Abstract
Background
Surgical resection remains the mainstay of curative treatment for intrahepatic cholangiocarcinoma (ICC). Prognosis after surgery is unsatisfactory despite improvements in treatment and post-operative clinical management. Despite developments in the molecular profiling of ICC, the preoperative prediction of prognosis remains a challenge. This study aimed to identify clinical prognostic indicators by investigating the molecular profiles of ICC and evaluating the preoperative imaging data of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET).
Methods
A retrospective analysis was performed on 50 consecutive patients with ICC who underwent curative hepatectomy after 18F-FDG-PET examination. To evaluate the molecular profiles of ICC, KRAS mutation status was assessed in resected specimens. For the assessment of glucose uptake, we observed the expression of glucose transporter-1 (GLUT-1) by immunohistochemistry. The data of 18F-FDG-PET were re-evaluated as follows: maximum standardized uptake value, metabolic tumor volume (MTV), and total lesion glycolysis (TLG). Cut-off values were determined using receiver operating characteristic (ROC) curve analysis. Cumulative overall survival (OS) was analyzed using the Kaplan–Meier analysis.
Results
Overall, 16 (32.0%) patients had mutations in KRAS. Patients with mutated KRAS exhibited shorter OS than those with wild-type KRAS (5-year OS, 0% vs. 35.1%, P < 0.001). GLUT-1 expression was significantly higher in tumors with mutated KRAS than in tumors with wild-type KRAS (median, 4.0 vs. 1.0, P < 0.001). Survival was significantly different when stratified by expression of GLUT-1 (5-year OS, 0% vs. 46.5%, P <0.001). Among the 18F-FDG-PET parameters, the MTV and TLG were significantly higher in the mutated KRAS group than in the wild-type KRAS group (P = 0.013 and P = 0.026, respectively). ROC curve analysis revealed a cut-off value of 38 for the MTV, with the highest accuracy (area under the curve = 0.789; 95% confidence interval, 0.581–0.902) for predicting KRAS mutation. This cut-off value permitted stratification of OS (high vs. low: 5-year OS, 13.1% vs. 36.7%, P = 0.008).
Conclusions
High MTV is associated with KRAS mutation and poor postoperative outcomes in patients with ICC, suggesting that the MTV of ICC measured by 18F-FDG-PET may provide useful information for tumor molecular profiles and prognosis.
Similar content being viewed by others
Background
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver malignancy, with an increasing incidence and mortality worldwide [1,2,3]. Currently, surgical resection represents the curative treatment option, but surgery is only possible for selected patients. Even with optimal surgery, the 5-year overall survival (OS) rate is 15–40% [4, 5]. The incidence of post-surgical recurrence is 50–60% [3, 4], and recurrence is associated with a poor prognosis. It is thus essential to develop a multidisciplinary strategy for ICC to improve prognosis [6,7,8,9].
Recently, new therapies based on the molecular or genetic characteristics of ICC have been developed. In theory, it should be possible to select the appropriate therapy for each patient depending on the molecular or genetic characteristics of their individual tumors. Without surgical resection, however, there is little biological information in ICC, so it is difficult to select treatment before surgery.
In the era of genetic landscape and precision medicine, increasing evidence suggests that genetic mutation profiles allow the evaluation of prognosis of cancer postoperatively [10,11,12,13,14]. ICC has a relatively large number of actionable mutations compared to other gastrointestinal carcinomas. Several studies have demonstrated that KRAS mutation in ICC could affect prognosis [12,13,14]. Therefore, assessment of KRAS mutation status may contribute to the development of treatment strategies. A surrogate marker for KRAS mutation would provide genomic information without the need for biopsy or surgery. To identify this surrogate marker, it may be helpful to assess the biological effects of KRAS mutation.
Investigators have reported that tumor cells with KRAS mutation exhibit enhanced glucose uptake and glycolysis to survive in severe conditions (i.e., low-glucose and hypoxia) [15,16,17]. Glucose transporter-1 (GLUT-1) is a major glucose transporter in cholangiocarcinoma, and its expression is correlated with higher malignant potential in ICC [18, 19]. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG), a glucose analog, is a less invasive modality that determines the glucose metabolism potential of tumors by quantifying 18F-FDG uptake. However, to date, the association between KRAS mutation and 18F-FDG-PET has not been reported in ICC.
We investigated the presence of KRAS mutation, the expression of GLUT-1, and 18F-FDG-PET parameters in 50 ICC patients, and examined whether there was an association between ICC prognosis and these factors.
Methods
Patients
The study was performed as a retrospective review of patients with mass-forming ICC who underwent hepatectomy at the Department of Surgery, Kyoto University Hospital (Kyoto, Japan) between May 2009 and August 2016. Inclusion criteria in the present study were: (1) diagnosis of ICC pathologically confirmed by two experienced pathologists, and (2) patients who underwent hepatectomy within 2 weeks of 18F-FDG-PET analysis. ICC was defined as a tumor developing from the intrahepatic bile duct at the secondary or more distal branches. Exclusion criteria were: (1) patients with apparent distant metastasis detected on preoperative imaging, and (2) patients with histologically different diseases. The clinicopathological characteristics and survival data of these patients were extracted from a prospectively maintained institutional database. Tumor stages were assessed according to the American Joint Committee on Cancer classification system, 7th edition [20]. Post-operative adjuvant chemotherapy was principally administered using gemcitabine for tumors in II–IV stages. Preoperative chemotherapy was not administered to patients in this study. Operative mortality was defined as death within 30 days of surgery; morbidity was evaluated according to the Clavien-Dindo classification system [21]. The latest survival data were collected on September 1, 2017. The study protocol was approved by the ethical committee of the Graduate School of Medicine, Kyoto University (G1019). Written informed consent was obtained from all study participants.
KRAS mutational analysis
DNA extraction and mutation detection were performed using a modified protocol, as described previously [22, 23]. Briefly, DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) tumor tissue sections by the QIAamp FFPE Tissue Kit (Qiagen, Venlo, Netherlands). Following DNA extraction, an assay with RASKET KIT (MBL, Nagoya, Japan) was performed according to the manufacturer’s protocol. We simultaneously examined 146 KRAS mutations located in exon 2 codons 12 and 13, exon 3 codons 59 and 61, and exon 4 codon 117.
GLUT-1 expression analysis
Immunohistochemical staining was performed on 4-μm-thick FFPE sections as previously described, using GLUT-1 antibody (#ab15309, Abcam, Tokyo, Japan) diluted at 1:200 [24]. Membrane-predominant staining was regarded as positive. The grade of GLUT-1 expression was semi-quantitatively assessed according to the following scoring scheme: the percentages of tumor cells with strong staining were calculated in 10 fields (magnification 200×), and the mean percentage of stained tumor cells was calculated and scored on a 5-point scale (0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%) [24]. The evaluation of immunohistochemical analysis was performed by two independent, experienced researchers who were blinded to the clinicopathological data. Using the median value of immunostaining grade as the cut-off, patients were classified into high and low GLUT-1 expression groups.
18F-FDG-PET study and image analysis
18F-FDG-PET studies were performed using a PET/computed tomography (CT) scanner (Discovery ST Elite or Discovery IQ; GE Healthcare, Milwaukee, WI, USA). Patients fasted for at least 4 h before undergoing 18F-FDG-PET. The plasma glucose level was checked before the injection of 18F-FDG, and there were no patients with blood glucose level > 150 mg/dL in this study. Data acquisition started approximately 60 min after intravenous administration of 18F-FDG (injected dose: approximately 3.7 MBq/kg body weight). Initially, starting at the level of the upper thigh, low-dose CT scans were obtained with the following parameters: 40–60 mA, 120 kV, 0.6-s tube rotation, and 3.75-mm section thickness. The CT images were acquired during shallow breathing, and scanning included the area from the upper thigh to the skull. Immediately after the CT scans were acquired, PET emission scanning was performed with an acquisition time of 2–3 min per bed position. Whole-body PET images were attenuation-corrected using CT data and reconstructed using a 3D ordered-subsets expectation–maximization algorithm called VUE Point Plus (Discovery ST Elite: 14 subsets, two iterations, a matrix size of 128 × 128, a voxel size of 4.7 × 4.7 × 3.3 mm, and post-filtering at 5-mm full width at half maximum; Discovery IQ: 12 subsets, four iterations, a matrix size of 192 × 192, a voxel size of 3.3 × 3.3 × 3.3 mm, and post-filtering at 5-mm full width at half maximum). For quantitative analysis, at least two board-certified radiologists/nuclear medicine physicians assessed 18F-FDG accumulation on a workstation (Advantage Workstation 4.6; GE Healthcare) by calculating the standardized uptake value (SUV) in the regions of interest placed over the suspected lesions using all available clinical information and correlative conventional imaging for anatomic guidance. The SUV was calculated for the quantitative analysis of tumor 18F-FDG uptake as follows: SUV = C (kBq/mL)/ID (kBq)/body weight (kg), where C is the tissue activity concentration measured by PET and ID is the injected dose.
18F-FDG uptake was also quantitatively assessed by SUVs calculated in volumes of interest (VOIs) that were placed over regions of abnormal 18F-FDG uptake. The boundaries of each VOI were checked by comparison with fused CT to exclude adjacent 18F-FDG avid structures. The maximum SUV (SUVmax) within the VOI was recorded for the primary tumor. Metabolic tumor volume (MTV) was defined as the total tumor volume segmented via the threshold SUV. The threshold of the mediastinal blood pool activity was used to define the lesions. For the threshold SUV established using mediastinal blood pool activity, a VOI of more than 5 × 5 × 5 voxels was drawn manually at the aortic arch. The average SUV at the aortic arch plus two standard deviations of the VOI was adopted as the threshold SUV for the tumor using the mediastinal blood pool. Total lesion glycolysis (TLG) was determined as a product of the average SUV (SUVmean) segmented via the threshold SUV multiplied by the number of voxels in the MTV (i.e., SUVmean × MTV). For each patient, we defined the MTV and TLG as the sum of the MTVs and TLGs of all lesions, respectively.
Statistical analysis
Continuous values are expressed as median (range) and were compared using the Mann–Whitney U test. Categorical variables were compared using Fisher’s exact test. The prognostic values of clinicopathological factors for survival were assessed using a Cox proportional hazard regression model for univariate and multivariate analyses. Hazard ratios with Wald 95% confidence intervals (CIs) were provided for the Cox regression models. OS was calculated from the date of surgery to the date of death or last follow-up according to the Kaplan–Meier method and analyzed by the log-rank test. Cut-off values of 18F-FDG-PET parameters to discriminate KRAS mutation status were determined by receiver operating characteristic (ROC) curve analysis. The optimal cut-off values, sensitivities, and specificities of 18F-FDG-PET parameters were determined using the Youden index. All analyses were two-sided, and differences were considered significant when P was < 0.05. All statistical analyses were performed using the JMP statistical software package (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
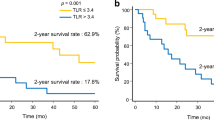
The clinicopathologic features of the patients are summarized in Table 1. No patients received any treatment before operation, and postoperative adjuvant chemotherapy was performed in 30 patients (60.0%). The overall morbidity rate was 36.0% (n = 18), and the class III/VI morbidity rate was 24.0% (n = 12). Follow-up was available in all cases and ranged from 9.0 to 91.0 months (median, 32.0 months). The median survival time was 22.5 months, with 3- and 5-year survival rates of 32.6 and 8.7%, respectively. Overall, 16 (32.0%) patients had mutations in KRAS. In multivariate analysis, KRAS mutation was an independent prognostic factor for OS (Additional file 1: Table S1). Furthermore, patients with mutated KRAS exhibited impaired OS compared with those with wild-type KRAS (5-year survival, 0% vs. 35.1%, P < 0.001, Fig. 1).
The association between KRAS mutation and clinicopathological factors
Patients were divided into two groups based on KRAS mutation status (Table 2). There were no significant differences in clinical and treatment factors between the two groups. No correlation was observed between pathological factors, histologic type, vascular invasion, biliary invasion, lymph node metastasis, or tumor size or stage and KRAS mutation status. However, there were significant differences in intrahepatic tumor number (P = 0.008). To assess the impact of KRAS mutation on tumor glucose uptake, we examined the expression of GLUT-1 by immunohistochemistry in resected ICC specimens (Fig. 2). The GLUT-1 expression score was significantly higher in the mutated KRAS group than in the wild-type KRAS group (median, 4.0 and 1.0, respectively, P < 0.001). Furthermore, patients were divided into two groups according to expression of GLUT-1. High expression of GLUT-1 was detected in 26 of 50 ICC patients, and there were no significant differences in clinical or treatment characteristics between the two groups. However, patients with high expression of GLUT-1 had a higher SUVmax (P < 0.001), MTV (P = 0.008), and TLG (P < 0.001) of 18F-FDG-PET parameters compared to patients with low expression (Table 3). Survival after surgery was clearly divided when stratified by expression of GLUT-1 (5-year survival, 0% vs. 46.5%, P <0.001, Fig. 3).
Immunohistochemical analysis for GLUT-1 expression in resected intrahepatic cholangiocarcinoma specimens and 18F-FDG-PET/CT scans. a A tumor with wild-type KRAS showed negative staining (score 0) for GLUT-1, and 18F-FDG-PET/CT scans showed modest accumulation of 18F-FDG in the tumor (MTV, 3.6; TLG, 31.9). b A tumor with mutated KRAS showed moderate membranous staining (score 3) for GLUT-1, and 18F-FDG-PET/CT scans showed intense accumulation of 18F-FDG in the tumor (MTV, 76; TLG 213). The scale bar represents 100 μm. GLUT-1 glucose transporter-1, 18F-FDG-PET 18F-fluorodeoxyglucose positron emission tomography, MTV metabolic tumor volume, TLG total lesion glycolysis
Survival of patients after curative surgery according to the grade of GLUT-1 expression. The Kaplan–Meier method was used to determine the patient survival and the log-rank test was used to compare survival of intrahepatic cholangiocarcinoma patients with high and low expression of GLUT-1. GLUT-1 glucose transporter-1
The association of 18F-FDG-PET parameters with KRAS mutation status and prognosis
Next, we analyzed the association between KRAS mutation and 18F-FDG-PET parameters. There were no significant differences in SUVmax between the mutated KRAS group and the wild-type KRAS group (median, 5.7 and 5.8, respectively, P = 0.370, Fig. 4a). However, the MTV and TLG were significantly higher in the mutated KRAS group than in the wild-type KRAS group (median MTV, 75.9 vs 20.3, P = 0.013; median TLG, 259.1 vs 61.9, P = 0.026, Figs. 2, 4b, c).
The association between KRAS mutation status and 18F-fluorodeoxyglucose positron emission tomography parameters. a Analysis of the maximum standardized uptake value (SUVmax), b metabolic tumor volume (MTV), and c total lesion glycolysis (TLG) according to KRAS mutation status. Bars = means. Assessed using the Mann–Whitney U test
Finally, we examined the association between 18F-FDG-PET parameters and patient survival after surgery. To determine the optimal cutoff of the 18F-FDG-PET parameters, we performed a ROC curve analysis to discriminate KRAS mutation status (Additional file 2: Figure S1). This analysis showed the highest accuracy among the 18F-FDG-PET parameters, (area under the curve = 0.789; 95% CI 0.581–0.902) for an MTV cutoff value of 38. The sensitivity, specificity, and accuracy for predicting KRAS mutation status were 77.8, 67.9, and 68.0%, respectively. Patients with a high MTV (≥ 38) exhibited worse OS than those with a low MTV (< 38) (high vs. low: 5-year OS, 13.1% vs. 36.7%, P = 0.008, Fig. 5).
Discussion
In this study, we investigated the KRAS mutation status in a cohort of 50 consecutive ICC patients who underwent radical hepatectomy and identified KRAS mutations in 32.0% of cases. The following features of our study are significant: (1) correlation between KRAS mutation and glucose uptake is recapitulated in ICC tumors, and (2) metabolic tumor volume on 18F-FDG-PET may provide useful information as a surrogate for prognosis, reflecting the impact of KRAS mutation on survival.
Malignant tumors can enhance tumor cell survival by genetic changes or modify glucose metabolism by cellular responses [25]. A number of genetic mutations in ICC have been identified [10,11,12,13,14]. In particular, KRAS mutation has been reported as a representative factor indicative of poor prognosis in ICC [12,13,14], and our patients with mutated KRAS showed significantly worse survival compared to those with wild-type KRAS. Regarding glucose metabolism, Warburg discovered that, even in the presence of oxygen, cancer cells undergo aerobic glycolysis rather than the normal oxidative phosphorylation. Aerobic glycolysis produces just two molecules of adenosine triphosphate (ATP) per molecule of glucose, while 36 ATP molecules are produced by oxidative phosphorylation. Cancer cells have an accelerated metabolism and increased requirements for ATP production. The reason why cancer cells, which need high ATP levels, take this inefficient pathway is not clear. To maintain high ATP levels for energy utilization, cancer cells may increase glucose transport through overexpression of GLUT-1. In this study, 52.0% of patients with ICC showed high expression of GLUT-1, which was associated with KRAS mutation. Whether the upregulation of GLUT-1 is attributable to KRAS mutation or whether GLUT-1 expression contributes to KRAS mutation remains to be established in ICC. High expression of GLUT-1 is associated with multiple tumors and tumor stage, and has been reported as a prognostic factor [26,27,28]. Patients with high GLUT-1-expressing tumors have a significantly poorer survival compared to patients with low GLUT-1 expression.
It has been reported that 18F-FDG accumulation reflects the KRAS mutational status of cancers [29,30,31]. We assessed three parameters measured by 18F-FDG-PET (SUVmax, MTV, and TLG). In practice, SUVmax is the most commonly assessed parameter of 18F-FDG-PET, and previous reports have suggested that this parameter is associated with survival in patients with various cancers [32, 33]. Recently, several reports suggested that the volumetric parameters of tumors measured by 18F-FDG-PET, such as the MTV and TLG, are more accurate prognostic factors than SUVmax in patients with various malignancies [34, 35]. In this study, we have shown that KRAS mutations were significantly associated with high 18F-FDG uptake as calculated by the MTV and TLG, while SUVmax was comparable between the mutated KRAS group and wild-type group. Volumetric parameters measured by 18F-FDG-PET have advantages in terms of predicting KRAS mutation status. First, 18F-FDG-PET is non-invasive and harmless compared to performing a liver tumor biopsy. Second, volumetric parameters reflect the metabolic activity of the entire tumor mass in a three-dimensional manner. There was no association between SUVmax and KRAS mutation in this study, but this could be due to the intratumoral heterogeneity of the KRAS mutation status [13, 36]. SUVmax exhibits only the highest intensity of 18F-FDG uptake in the tumor and cannot reflect the metabolic activity of the entire tumor. Of the three parameters, the MTV and TLG were associated with KRAS mutation, and ROC analysis showed that the MTV was the best predictor of KRAS mutation.
In this study, the median SUVmax of the tumor lesions was 5.8 (range, 2.9–14.7). No patient with ICC had too little 18F-FDG uptake to detect the tumor lesion by FDG-PET despite of detectable tumor by CT or magnetic resonance imaging (MRI). When it was difficult to determine boundaries for obscure tumors, the tumor margin was identified using preoperative imaging, such as CT and/or MRI. Morphological size measured by CT or MRI was not significantly different between tumors with mutated KRAS and those with wild-type KRAS. In addition, it is well known that ICC tumors are frequently accompanied by central necrosis as they increase in size. Metabolic volume measured by 18F-FDG-PET more accurately reflects tumor viability than does radiographic volume, particularly as it takes into account tumor activity.
The current study has a limitation. It was a retrospective design conducted in a single institutional cohort of patients and involved a small study population of 50 consecutive ICC patients, including only 16 patients with KRAS mutation. This weakened the statistical power of our analysis. In addition, patient-selection bias might have influenced the statistical results. However, this study focused exclusively on ICC, rather than on biliary tract cancer, contributing to a better understating of KRAS-related molecular biology. Therefore, this should be considered a preliminary report, and further prospective studies with larger patient cohorts are required to validate the combination of 18F-FDG-PET parameters in association with somatic mutations and prognosis for patients with ICC.
Conclusions
In this study, we demonstrated that KRAS mutation is associated with GLUT-1 expression and volumetric parameters of 18F-FDG-PET in ICC tumors. KRAS mutation affects the prognosis of ICC patients undergoing surgical resection and is associated with tumor glucose uptake. Moreover, our results suggest that 18F-FDG-PET may serve as a potential biomarker for KRAS mutation status and survival in ICC.
Abbreviations
- ICC:
-
intrahepatic cholangiocarcinoma
- GLUT-1:
-
glucose transporter-1
- 18F-FDG-PET:
-
18F-fluorodeoxyglucose positron emission tomography
- FFPE:
-
formalin-fixed, paraffin-embedded
- CT:
-
computed tomography
- MRI:
-
magnetic resonance imaging
- SUVmax :
-
maximum standardized uptake value
- VOI:
-
volume of interest
- MTV:
-
metabolic tumor volume
- TLG:
-
total lesion glycolysis
- 95% CIs:
-
95% confidence intervals
- OS:
-
overall survival
- ROC:
-
receiver operating characteristic
- ATP:
-
adenosine triphosphate
References
De Oliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149:565–74.
Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R, AISF Cholangiocarcinoma Committee. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490–5.
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–95.
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96.
Maithel SK, Gamblin TC, Kamel I, Corona-Villalobos CP, Thomas M, Pawlik TM. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119:3929–42.
Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2017. https://doi.org/10.1002/bjs.10641.
Buettner S, Koerkamp BG, Ejaz A, Buisman FE, Kim Y, Margonis GA, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-A multi-institutional analysis. J Surg Oncol. 2017;115:312–8.
Omichi K, Cloyd JM, Yamashita S, Tzeng CD, Conrad C, Chun YS, et al. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752–65.
Zhu AX, Borger DR, Kim Y, Cosgrove D, Ejaz A, Alexandrescu S, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21:3827–34.
Ruzzenente A, Fassan M, Conci S, Simbolo M, Lawlor RT, Pedrazzani C, et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patients. Ann Surg Oncol. 2016;23:1699–707.
Chaitanya RC, Rachna S, Ying W, Asif R, HyunSeon CK, Jacqueline W. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS ONE. 2014;9:1371–94.
Zou S, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696.
Robertson S, Hyder O, Dodson R, Nayar SK, Poling J, Beierl K, et al. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013;44:2768–73.
Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22.
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9.
Pylayeva-Gupta YE, Grabocka D, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–74.
Paudyal B, Oriuchi N, Paudyal P, Higuchi T, Nakajima T, Endo K. Expression of glucose transporters and hexokinase II in cholangiocellular carcinoma compared using [18F]-2-fluro-2-deoxy-d-glucose positron emission tomography. Cancer Sci. 2008;99:260–6.
Kubo Y, Aishima S, Tanaka Y, Shindo K, Mizuuchi Y, Abe K, et al. Different expression of glucose transporters in the progression of intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1610–7.
Edge SB, Compton CC. The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Bando H, Yoshino T, Shinozaki E, Nishina T, Yamazaki K, Yamaguchi K. Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit. BMC Cancer. 2013;13:405.
Yoshino T, Muro K, Yamaguchi K, Nishina T, Denda T, Kudo T, et al. Clinical validation of a multiplex kit for RAS mutations in colorectal cancer: results of the RASKET (RAS KEy testing) prospective, multicenter study. EBioMedicine. 2015;2:317–23.
Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, et al. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol. 2011;55:846–57.
Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formationfrom orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011;71:3110–20.
Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22:1644–52.
Marubashi S, Gotoh K, Takahashi H, Ohigashi H, Yano M, Ishikawa O, et al. Prediction of the postoperative prognosis of intrahepatic cholangiocarcinoma (ICC): importance of preoperatively- determined anatomic invasion level and number of tumors. Dig Dis Sci. 2014;59:201–13.
Yoh T, Seo S, Hatano E, Taura K, Fuji H, Ikeno Y, et al. A novel biomarker-based preoperative prognostic grading system for predicting survival after surgery for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24:1351–7.
Kawada K, Nakamoto Y, Kawada M, Hida K, Matsumoto T, Murakami T, et al. Relationship between 18F-fluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res. 2012;18:1696–703.
Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil Diaz C, Lopez-Picazo JM, et al. Role of [18F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:2058–65.
Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820–7.
Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255–60.
Bahri H, Laurence L, Edeline J, Leghzali H, Devillers A, Raoul JL, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med. 2014;55:1786–90.
Ryu IS, Kim JS, Roh JL, Cho KJ, Choi SH, Nam SY, et al. Prognostic significance of preoperative metabolic tumour volume and total lesion glycolysis measured by (18)F-FDG PET/CT in squamous cell carcinoma of the oral cavity. Eur J Nucl Med Mol Imaging. 2014;41:452–61.
Ogawa S, Itabashi M, Kondo C, Momose M, Sakai S, Kameoka S. Prognostic value of total lesion glycolysis measured by 18F-FDG-PET/CT in patients with colorectal cancer. Anticancer Res. 2015;35:3495–500.
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79.
Authors’ contributions
YI, SS, and KI designed the study, interpreted the results, and wrote the manuscript. YI, SS, and YN were responsible for the collection and assembly of data. All authors are responsible for the provision of study material or patients. All authors have made a substantial contribution to the study. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Noriko Sakaguchi (Department of Experimental Immunology, Immunology Frontier Research Center, Osaka University) for her helpful discussion.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the ethical committee of the Graduate School of Medicine, Kyoto University (G1019). Written informed consent was obtained from all study participants.
Funding
This work was supported by the Project for Cancer Research and Therapeutic Evolution (P-CREATE) from the Japan Agency for Medical Research and Development (AMED), and Grants-in-Aid for Scientific Research funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 26461909 and No. 17K10510).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1.
Univariate and Multivariate analyses of prognostic factors for overall survival.
Additional file 2: Figure S1.
The ROC curve analysis of the performance of 18F-FDG-PET parameters for predicting KRAS mutation status. (a) Maximum standardized uptake value (SUVmax), (b) metabolic tumor volume (MTV), and (c) total lesion glycolysis (TLG). Note the high area under the ROC curve (AUC), 95% confidence interval (CI), and cutoff value (red font). ROC receiver operating characteristic, AUC area under the curve.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ikeno, Y., Seo, S., Iwaisako, K. et al. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18F-FDG-PET is associated with the KRAS mutation status and prognosis. J Transl Med 16, 95 (2018). https://doi.org/10.1186/s12967-018-1475-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-018-1475-x