Abstract
Background
To investigate venous thromboembolism (VTE) in hospitalized patients with severe high altitude pulmonary edema (HAPE), we performed a single center retrospective study to evaluate its clinical characteristics, prognosis, and potential thromboprophylaxis strategies in a large referral and treatment center in plateau regions.
Methods
We studied a total of 18 patients with severe HAPE from January 1, 2012 to December 31, 2021. Demographic and clinical data, laboratory data, including ultrasound scans of the lower extremities and cardiac ultrasound, and computed tomographic pulmonary angiography (CTPA) variables were obtained, and comparisons were made between groups with and without VTE.
Results
Of the 18 patients hospitalized with severe HAPE (age 43 (range, 34–54) years, 14 [77.8%] men), 7 patients developed VTE (38.9%), including 5 with deep vein thrombosis (DVT) and pulmonary embolism (PE), 2 of whom had DVT only. Eighteen patients are all firstly rapid ascent to high altitudes which the mean altitude was 3700 m (3656–4050 m). Compared with patients who did not have VTE, patients with VTE had a longer time in hospital (13 [11, 19] versus 9 [7, 12]; P = 0.027), respiratory failure (6 [85.7%] versus 2 [18.2%]; P = 0.013), the shortened APTT (21.50 [19.00, 27.50] versus 26.30 [24.80, 30.10]; P = 0.044) and the higher level of D-dimer (7.81 [4.62, 9.60] versus 2.90 [1.75, 3.37]; P = 0.003). The proportion of thromboprophylaxis is too low in our cohort which 2 of 18 (11.1%) patients were given VTE prophylaxis. There was no statistically significant difference between the VTE and non-VTE groups (0 [0.0%] versus 2 [18.2%]; P = 0.497).
Conclusions
The prevalence of VTE is high in hospitalized patients with severe high altitude pulmonary edema (HAPE). Prophylaxis for venous thromboembolism may be protective in severe HAPE patients after admission. Our data seem to suggest that VTE is probably an additional prognostic factors in patients with severe HAPE.
Similar content being viewed by others
Introduction
High altitude pulmonary edema (HAPE) is a series of pulmonary disorders caused by pulmonary vasoconstriction due to hypoxia when a person first enters a plateau (usually > 2500 m above sea level). HAPE is the most common cause of death related to high altitude. The reported incidence of HAPE ranges from an estimated 0.01% of skiers traveling from low altitude to Vail, CO (2500 m), to 15.5% of Indian soldiers rapidly transported to altitudes of 3355 and 5940 m [1]. It is a non-cardiogenic pulmonary edema and a potentially fatal disease of altitude. Exaggerated hypoxic pulmonary vasoconstriction, elevated pulmonary artery pressures, and high-permeability noncardiogenic edema resulting from stress failure of pulmonary capillaries in focal areas of the lung characterize HAPE. Early symptoms of HAPE include exertional dyspnea, cough and reduced mobility. As the disease progresses, dyspnea at rest, worsening cough and the appearance of distinct pink frothy sputum suggest the development of significant pulmonary edema [2]. Deep vein thrombosis (DVT), a subset of venous thromboembolism (VTE), is a major preventable cause of morbidity and mortality worldwide. The incidence of VTE is estimated to be 1 per 1000 people annually [3, 4]. Virchow’s Triad, first described in 1856, implicates three contributing factors in the formation of thrombosis: venous stasis, vascular injury, and hypercoagulability. However, the concurrent presence of venous stasis and vascular injury or hypercoagulability greatly increases the risk for clot formation [5].
Previous studies demonstrated an increased risk of venous stasis, vascular injury, and hypercoagulability in HAPE patients. The prevalence of VTE and its pathophysiology, clinical characteristics, prognosis, screening, and preventive strategies have not been investigated in this HAPE illness that can cause severe and critical disease and death. We performed a single institutional retrospective study in patients with confirmed severe HAPE to identify the prevalence, clinical characteristics, and prognosis of VTE in the cohort of hospitalized patients.
Methods
Study design and patients
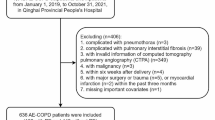
Two hundred and sixty-four patients diagnosed with HAPE at Qinghai Provincial People’s Hospital between January 1, 2012 and December 31, 2021 were retrospectively collected.
The inclusion criteria were 1) diagnosed with HAPE according to the Guideline [6]; 2) aged ≥18 years; 3) any of the following conditions: respiratory failure requiring mechanical ventilation, shock, or other organ failure requiring admission to intensive care unit (ICU) admissions. Therefore, 26 patients with severe HAPE were included. Then, we excluded those without available computed tomographic pulmonary angiography (CTPA), cardiac ultrasound or venous ultrasound scanning. We divided the above population into VTE and non-VTE groups in order to effectively explore the clinical characteristics of VTE. Finally,18 patients with severe HAPE were included in the analysis (Fig. 1).
Data collection
The baseline characteristics of the patients were collected from the medical records, including age, sex, weight, height, smoking history, altitude (permanent residence, birthplace and affected area), altitude difference, disease course, hospital stay, medical history and complications. Body mass index (BMI) was calculated as weight (kg) divided height squared (m2). The symptoms, physical examination findings, laboratory examination findings at admission, echocardiographic detection and treatments were collected.
Statistical analysis
The normality of the continuous variables was tested using the Shapiro-Wilk test. The data in a normal distribution were described as means ± standard deviations and compared using the independent students’ t-test. The data not in a normal distribution were described as medians (P25, P75) and compared using the nonparametric Wilcoxon test. The categorical data were described as n (%) and compared using the Fisher exact probability test. We used two-tailed test, and P < 0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R (version 3.6.3, https://www.r-project.org/).
Results
The occurrence rate of VTE in severe HAPE patients was 38.9% (7/18), including 5 with deep vein thrombosis (DVT) and pulmonary embolism (PE), 2 of whom had DVT only. We followed up the survival rates of all patients within 28 days after a diagnosis of severe HAPE. No patients were lost to follow-up. Moreover, the 18 patients with severe HAPE were no 28-day mortality after patients received anticoagulant therapy (Fig. 2).
High altitude pulmonary edema (HAPE) in a 39-year-old boy who had dyspnea at rest and worsening cough for 3 days before admission. A-D Axial image of CT pulmonary angiogram showing thrombi as filling defects in right main pulmonary artery extending into its branch and in distal left pulmonary artery with extension into its superior branch. E-H Multiple lesions of ground glass opacity, patchy lesions and partial consolidation in the CT imaging were presented in the bilateral lung center along the bronchovascular bundle
Table 1 shows the clinical data for our cohort. The mean age was 43 (34–54) years; 14 (77.8%) patients were men. Comorbidities included respiratory failure, acute high altitude cerebral edema, high altitude polycythemia, pulmonary hypertension. No patient had a history of VTE. Common symptoms at the onset of illness were fever, dry cough, fatigue, dyspnea, diarrhea, headache, Lower extremity swelling and Lower extremity pain. Days from entry into the plateau to onset of illness was 1 (1–2) day. Compared with the non- VTE group, patients with VTE were longer time in hospital (9 (7, 12) vs 13 (11, 19), P = 0.027) and a higher proportion of respiratory failure (85.7% [6/7] vs 18.2% [2/11], P = 0.013). There were no differences in altitude (permanent residence, birthplace, affected area, difference), symptoms, physical examination, imaging examination and echocardiographic detection (P > 0.05 for all) between the VTE and the non- VTE groups.
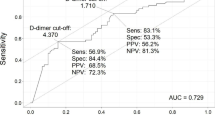
Table 2 shows laboratory data and abnormalities at admission, including D-dimer, Activated partial thromboplastin time, Cardiac troponin I and B-type natriuretic peptide. There were no differences in Blood gas analysis, routine blood tests and other blood chemistry tests (P > 0.05 for all) between the DVT and the non-DVT groups.
Treatments of patients with high altitude pulmonary edema are shown in Table 3. Of the 18 patients, 18 (100%) all received oxygen therapy, noninvasive mechanical ventilation and diuretics therapy, 4 (36.4%) received aminophylline therapy, 1 (5.6%) received vasodilator drugs therapy, 7 (38.9%) patients were given anticoagulant therapy, and 3 (16.7%) patients received Antiplatelet therapy before CTPA, cardiac ultrasound or venous ultrasound scanning for VTE. Most patients (10/18 [55.6%]) received hormone therapy, 4 (22.2%) with sedative treatment and 5 (27.8%) with gamma globulin. Compared with the non-VTE group, patients with VTE have similar proportion of VTE prophylaxis (0 [0.0%] versus 2 [18.2%]; P = 0.497). There was no statistically significant difference in the other treatments between the VTE and non-VTE groups (P > 0.05 for all).
Discussion
This study explored the prevalence and clinical characteristics of venous thromboembolism from severe high altitude pulmonary edema in plateau regions. We performed a single-institution retrospective study of patients with confirmed severe HAPE and found a high prevalence of VTE and an association between VTE and longer time in hospital, respiratory failure, the shortened APTT and the higher level of D-dimer in hospitalized patients with severe HAPE. In our current study of the largest sample size to date, little is known about the occurrence rate and the association of VTE in severe HAPE.
We observed that the occurrence rate of VTE in our study population was 38.9% (7/18 studied), 26.9% (7/26 of all severe HAPE studied) and 2.65% (7/264 of all patients in our center). This prevalence appears to be higher than that reported in the literature [7,8,9,10], which reported that the rates of objectively confirmed VTE in 4 prospective studies ranged from 13 to 31% and suggested a potential role for thromboprophylaxis in patients requiring critical care. This rate was also higher than that reported for many other hospitalized patients [11, 12] and series of patients in ICUs reported from China [13].
Several reasons probably account for the high prevalence of DVT in severe HAPE patients. First, most of the previously mentioned studies focused on critically ill patients who were in the ICU for different diseases. HAPE is an abnormally high pulmonary artery pressures due to multiple factors. Most studies suggest that HAPE is associated with damaged endothelium and alveolar epithelium caused by inflammatory responses under hypoxic conditions, which involve several pathways and mediators, including hypoxia-inducible factor, vascular endothelial growth factor, endothelin-1, and inducible nitric oxide synthase, and also involving sodium channels that regulate water transport, Na-K-ATPase, and aquaporin, pulmonary hemodynamic changes and higher hydrostatic pressure play a vital role in the acute and rapid progression of HAPE [14,15,16]. Second, there are aspects of altitude excursions that increase blood viscosity, for example, dehydration causing hemoconcentration, and polycythemia, in addition to the compensatory rise in hematocrit with acclimatization, which are likely to increase VTE risk. Furthermore, Studies have shown hypoxia and low temperature at high altitude can induce hypercoagulabilitys to increase thromboembolic events at high-altitude [17].
Outcome analyses clinical characteristics of venous thromboembolism onset from severe high altitude pulmonary edema in plateau regions, including longer time in hospital, respiratory failure, the shortened APTT and the higher level of D-dimer. Therefore, we speculate that the inflammatory state may promote venous thrombosis under hypoxic conditions. Coagulation activation could also be associated with a sustained infammatory response [18]. D-dimer is an important indicator for diagnosing of patients with VTE, and its increase is important significance for the differential diagnosis of patients with symptomatic VTE. Le Roux et al. demonstrated that the levels of D-dimer levels increased significantly at 6542 m after 1 week and at 3 weeks compared to those observed at sea level in seven climbers [19]. Analogously, Pichler Hefti et al. found that D-dimer levels increased with increasing altitude in Muztagh Ata, China [20]. Our data showed that serum D-dimer levels in patients with VTE in severe high altitude pulmonary edema were higher than non-VTE groups. This suggests that D-dimer, as an important differential index for VTE diagnosis, still has diagnostic efficacy in plateau regions. A transcriptomic and proteomic analysis of platelets demonstrated that plateau regions were associated with the upregulation of proteins with thrombosis and platelet activation without thrombosis in plateau regions -residing patients compared to subjects residing at low-altitude [21]. Moreover, a novel genome-wide expression analysis performed by Jha et al. showed that genes associated with the coagulation cascade and platelet activation were significantly upregulated in patients with VTE at plateau regions [22]. These studies indicate that platelet activation may induce thrombosis.
Prophylaxis for VTE [23, 24] and for extended-duration VTE [25, 26] has been investigated in clinical trials to improve clinical outcomes in severely or critically ill patients. Regrettably, the proportion of thromboprophylaxis is too low in our cohort which 2 of 18 (11.1%) patients were given VTE prophylaxis. There was no statistically significant difference between the VTE and non-VTE groups (0 [0.0%] versus 2 [18.2%]; P = 0.497). Our data suggest that there is a possible protective effect of prophylaxis for VTE in the higher risk in this cohort. This also suggests that for HAPE, thromboprophylaxis strategies should fully be recognized and strengthened, including moderately increasing the dose of anticoagulant drugs. Because our sample size is limited, the effect of VTE prophylaxis on hospitalized patients with COVID-19 warrants further investigation.
Although these findings are not surprising, given that our patient population represented severely ill patients at high risk for VTE, our data raised the question of screening for VTE, risk stratification, and potential VTE prophylaxis to improve outcomes in hospitalized patients with HAPE. Meanwhile, since VTE has no specific clinical manifestations, it can be easily diagnosed as other respiratory diseases at the initial stage, and a combination of these diseases cannot be ruled out. These results make it difficult to diagnose VTE. Therefore, more attention should be paid to the high-risk VTE groups in HAPE groups and VTE prophylaxis.
This study has some limitations. First, this is a single center retrospective study, as the sample size is small, and it is difficult to exclude accidental errors. Second, due to the critical condition of patients with severe HAPE, CTPA examinations were restricted, which significantly underestimated the prevalence of VTE. Thus, Prospective multi-center large sample studies might be needed in the future to further confirm the findings in our current study.
Conclusions
The incidence of VTE is extremely high in patients with severe HAPE and may be associated with adverse outcomes. The clinical characteristics for VTE are longer time in hospital, respiratory failure, the shortened APTT and the higher level of D-dimer in severe HAPE. We suspect that VTE is probably an additional risk factor for the death of severe HAPE in hospitalized patients. The analysis of severe HAPE may help to provide more accurate screening for VTE and lead to corresponding measures to improve the clinical outcome of patients with severe HAPE.
Availability of data and materials
All data analyzed during the study are presented in the main manuscript. The anonymous dataset is available from the corresponding author upon reasonable request.
Abbreviations
- VTE:
-
Venous thromboembolism
- HAPE:
-
High altitude pulmonary edema
- CTPA:
-
Computed tomographic pulmonary angiography
- DVT:
-
Deep vein thrombosis
- PE:
-
Pulmonary embolism
- APTT:
-
Activated partial thromboplastin time
- BMI:
-
Body mass index
- ICU:
-
Intensive care unit
References
Pennardt A. High-altitude pulmonary edema: diagnosis, prevention, and treatment. Curr Sports Med Rep. 2013;12(2):115–9.
Maggiorini M. Prevention and treatment of high-altitude pulmonary edema. Prog Cardiovasc Dis. 2010;52(6):500–6.
Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501.
White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8.
Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow's contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010;8(3–4):168–72.
Bärtsch P. High altitude pulmonary edema. Respiration. 1997;64(6):435–43.
Moser KM, LeMoine JR, Nachtwey FJ, Spragg RG. Deep venous thrombosis and pulmonary embolism. Frequency in a respiratory intensive care unit. Jama. 1981;246(13):1422–4.
Geerts W, Cook D, Selby R, Etchells E. Venous thromboembolism and its prevention in critical care. J Crit Care. 2002;17(2):95–104.
Wilasrusmee C, Kiranantawat K, Horsirimanont S, et al. Deep venous thrombosis in surgical intensive care unit: prevalence and risk factors. Asian J Surg. 2009;32(2):85–8.
Angral R, Islam MS, Kundan S. Incidence of deep vein thrombosis and justification of chemoprophylaxis in Indian patients: a prospective study. Bangladesh Med Res Counc Bull. 2012;38(2):67–71.
Dagadaki O, Birbas K, Mariolis T, Baltopoulos G, Myrianthefs P. Necessity of the periodical ultrasound assessment of the peripheral venous system in intensive care unit patients. Ultrasound Med Biol. 2019;45(2):367–73.
Boddi M, Peris A. Deep vein thrombosis in intensive care. Adv Exp Med Biol. 2017;906:167–81.
Zhang C, Zhang Z, Mi J, et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine. 2019;98(23):e15833.
Alam P, Agarwal G, Kumar R, et al. Susceptibility to high-altitude pulmonary edema is associated with circulating miRNA levels under hypobaric hypoxia conditions. Am J Physiol Lung Cell Mole Physiol. 2020;319(2):L360–l8.
Kanipakam H, Sharma K, Thinlas T, Mohammad G, Pasha MAQ. Structural and functional alterations of nitric oxide synthase 3 due to missense variants associate with high-altitude pulmonary edema through dynamic study. J Biomol Struct Dyn. 2021;39(1):294–309.
Zong HF, Guo G, Liu J, Yang CZ, Bao LL. Influence of alveolar fluid on Aquaporins and Na+/K+-ATPase and its possible theoretical or clinical significance. Am J Perinatol. 2022;29(14):1586–95.
Li M, Tang X, Liao Z, et al. Hypoxia and low temperature up-regulate transferrin to induce hypercoagulability at high altitude. Blood. 2022;140(19):2063–75.
Beristain-Covarrubias N, Perez-Toledo M, Thomas MR, Henderson IR, Watson SP, Cunningham AF. Understanding infection-induced thrombosis: lessons learned from animal models. Front Immunol. 2019;10:2569.
Le Roux G, Larmignat P, Marchal M, Richalet JP. Haemostasis at high altitude. Int J Sports Med. 1992;13(Suppl 1):S49–51.
Pichler Hefti J, Risch L, Hefti U, et al. Changes of coagulation parameters during high altitude expedition. Swiss Med Wkly. 2010;140(7–8):111–7.
Shang C, Wuren T, Ga Q, et al. The human platelet transcriptome and proteome is altered and pro-thrombotic functional responses are increased during prolonged hypoxia exposure at high altitude. Platelets. 2020;31(1):33–42.
Jha PK, Sahu A, Prabhakar A, et al. Genome-wide expression analysis suggests hypoxia-triggered hyper-coagulation leading to venous thrombosis at high altitude. Thromb Haemost. 2018;118(7):1279–95.
Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Vaitkus PT, Goldhaber SZ. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–9.
Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended Thromboprophylaxis with Betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(6):534–44.
Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513–23.
Acknowledgements
Not applicable.
Funding
This work was supported by the Planning Project of Qinghai Department of Science and Technology (Grant NO. 2023-ZJ-719 to Dr. XiaoKai Feng).
Author information
Authors and Affiliations
Contributions
Drs Feng, Drs Pu and Drs Sun had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs XK Feng, XY Pu, YQ Sun completed the Concept and design of the research. Drs YM Liu, XK Feng wrote the main manuscript text and Drs YQ Sun, XW Feng and YX Tang. prepared the data and figures 1-2. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics and approval and consent to participate
This retrospective study involving human participants was approved by the Research Ethics Board at Qinghai Provincial People’s Hospital, Qinghai University (Ethical number: 2022–28) and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Feng, X., Tang, Y. et al. Clinical characteristics of venous thromboembolism onset from severe high altitude pulmonary edema in plateau regions. Thrombosis J 21, 22 (2023). https://doi.org/10.1186/s12959-023-00469-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-023-00469-4