Abstract
The novel variants of the SARS-CoV-2 are a great global concern for the ongoing COVID-19 pandemic. However, how the novel variants predominate and replace existing strains remains elusive. In this study, I simulated the infection spread to investigate what kinds of viral, immunological, and epidemiological factors affect the predominance of SARS-CoV-2 novel variants. The results showed that the increase of the transmissibility of the novel variant substantially enhanced the predominance probability. In addition, the increasing trend of the infection spread, the large case number of the epidemic, and the ability of immune escape of the novel variant increased the predominance probability. A small number of cases and a decreasing trend of an entire epidemic, including not only the novel variant but also earlier strains, are especially important to reduce the chance of the predominance of the novel variant and delay the process. Good control of the COVID-19 epidemic could make the disease burden small and sequester the spread of the SARS-CoV-2 novel variants.
Similar content being viewed by others
Introduction
The novel variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) keep emerging and hence are a great global concern for the ongoing pandemic of coronavirus disease 2019 (COVID-19). A variant with D614G mutation in the spike protein encoded in the S gene emerged, and its descendants became the major circulating strains in early 2020 possibly due to its high infectivity [1, 2]. The novel variants named Alpha, Beta, and Gamma with characteristic mutations, such as N501Y in the S gene, have emerged and spread worldwide [3]. These novel variants are considered to have high transmissibility, high pathogenicity, and/or the ability to escape from the immunity generated by prior infection with earlier strains or vaccination [4,5,6,7,8,9].
The emergence and spread of those variants have caused the surge of COVID-19 cases from the end of 2020 to the beginning of 2021 in the United Kingdom, South Africa, and Brazil [5, 10, 11]. The introduction and predominance of those novel variants with the rise of the number of COVID-19 cases were also observed in other parts of the world [12,13,14]. Many more variants of concern and variants of interest continue to emerge, including the Delta variant, which has even higher transmissibility and has caused a significant impact around the world in 2021 [15]. However, factors that affect the process of the predominance of the novel variants remain elusive.
Here, I performed a simulation study to investigate the causative relationship between viral, immunological, and epidemiological conditions and the process of the predominance of the SARS-CoV-2 novel variants.
Methods
Simulation of infection spread
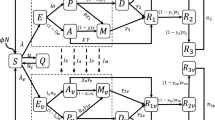
The infection spread was simulated by a stochastic time-discrete compartment model with S (susceptible), I (infectious), and R (removed) compartments in a population of N = 1,000,000. On day t, the new infections are generated by each infected person on day t − 1 according to the reproduction number (REP) and the proportion of susceptible people in the population, assuming a negative binomial distribution as follows:
where int indicates the mean serial interval of infection set to 5 days [16] and k represents the dispersion parameter of a negative binomial distribution. Heterogeneity in the transmission is determined by k; the parameter was set to 0.3 in the simulation according to previous reports on COVID-19 [17, 18].
I people move to R according to a probability of 1/int each day. R people are protected from an infection with the already infected strain (either an existing strain or a novel variant). They are also protected from an infection with the other strain on the basis of the degree of cross-immunity described later in the “Methods” section.
Not an infectious period but a serial interval was used for the reproduction of secondary cases. The mean interval between transmission generations (i.e., serial interval) should reproduce the process of infection spread well. Inclusion of the probability density of secondary transmission during an infectious period was not regarded as needed in the model to investigate the predominance process of a novel variant.
Two viral strains were included in the simulation; one is an earlier existing strain, and the other is an emerging novel variant. Five parameters for the precondition in the simulation were investigated. (1) Coefficient to increase the transmissibility of the novel variant (1.0/1.5/2.0). For example, the SARS-CoV-2 Alpha variant was reported to have ~ 1.3–1.5 times higher transmissibility than the original strain. The Delta variant was regarded to have ~ 2.0 times higher transmissibility than the original strain and ~ 1.5 times than the Alpha variant [5, 15]. (2) Epidemic trend defined by reproduction number of the existing strain (0.67/1.0/1.5). For example in Japan, the effective reproduction number of COVID-19 ranges between 0.5 (under a state of emergency) and 2 (when public health interventions were relaxed) [19]. (3) The total number of people infected with the existing strain and the novel variant on day 0 (100/500/2500). 4) Cross-immunity between the existing strain and the novel variant (0.25/0.5/0.7). “0.25” means that only 25% of people infected with the existing strain can develop an immunity to protect them from infection with the novel variant (i.e., the novel variant can escape an immunity made by the earlier strain.) “0.75” means that 75% of people infected with the existing strain become resistant for both the existing strain and the novel variant (i.e., good cross-immunity between the two strains). (5) Prevalence of immuned people in the population on day 0 (0%/40%/80%). Most preexisting immunity was assumed to be generated by vaccination for the existing strain. Cross-protection for the novel variant by vaccination was determined by the cross-immunity parameter aforementioned. The combination of these parameters yielded 243 patterns of preconditions for the simulation.
On day 0, 5% of the infected people were assumed to be infected with the novel variant. Stochastic simulations were run for 200 days by 100 times for each precondition. The predominance of the novel variant was determined when the proportion of people infected with the novel variant surpassed 80% of all infected people. And, the frequency and days for the predominance in the simulations were analyzed for each precondition.
Data availability
A computer script for the simulations is available at GitHub (https://github.com/yukifuruse1217/variant_simulation).
Results
I investigated the probability of the SARS-CoV-2 novel variant predominance in the stochastic simulation model exploring conditions that contribute to the process of the predominance. Examined factors included the increase of transmissibility of the novel variant, the trend of the epidemic, the epidemic size including both the existing strain and the novel variant, the cross-immunity between the existing strain and the novel variant, and the prevalence of already immuned people in the population. It should be noted that the present study does not consider a particular variant; rather, I investigated the infection spread dynamics of SARS-CoV-2 novel variants at a general level.
As expected, the increase of the transmissibility of the novel variant substantially enhanced the predominance probability (Fig. 1A). In addition, the increasing trend of the infection spread of the entire epidemic (i.e., large reproduction number) (Fig. 1B), the large case number of the epidemic (Fig. 1C), and the novel variant’s ability to escape from immunity made by the earlier strain (Fig. 1D) increased the predominance probability.
Probability of the predominance of SARS-CoV-2 novel variant by preconditions. The frequencies of predominance in 100 stochastic simulations were plotted for each precondition. The corresponding preconditions with the same parameters, except the variable indicated in the X-axis, were connected with a line. Lines were colored by transmissibility of the novel variant except for panel F, in which lines were colored by a parameter of cross-immunity
The proportion of already immuned people showed complicated effects on the predominance probability (Fig. 1E). The high proportion of the preexisting immunity for the earlier strain increased the predominance probability when the novel variant had an immune escape ability (pink lines in Fig. 1F). When the preexisting immunity could prevent the infection with not only the earlier strain but also the novel variant (i.e., good cross-immunity), a higher preexisting immunity then decreased the predominance probability (blue lines in Fig. 1F). There are also Λ-shape patterns in some cases (Fig. 1E, F), suggesting that ~ 40% vaccination coverage could have a higher predominance probability of the novel variant than a very low or very high coverage scenario.
The simulations also found that those factors affect the duration for the novel variant to achieve predominance (Fig. 2). Especially, the increase of the transmissibility of the novel variant (Fig. 2A), the weak cross-immunity between the earlier strain and the novel variant (Fig. 2D), and the high proportion of already immuned people (Fig. 2E, F) shortened the predominance process.
Days for the predominance of SARS-CoV-2 novel variant by preconditions. The median days for the novel variant to predominate in 100 stochastic simulations were plotted for each precondition. The data of simulations with preconditions in which the predominance frequency was less than 20% were excluded from the figure. The corresponding preconditions with the same parameters, except the variable indicated in the X-axis, were connected with a line. Lines were colored by transmissibility of the novel variant except for panel F, in which lines were colored by a parameter of cross-immunity
Moreover, it is interesting that the novel variant could predominate even when the variant did not have increased transmissibility (i.e., variant transmissibility = 1.0, red dots in Fig. 1). In contrast, the novel variant could fail to predominate even with two-fold transmissibility (i.e., variant transmissibility = 2.0. blue dots in Fig. 1), especially when the entire epidemic size was small and decreasing. When the number of infected people and reproduction number were small (i.e., initial size = 100 and reproduction number = 0.67. blue dots in Fig. 1B, C), the predominance probability of the novel variant with two-fold transmissibility substantially dropped for some occasions.
Discussion
Overall, good control of the ongoing COVID-19 epidemic, represented by a small number of cases and its decreasing trend of the entire epidemic including existing and novel strains, can reduce the predominance probability of the SARS-CoV-2 novel variants. Even a variant with no increase in transmissibility can predominate when the epidemic is growing and the variant is able to escape from immunity generated by earlier strains (Fig. 1).
The intervention on the infection spread by public health measures, such as wearing a face mask, physical distancing, rapid case detection, contact tracing, and isolation, is important for not only making the disease burden small but also sequestering the emergence and predominance of the novel variants. Even if it seems impossible to completely stop the spread of the novel variants, we could delay the predominance process. Although the present theoretical study showed the causal relationship between those factors and the predominance of SARS-CoV-2 novel variants, their detailed mechanisms should be further studied in the future.
It is worrisome that the present study found that “halfway” vaccination coverage may increase the predominance probability of novel variants. A previous report also suggested that “intermediate” immune pressure could maximize the possibility of viral adaptation [20]. Therefore, good control of the epidemic will become more crucial to prevent the spread of future variants of SARS-CoV-2 as vaccination is being rolled out.
Availability of data and materials
A computer script for the simulations is available at GitHub (https://github.com/yukifuruse1217/variant_simulation).
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Volz E, Hill V, McCrone JT, Price A, Jorgensen D, O’Toole Á, et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2020;184(1):64–75.e11.
Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–27.
Konings F, Perkins MD, Kuhn JH, Pallen MJ, Alm EJ, Archer BN, et al. SARS-CoV-2 variants of interest and concern naming scheme conducive for global discourse. Nat Microbiol 2021 67. 2021 Jun 9 [cited 2021 July 19];6(7):821–3. Available from https://www.nature.com/articles/s41564-021-00932-w.
Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021 Mar 9 [cited 2021 Mar 31];372:n579. Available from https://www.ncbi.nlm.nih.gov/pubmed/33687922.
Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 Mar 3 [cited 2021 Mar 31]; Available from https://www.ncbi.nlm.nih.gov/pubmed/33658326.
Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021 Mar 25 [cited 2021 Mar 31];1–17. Available from https://www.ncbi.nlm.nih.gov/pubmed/33767447.
Abdool Karim SS, de Oliveira T. New SARS-CoV-2 Variants—clinical, public health, and vaccine implications. N Engl J Med. 2021 Mar 24 [cited 2021 Mar 31];NEJMc2100362. Available from https://www.nejm.org/doi/10.1056/NEJMc2100362.
Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021 Mar 26 [cited 2021 Mar 31];1–8. Available from https://www.nature.com/articles/s41591-021-01318-5.
Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021 Mar 16 [cited 2021 Mar 31];NEJMoa2102214. Available from https://www.nejm.org/doi/10.1056/NEJMoa2102214.
Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021 [cited 2021 Apr 14]; Available from https://pubmed.ncbi.nlm.nih.gov/33690265/.
Faria NR, Mellan TA, Whittaker C, Claro IM, Candido D da S, Mishra S, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science (80-). 2021 Apr 14 [cited 2021 Apr 16];eabh2644. Available from https://www.sciencemag.org/lookup/doi/10.1126/science.abh2644.
CoVariants. 2021 [cited 2021 Mar 31]. Available from https://covariants.org/.
Washington NL, Gangavarapu K, Zeller M, Bolze A, Cirulli ET, Schiabor Barrett KM, et al. Emergence and rapid transmission of SARS-CoV-2 B.1.1.7 in the United States. Cell. 2021 Mar 30 [cited 2021 Apr 14]; Available from https://linkinghub.elsevier.com/retrieve/pii/S0092867421003834.
Gaymard A, Bosetti P, Feri A, Destras G, Enouf V, Andronico A, et al. Early assessment of diffusion and possible expansion of SARS-CoV-2 lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Eurosurveillance. 2021 Mar 4 [cited 2021 Apr 14];26(9):2100133. Available from https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.9.2100133.
Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021 Jun 17 [cited 2021 Jul 19];26(24):2100509. Available from https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.24.2100509.
Rai B, Shukla A, Dwivedi LK. Estimates of serial interval for COVID-19: A systematic review and meta-analysis. Clin Epidemiol Glob Heal. 2021 Jan 1 [cited 2021 Feb 22];9:157–61. Available from https://pubmed.ncbi.nlm.nih.gov/32869006/.
Sun K, Wang W, Gao L, Wang Y, Luo K, Ren L, et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science (80-). 2020 Nov 24 [cited 2020 Nov 26];eabe2424. Available from https://www.sciencemag.org/lookup/doi/10.1126/science.abe2424.
Laxminarayan R, Wahl B, Reddy Dudala S, Gopal K, Mohan C, Neelima S, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Vol. 370, Science. American Association for the Advancement of Science; 2020 Nov [cited 2020 Nov 11]. Available from https://science.sciencemag.org/.
Furuse Y. Simulation of future COVID-19 epidemic by vaccination coverage scenarios in Japan. J Glob Health. 2021;11:05025.
Cobey S, Larremore DB, Grad YH, Lipsitch M. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat Rev Immunol 2021 215. 2021 Apr 1 [cited 2021 Dec 3];21(5):330–5. Available from https://www.nature.com/articles/s41577-021-00544-9.
Acknowledgements
I thank Yoshio Koyanagi, Koji Maemura, Kayoko Matsushima, Hisayuki Hamada, and Noriyuki Nishida for their support.
Funding
This study was funded in part by the Leading Initiative for Excellent Young Researchers (Grant Number 16809810) from the Ministry of Education, Culture, Sports, Science and Technology in Japan and by the Research Program on Emerging and Re-emerging Infectious Diseases (21fk0108108h9903 and 20fk0108451s0301) from the Japan Agency for Medical Research and Development. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
YF conceptualized the study, performed simulations, analyzed and interpreted data, and wrote the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Furuse, Y. Simulation study reveals factors that affect the predominance of SARS-CoV-2 novel variant. Virol J 18, 253 (2021). https://doi.org/10.1186/s12985-021-01726-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-021-01726-6