010) found that a 1.8 kb region between HOXD11 and HOXD12 has an activator function in the differentiation of human embryonic stem cells into mesenchymal stem cells (MSCs) and then into osteoblasts [59]. In humans, mutations in HOXD13 cause dominantly inherited limb malformation synpolydactyly (SPD) [60]. Although no specific function of HOXD family genes in teratomas has been reported in humans, due to the relationship between these genes and differentiation in chondrogenesis and osteogenesis, we speculate that amplifications in HOXD family genes are associated with bone differentiation in teratomas. Only one previous study on teratocarcinoma embryoid bodies has suggested a possible role for HOXD12 in establishing extraembryonic endoderm lineage in mice [61].
Teratoma, means strange tumor, as it contains tissues found in other parts of the body. Characteristic elements include skin, hair, fat, teeth, and bone [62]. It is interesting to understand the mechanisms involved in the development of so many different types of tissues in this tumor. From our previous report, DUSP5 and PHLDA1 mutations in mature cystic teratomas of the ovary identified on whole‑exome sequencing may explain teratoma characteristics in terms of osteogenic differentiation and hair growth [17]. Some intrinsic factors may also be involved, such as HOXD genes which are associated with bone development. In this study, HODX9 (which affects cartilage formation [63]) to HOXD13 (which affects the ossification of mature bone [64]) were amplified in all our teratoma samples. This may be the reason why the bone component is so common in teratomas and used for preoperative diagnosis. This is the first study on humans to support previous findings of chondrogenesis and osteogenesis on mouse model [63, 64]. Here, we provide new insights into mature cystic teratomas of the ovary, which increase our understanding of teratoma polydermal differentiation.
Conclusions
In summary, amplifications of EVX2, HOXD13, HOXD12, HOXD11, HOXD10, and HOXD9 on 2q31.1, NDUFV1 on 11q13.2, and RPL10, SNORA70, DNASE1L1, TAZ, ATP6AP1, and GDI1 on Xq28 were found in all nine mature cystic teratomas in this study. Our results indicated that amplifications of these genes play an important etiological role in teratoma formation. Moreover, amplifications of EVX2 and HOXD9-HOXD13 on 2q31.1 may help to explain the characteristics of teratomas in chondrogenesis and osteogenesis.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- CGH:
-
comparative genomic hybridization
- DNASE1L1:
-
deoxyribonuclease 1 Like 1
- DNA:
-
deoxyribonucleic acid
- EVX2:
-
Even-Skipped Homeobox 2
- GDI1:
-
GDP dissociation inhibitor 1
- HOXD:
-
homeobox D cluster
- NDUFV1:
-
NADH ubiquinone oxidoreductase complex I
- RPL10:
-
ribosomal protein L10
- SNORA70:
-
small nucleolar RNA, H/ACA Box 70
- TAZ:
-
Tafazzin
- UV-VIS:
-
Ultraviolet–visible
References
Goyal LD, Kaur B, Badyal RK. Malignant mixed germ cell tumors of the ovary: a series of rare cases. J Reprod Infertil. 2019;20:231–6.
Euscher ED. Germ cell tumors of the female genital tract. Surg Pathol Clin. 2019;12:621–49.
Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21:475–90.
O’Neill KE, Cooper AR. The approach to ovarian dermoids in adolescents and young women. J Pediatr Adolesc Gynecol. 2011;24:176–80.
Western PS, Ralli RA, Wakeling SI, Lo C, van den Bergen JA, Miles DC, et al. Mitotic arrest in teratoma susceptible fetal male germ cells. PLoS ONE. 2011;6:e20736.
Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–8.
Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27:281–7.
Su W, Zhou M, Zheng Y, Fan Y, Wang L, Han Z, et al. Bioluminescence reporter gene imaging characterize human embryonic stem cell-derived teratoma formation. J Cell Biochem. 2011;112:840–8.
Stachelscheid H, Wulf-Goldenberg A, Eckert K, Jensen J, Edsbagge J, Bjorquist P, et al. Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. J Tissue Eng Regen Med. 2013;7:729–41.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Aleckovic M, Simon C. Is teratoma formation in stem cell research a characterization tool or a window to developmental biology? Reprod Biomed Online. 2008;17:270–80.
Michels E, De Preter K, Van Roy N, Speleman F. Detection of DNA copy number alterations in cancer by array comparative genomic hybridization. Genet Med. 2007;9:574–84.
Riopel MA, Spellerberg A, Griffin CA, Perlman EJ. Genetic analysis of ovarian germ cell tumors by comparative genomic hybridization. Cancer Res. 1998;58:3105–10.
Veltman I, Veltman J, Janssen I, Hulsbergen-van de Kaa C, Oosterhuis W, Schneider D, et al. Identification of recurrent chromosomal aberrations in germ cell tumors of neonates and infants using genomewide array-based comparative genomic hybridization. Genes Chromosomes Cancer. 2005;43:367–76.
Wang WC, Lai YC. Genetic analysis results of mature cystic teratomas of the ovary in Taiwan disagree with the previous origin theory of this tumor. Hum Pathol. 2016;52:128–35.
Wang WC, Lai YC. Evidence of metachronous development of ovarian teratomas: a case report of bilateral mature cystic teratomas of the ovaries and systematic literature review. J Ovarian Res. 2017;10:17.
Wang WC, Lai YC. DUSP5 and PHLDA1 mutations in mature cystic teratomas of the ovary identified on whole-exome sequencing may explain teratoma characteristics. Hum Genomics. 2022;16:50.
Schmid-Braz AT, Cavalli LR, Cornelio DA, Wuicik L, Ribeiro EM, Bleggi-Torres LF, et al. Comprehensive cytogenetic evaluation of a mature ovarian teratoma case. Cancer Genet Cytogenet. 2002;132(2):165–8.
Amiel A, Atzmon H, Klein Z, Kidron D, Gaber E, Vassilyev O, et al. Application of comparative genomic hybridization technique for detection of chromosomal aberrations in benign cystic teratoma. Cancer Genet Cytogenet. 2003;144(1):73–5.
Schneider DT, Schuster AE, Fritsch MK, Calaminus G, Harms D, Gobel U, et al. Genetic analysis of childhood germ cell tumors with comparative genomic hybridization. Klin Padiatr. 2001;213:204–11.
Looijenga LH, Zafarana G, Grygalewicz B, Summersgill B, Debiec-Rychter M, Veltman J, et al. Role of gain of 12p in germ cell tumour development. APMIS: Acta Pathologica Microbiol et Immunol Scand. 2003;111:161–71. discussion 72 – 3.
Lund RJ, Narva E, Lahesmaa R. Genetic and epigenetic stability of human pluripotent stem cells. Nat Rev Genet. 2012;13:732–44.
Ali ST, Duncan AM, Schappert K, Heng HH, Tsui LC, Chow W, et al. Chromosomal localization of the human gene encoding the 51-kDa subunit of mitochondrial complex I (NDUFV1) to 11q13. Genomics. 1993;18:435–9.
Schuelke M, Smeitink J, Mariman E, Loeffen J, Plecko B, Trijbels F, et al. Mutant NDUFV1 subunit of mitochondrial complex I causes leukodystrophy and myoclonic epilepsy. Nat Genet. 1999;21:260–1.
Woodbury-Smith M, Paterson AD, Thiruvahindrapduram B, Lionel AC, Marshall CR, Merico D, et al. Using extended pedigrees to identify novel autism spectrum disorder (ASD) candidate genes. Hum Genet. 2015;134:191–201.
Gad A, Besenfelder U, Rings F, Ghanem N, Salilew-Wondim D, Hossain MM, et al. Effect of reproductive tract environment following controlled ovarian hyperstimulation treatment on embryo development and global transcriptome profile of blastocysts: implications for animal breeding and human assisted reproduction. Hum Reprod. 2011;26:1693–707.
Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteom. 2005;4:1920–32.
Vandewalle J, Van Esch H, Govaerts K, Verbeeck J, Zweier C, Madrigal I, et al. Dosage-dependent severity of the phenotype in patients with mental retardation due to a recurrent copy-number gain at Xq28 mediated by an unusual recombination. Am J Hum Genet. 2009;85:809–22.
Ullrich O, Stenmark H, Alexandrov K, Huber LA, Kaibuchi K, Sasaki T, et al. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–50.
D’Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, et al. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet. 1998;19:134–9.
Dorus S, Vallender EJ, Evans PD, Anderson JR, Gilbert SL, Mahowald M, et al. Accelerated evolution of nervous system genes in the origin of Homo sapiens. Cell. 2004;119:1027–40.
Gong X, Delorme R, Fauchereau F, Durand CM, Chaste P, Betancur C, et al. An investigation of ribosomal protein L10 gene in autism spectrum disorders. BMC Med Genet. 2009;10:7.
Klauck SM, Felder B, Kolb-Kokocinski A, Schuster C, Chiocchetti A, Schupp I, et al. Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol Psychiatry. 2006;11:1073–84.
Brooks SS, Wall AL, Golzio C, Reid DW, Kondyles A, Willer JR, et al. A novel ribosomopathy caused by dysfunction of RPL10 disrupts neurodevelopment and causes X-linked microcephaly in humans. Genetics. 2014;198:723–33.
De Keersmaecker K, Atak ZK, Li N, Vicente C, Patchett S, Girardi T, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet. 2013;45:186–90.
Shi J, Zhang L, Zhou D, Zhang J, Lin Q, Guan W, et al. Biological function of ribosomal protein L10 on cell behavior in human epithelial ovarian Cancer. J Cancer. 2018;9:745–56.
Shen XJ, Ali-Fehmi R, Weng CR, Sarkar FH, Grignon D, Liao DJ. Loss of heterozygosity and microsatellite instability at the Xq28 and the A/G heterozygosity of the QM gene are associated with ovarian cancer. Cancer Biol Ther. 2006;5:523–8.
Supek F, Supekova L, Mandiyan S, Pan YC, Nelson H, Nelson N. A novel accessory subunit for vacuolar H(+)-ATPase from chromaffin granules. J Biol Chem. 1994;269:24102–6.
Jansen EJ, Timal S, Ryan M, Ashikov A, van Scherpenzeel M, Graham LA, et al. ATP6AP1 deficiency causes an immunodeficiency with hepatopathy, cognitive impairment and abnormal protein glycosylation. Nat Commun. 2016;7:11600.
Schoonderwoert VT, Martens GJ. Targeted disruption of the mouse gene encoding the V-ATPase accessory subunit Ac45. Mol Membr Biol. 2002;19:67–71.
Ganot P, Caizergues-Ferrer M, Kiss T. The family of box ACA small nucleolar RNAs is defined by an evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev. 1997;11:941–56.
Liang J, Wen J, Huang Z, Chen XP, Zhang BX, Chu L. Small nucleolar RNAs: insight into their function in Cancer. Front Oncol. 2019;9:587.
Rodriguez AM, Rodin D, Nomura H, Morton CC, Weremowicz S, Schneider MC. Identification, localization, and expression of two novel human genes similar to deoxyribonuclease I. Genomics. 1997;42:507–13.
Laukova L, Konecna B, Janovicova L, Vlkova B, Celec P. Deoxyribonucleases and their applications in Biomedicine. Biomolecules. 2020;10:1036.
Keyel PA. Dnases in health and disease. Dev Biol. 2017;429:1–11.
Garlid AO, Schaffer CT, Kim J, Bhatt H, Guevara-Gonzalez V, Ping P. TAZ encodes tafazzin, a transacylase essential for cardiolipin formation and central to the etiology of Barth syndrome. Gene. 2020;726:144148.
Chen M, Zhang Y, Zheng PS. Tafazzin (TAZ) promotes the tumorigenicity of cervical cancer cells and inhibits apoptosis. PLoS ONE. 2017;12:e0177171.
Pathak S, Banerjee A, Meng WJ, Kumar Nandy S, Gopinath M, Sun XF. Significant expression of tafazzin (TAZ) protein in colon cancer cells and its downregulation by radiation. Int J Radiat Biol. 2018;94:79–87.
Tarchini B, Duboule D, Kmita M. Regulatory constraints in the evolution of the tetrapod limb anterior-posterior polarity. Nature. 2006;443:985–8.
Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–16.
D’Esposito M, Morelli F, Acampora D, Migliaccio E, Simeone A, Boncinelli E. EVX2, a human homeobox gene homologous to the even-skipped segmentation gene, is localized at the 5’ end of HOX4 locus on chromosome 2. Genomics. 1991;10:43–50.
Herault Y, Hraba-Renevey S, van der Hoeven F, Duboule D. Function of the Evx-2 gene in the morphogenesis of vertebrate limbs. EMBO J. 1996;15:6727–38.
Goodman FR, Majewski F, Collins AL, Scambler PJ. A 117-kb microdeletion removing HOXD9-HOXD13 and EVX2 causes synpolydactyly. Am J Hum Genet. 2002;70:547–55.
Cantile M, Pettinato G, Procino A, Feliciello I, Cindolo L, Cillo C. In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer. 2003;39:257–64.
Lv X, Li L, Lv L, Qu X, Jin S, Li K, et al. HOXD9 promotes epithelial-mesenchymal transition and cancer metastasis by ZEB1 regulation in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:133.
Liu M, Xiao Y, Tang W, Li J, Hong L, Dai W, et al. HOXD9 promote epithelial-mesenchymal transition and metastasis in colorectal carcinoma. Cancer Med. 2020;9:3932–43.
Yuan YH, Wang HY, Lai Y, Zhong W, Liang WL, Yan FD, et al. Epigenetic inactivation of HOXD10 is associated with human colon cancer via inhibiting the RHOC/AKT/MAPK signaling pathway. Cell Commun Signal. 2019;17:9.
Bhatlekar S, Addya S, Salunek M, Orr CR, Surrey S, McKenzie S, et al. Identification of a developmental gene expression signature, including HOX genes, for the normal human colonic crypt stem cell niche: overexpression of the signature parallels stem cell overpopulation during colon tumorigenesis. Stem Cells Dev. 2014;23:167–79.
Woo CJ, Kharchenko PV, Daheron L, Park PJ, Kingston RE. A region of the human HOXD cluster that confers polycomb-group responsiveness. Cell. 2010;140:99–110.
Muragaki Y, Mundlos S, Upton J, Olsen BR. Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science. 1996;272:548–51.
Labosky PA, Weir MP, Grabel LB. Homeobox-containing genes in teratocarcinoma embryoid bodies: a possible role for Hox-D12 (Hox-4.7) in establishing the extraembryonic endoderm lineage in the mouse. Dev Biol. 1993;159(1):232–44.
Ellenson LH, Pirog EC. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. The female genital tract. 8 ed. Saunders; 2010.
Hong Q, Li XD, Xie P, Du SX. All-trans-retinoic acid suppresses rat embryo hindlimb bud mesenchymal chondrogenesis by modulating HoxD9 expression. Bioengineered. 2021;12:3900–11.
Kuss P, Kraft K, Stumm J, Ibrahim D, Vallecillo-Garcia P, Mundlos S, et al. Regulation of cell polarity in the cartilage growth plate and perichondrium of metacarpal elements by HOXD13 and WNT5A. Dev Biol. 2014;385:83–93.
Acknowledgements
The authors would like to thank GenePhile Bioscience Laboratory of Ko’s Obstetrics and Gynecology Clinic for help with acquisition and analysis of data.
Author information
Authors and Affiliations
Contributions
WWC designed the experiments, provided samples and clinical data, interpreted the results, and made critical revisions to the manuscript. TCH and CYK reviewed the tumor sections. YCL designed the experiments, performed the experiments, interpreted the results, and drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study were approved by the Institutional Review Board of Chung Shan Medical University Hospital via grant reference CS15060 and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All cases included in this study were fully anonymized.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13048_2024_1458_MOESM1_ESM.docx
Supplementary Material 1: Histological characteristics of mature cystic teratomas of the ovary (hematoxylin and eosin, H&E) with ruler. Scale bar: 200 μm
13048_2024_1458_MOESM2_ESM.xlsx
Supplementary Material 2: Chromosomal aberrations on array comparative genomic hybridization (aCGH) analysis present 4 to 6 teratomas
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, WC., Hou, TC., Kuo, CY. et al. Amplifications of EVX2 and HOXD9-HOXD13 on 2q31 in mature cystic teratomas of the ovary identified by array comparative genomic hybridization may explain teratoma characteristics in chondrogenesis and osteogenesis. J Ovarian Res 17, 129 (2024). https://doi.org/10.1186/s13048-024-01458-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01458-5