Abstract
Ovarian cancer (OC) is one of the most prevalent and lethal gynecological malignancies, with high mortality primarily due to its aggressive nature, frequent metastasis, and resistance to standard therapies. Recent research has highlighted the critical role of extracellular vesicles (EVs) in these processes. EVs, secreted by living organisms and carrying versatile and bioactive cargoes, play a vital role in intercellular communication. Functionally, the transfer of cargoes orchestrates multiple processes that actively affect not only the primary tumor but also local and distant pre-metastatic niche. Furthermore, their unique biological properties position EVs as novel therapeutic targets and promising drug delivery systems, with potential profound implications for cancer patients.
This review summarizes recent progress in EV biology, delving into the intricate mechanisms by which EVs contribute to OC metastasis and drug resistance. It also explores the latest advances and therapeutic potential of EVs in the clinical context of OC. Despite the progress made, EV research in OC remains in its nascent stages. Consequently, this review presents existing research limitations and suggests avenues for future investigation. Altogether, the review aims to elucidate the critical roles of EVs in OC and spotlight their promising potential in this field.
Similar content being viewed by others
Introduction
Ovarian cancer (OC) is the second leading cause of global gynecologic cancer deaths [1]. Epithelial ovarian cancers (EOC) account for over 90% of ovarian tumors, with high-grade serous carcinoma being the most prevalent variant, typically originating from the distal end of the fallopian tube [2]. While cervical cancer has seen a reduction in morbidity and mortality due to decreased HPV infection rates and effective HPV vaccination [3], and most endometrial cancers can be cured by hysterectomy due to early clinical manifestations like postmenopausal bleeding [4], ovarian cancer remains insidious with no typical early symptoms, leading to late-stage diagnosis and posing significant threats to women’s lives [5]. The standard first-line treatment of ovarian cancer includes extensive debulking surgery, combined with platinum- and taxane-based chemotherapy, with or without the angiogenesis inhibitor bevacizumab [6]. The introduction of Poly (ADP-ribose) polymerase inhibitors (PARPis) has brought about a significant change in the treatment of ovarian cancer, opening a new era of ovarian cancer maintenance therapy. Several phase III clinical randomized controlled trials have shown that PARPis significantly prolong progression-free survival of ovarian cancer patients, especially in patients with BRCA gene mutation and/or homologous recombination deficiency (HRD) positivity, making them the preferred first-line maintenance therapy for some ovarian cancer patients [7,8,9,10,11]. Immunosuppressants and antibody-drug conjugates are also under investigation, offering new therapeutic hope [12]. An interim analysis of the Phase III DUO-O study presented at the 2023 American Society of Clinical Oncology annual meeting demonstrated for the first time that immunotherapy can provide tangible benefits in first-line maintenance therapy for ovarian cancer, altering the previously unsuccessful trend of immunotherapy in this disease [13]. Despite significant progress in surgical techniques, chemotherapy, and maintenance therapy over recent decades, more than 70% of patients with advanced disease will still experience recurrence after completion of standard initial therapy [14, 15]. And with more lines of treatment, patients develop multidrug resistance, leading to shorter remission periods and resulting in a 50% relative survival rate at five years globally [16]. Therefore, metastasis and chemotherapy resistance in ovarian cancer remain major obstacles to the treatment of ovarian cancer.
Extracellular vesicles (EVs) are cell-derived particles encapsulated by a lipid bilayer and incapable of self-replication. Depending on the biogenesis pathway, exosomes refer to EVs released from the interior of the cell via multivesicular vesicles, whereas ectosomes (a.k.a., microvesicle, microparticle) refer to EVs germinating from the surface of the cell. Moreover, some types of EVs are produced during specific cellular processes. Migrasomes are 500–3000 nm EVs left behind by migrating cells, while apoptotic bodies are formed by swelling and protrusion of apoptotic membranes during cell death [17]. In addition, based on diameter, small EVs typically refer to particles less than 200 nanometers in diameter, which includes small ectosomes and exosomes [18]. Among them, exosomes have been most intensively studied due to their small average particle size, narrow range of particle size distribution, complex composition and diverse functions [19] (Fig. 1). EVs carry a wide variety of cargoes, including RNAs, proteins, lipids, and DNA, and serve as important intercellular communication mediators that regulate gene expression profiles in the target cells [20]. In addition to delivering cargo to target cells to facilitate intercellular communication, EVs are involved in processes such as cell differentiation and proliferation, angiogenesis and immune signaling [21]. In recent years, an increasing body of research has emphasized the pivotal role of EVs in various aspects of cancer biology, including carcinogenesis, metastasis, drug resistance, and tumor immune response [22]. Furthermore, EVs have emerged as promising liquid biopsy markers due to their tumor-associated content and stable presence in body fluids, and have become a frontier hot spot in the field of tumor marker research, which is expected to help the accurate diagnosis and treatment of cancer [23].
Following this, the exploration will focus on the significance of tumor- and tumor microenvironment-derived EVs in orchestrating OC metastasis. The multifaceted roles of EVs in OC drug resistance will then be examined in greater depth, encompassing the intricate mechanisms of resistance and the potential therapeutic strategies targeting EVs. This in-depth discussion aims to elucidate the pivotal roles of EVs in the progression and treatment of OC.
Biogenesis of extracellular vesicles. Exosomes are produced via the endosomal pathway. The plasma membrane invaginates to form early endosomes, which can exchange materials with the Golgi apparatus or fuse with each other to form late endosomes. These late endosomes transition into multivesicular bodies (MVBs). MVBs can fuse with lysosomes for degradation or with the plasma membrane to release exosomes. Apoptotic bodies, large oncosomes, and ectosomes are formed through cell budding. Migrasomes are vesicular structures generated at the tips or branches of contractile fibers during cell migration. Released extracellular vesicles (EVs) can directly bind to the cell surface, fuse with the cell membrane to release their contents into the cytoplasm, or be internalized by cells through endocytosis
How do EVs affect ovarian cancer metastasis?
The metastasis of ovarian cancer is a dynamic and multifaceted process, requiring cancer cells to undergo a series of steps in the invasion-metastasis cascade. Initially, ovarian cancer cells from the primary lesion invade the extracellular matrix, locally invading to enable tumor cells to breach the basement membrane and vascular walls, subsequently entering blood or lymphatic vessels and thus accessing the circulatory system. Once tumor cells survive in the circulation system, they can potentially be transported to other parts of the body and establish in distant organs. Unlike most cancers that primarily metastasize through blood or lymphatic routes, the most common mode of ovarian metastasis is through implantation. Due to the lack of anatomical barriers, tumor cells can shed directly, implanting onto the surfaces of pelvic and abdominal organs, disseminating within the peritoneal cavity via peritoneal fluid [24] (Fig. 2).
EVs contribute to the aforementioned process of ovarian cancer metastasis by enhancing tumor cell invasiveness, promoting angiogenesis, modulating immunity, and inducing epithelial-mesenchymal transition. Additionally, prior to the formation of metastatic lesions, extracellular vesicles can flow within the circulatory system or peritoneal fluid, adjusting the microenvironment of distant organs, thereby inducing the formation of pre-metastatic niches and providing conducive conditions for the settlement and growth of ovarian cancer cells.
Metastatic process of ovarian cancer. In situ ovarian cancer can metastasize via blood and lymphatic vessels. However, due to the lack of anatomical barriers between the peritoneal cavity and ovaries, cancer cells can directly shed and implant within the peritoneum, the most common site of metastasis. EVs can travel with ascites, impacting the peritoneum and promoting the formation of pre-metastatic niches, thereby facilitating further OC spread
Improve dissemination potential of primary tumor cells
Migration refers to the process by which tumor cells move from their original location to other sites, typically involving cell motility and directional movement. Invasion, on the other hand, describes the penetration of tumor cells through surrounding tissues or blood vessel walls to invade neighboring tissues or blood vessels. For tumor cells to invade, they must possess migratory capability. EVs exert regulatory influence over the signaling pathways and genetic expression within tumor cells, consequently amplifying their migratory proficiency and expediting the process of invasion.
Exosomal LRP1 promotes the migration of ovarian cancer cells. The low-density lipoprotein receptor-related protein 1 (LRP1) is a high molecular weight transmembrane receptor widely expressed on cell surfaces, primarily involved in endocytosis and regulation of signaling pathways [25]. Proteomic analysis of serum exosomes from epithelial ovarian cancer (EOC) patients revealed significantly elevated LRP1 levels compared to healthy volunteers. In vitro experiments demonstrated that patient-derived serum exosomes promoted ovarian cancer cell migration, possibly through the p-ERK/MMP2/MMP9 signaling pathway. Subsequent in vivo experiments further confirmed the impact of exosomal LRP1 on tumor migration. Additionally, prognostic analysis indicated that elevated levels of LRP1 may lead to shortened overall survival in EOC patients, suggesting the potential diagnostic value of circulating exosomal LRP1. However, further studies with expanded sample sizes are needed for validation [26].
EVs can also enhance the migration of ovarian cancer cells through the transfer of microRNA. M2 macrophages, particularly tumor-associated macrophages (TAMs), are predominant immune cells in the peritoneum [27]. The interaction between TAMs and tumor cells is pivotal in OC progression, with EVs occupying a crucial position as carriers of micro-messages in this process. In M2 exosomes, microarray analyses revealed an enrichment of miR-221-3p, which is transferred to ovarian cancer cells, directly suppressing cyclin-dependent kinase inhibitor 1B (CDKN1B) and promoting cell proliferation and migration. Decreased CDKN1B levels correlate with ovarian cancer progression and poor prognosis [28]. A separate study found that miR-106a-5p expression is markedly higher in ovarian cancer cells compared to normal cells. Tumor-derived EVs carrying miR-106a-5p inhibit KLF6, promoting OC cell proliferation and migration, thus driving cancer progression and metastasis [29]. Notably, exosomal miR-106a-5p has also been reported to promote tumorigenesis in other cancers such as nasopharyngeal carcinoma, hepatocellular carcinoma, and breast cancer [30,31,32].
In addition to directly promoting tumor growth and metastasis, more aggressive subpopulations can confer a metastatic phenotype to less aggressive subpopulations through exosomes. CD44 expression is higher in ovarian cancer cells with greater metastatic ability and in the exosomes they secrete. It was found that exosomal CD44 from highly metastatic HO8910PM cells could be transferred to low metastatic HO8910 cells, and that overexpression of CD44 promoted migration, invasion, and proliferation of HO8910 cells as a promoter of metastatic behavior. The ability of ovarian cancer to metastasize extensively may develop by transferring high metastatic capacity between tumor cells. However, the study’s reliability appears to be insufficient, given that it only experimented with a single ovarian cancer cell line [33].
Conversely, some EVs may inhibit malignant biological behaviors. Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK), a member of the TNF superfamily, may mediate an anti-tumor effect on tumor-infiltrating macrophages [34]. Research demonstrated that exosomes from TWEAK-stimulated macrophages (TMs) attenuated the migration and invasion of EOC cells. TMs inhibit ovarian cancer invasion and metastasis both in vivo and in vitro by delivering exosomal miR-7 to epithelial ovarian cancer cells. miR-7, a tumor-suppressive microRNA, suppresses the activity of the EGFR/AKT/ERK1/2 signaling pathways in ovarian cancer cells. This study highlights TWEAK’s role in the enrichment of miR-7 in macrophages, although the precise mechanism by which TWEAK upregulates miR-7 remains undetailed [35].
Form pre-metastatic niche
The pre-metastatic niche (PMN) refers to the environment or conditions in distant organs that promote tumor cell colonization and growth before malignant tumor cells detach from the primary tumor site and spread through the blood or lymphatic system to these distant sites. The formation of the PMN is primarily driven by the secretion of EVs, cytokines, growth factors, and other signaling molecules [36]. In recent years, the role of EV-mediated PMN formation has garnered significant attention.
Current research on PMN focuses more on extracellular matrix remodeling and reprogramming. During EVs remodeling of stromal cells at the site of metastasis to facilitate metastasis, it is important to induce macrophage polarization and convert some normal stromal cells into CAFs. Macrophages are typically classified into two main groups, namely classically activated macrophages (M1) and alternatively activated macrophages (M2), with M1 macrophages exhibiting pro-inflammatory activity and M2 macrophages displaying pro-tumorigenic activity [37]. TAMs are a subset of immune cells in the TME and are involved in tumorigenesis, progression and metastasis. TAMs are predominantly of the M2 type and are situated in the TME, where they exert influence on metastasis in multiple cancer types through their interactions with cancer cells [38]. Studies have identified that circATP2B4-containing EVs generated by EOC cells are abnormally highly expressed in ovarian cancer metastasis and promoted M2 macrophage polarization through the miR-2-532p/SREBF3 axis, thereby promoting EOC metastasis [39]. In addition, miR-181c-5p is highly expressed in EVs from hypoxic EOC cells, leading to increased M2 polarization of TAMs and ultimately promoting EOC growth and metastasis. Further investigation revealed that miR-181c-5p upregulates HOXA10 by targeting KAT2B and activate the JAK1 / STAT3 signaling pathway, thereby promoting the polarization of TAMs [40].
EVs can also reprogram stromal cells in the PMN, including CAFs, into tumor-promoting phenotypes. Research has shown that exosomal miR-141, highly secreted by ovarian cancer cells, can reprogram mesenchymal fibroblasts into pro-inflammatory CAFs, thus promoting metastatic colonization. Additionally, tumor-secreted exosomal miR-141 activates tumor-stromal interactions and promotes PMN formation by mediating the YAP1/GROα/CXCRs signaling pathway [41]. Furthermore, OC-derived EVs were observed to transport miR-630 into NFs and through the KLF6/NF-κB axis, thereby promoting CAFs activation and facilitating ovarian cancer invasion and metastasis [42]. However, further in vivo experiments are required to further fully elucidate the role of miR-630/KLF axis in ovarian cancer.
The peritoneum is a thin membrane that covers the surfaces of intra-abdominal organs and the inner side of the abdominal wall. The shedding of ovarian cancer cells from the primary tumor and their carriage to the omentum and peritoneum through the physiological movement of peritoneal fluid is recognized as one of the most common metastatic routes for epithelial ovarian cancer [43]. The development of metastasis is not a random event, and it only occurs when tumor cells and organs are compatible, according to Stephan Paget’s seed-soil theory. In the microenvironment of the peritoneal cavity of ovarian cancer, human peritoneal mesothelial cells (HPMCs) are continuous monolayers of cells lining the peritoneal cavity, and the mesothelial layer composed of HPMCs is a natural barrier and an important line of defense for the body to resist the metastasis of tumors in the peritoneal cavity [44]. One of the critical steps in successful metastasis of ovarian cancer is the attachment and effective interaction of cancer cells with HPMCs to form secondary tumors. Reports have suggested that tumor cells release EVs carrying high concentrations of MMP1 mRNA in ascites, which induce apoptosis in mesothelial cells and disrupt the mesothelial cell barrier, facilitating peritoneal metastasis. Moreover, MMP1 in EVs may be a prognostic biomarker because the level of MMP1 expression in tumor tissues is closely associated with the prognosis of patients with early-stage ovarian cancer [45].
Apart from directly stimulating mesothelial cell apoptosis, EVs can also remodel peritoneal mesothelial cells through the mesothelial-mesenchymal transition (MMT) pathway. The peritoneum serves as the first barrier for intra-abdominal organ defense, and under some circumstances such as inflammatory or traumatic stimuli, MMT occurs in mesothelial cells, which facilitates tumor peritoneal colonization [46]. The researchers applied ovarian cancer cell-derived EVs to mesothelial cells and found that the EVs could enter recipient mesothelial cells, induce the MMT phenotype and enhance cancer cell adhesion to mesothelial cells. Further studies demonstrated that LncRNA SPOCD1-AS from ovarian cancer EVs remodels mesothelial cells to promote peritoneal metastasis via interacting with G3BP1 [47]. One study co-cultured exosomal annexin A2 (ANXA2) derived from ovarian cancer cells with HPMCs. ANXA2 from ovarian cancer cells could be transferred to HPMCs via exosomes, which not only promoted the migration, invasion, apoptosis, morphological changes and fibrosis of HPMCs, but also promoted MMT and extracellular matrix degradation of HPMCs through the PI5K/AKT/mTOR pathway, and ultimately facilitated peritoneal metastasis [48]. Another study reported that cancer-derived exosome circPUM1 effects on the peritoneum to upregulate the expression of NF-κB and MMP2 in mesothelial cells and induces MMT, thereby promoting peritoneal spread of the tumor [49].
Li et al. found that the EOC cells transfer the exosomal ITGA5B1/AEP complex to HPMCs, and these exosomes facilitate HPMCs proliferation and migration. And HPMCs ultimately favor EOC peritoneal metastasis through the FAK/Akt/Erk pathway and phosphorylation of EMT [50]. Additionally, EOC-derived exosomes can transfer CD44 to HPMCs, reprogramming them to a more EMT phenotype, thereby promoting cancer invasion and metastasis [51].
Fibronectin and vitronectin play crucial roles in the peritoneal dissemination of ovarian cancer. Exosomal miR-99a-5p from EOC cells promotes EOC cell invasion by upregulating fibronectin and vitronectin in HPMCs [52]. This underscores the intricate role of EVs in orchestrating ovarian cancer metastasis by modulating peritoneal mesothelial cells, offering a potential target for the treatment of ovarian cancer intraperitoneal metastasis.
The omentum, a structure composed of fat and connective tissue, is actually a part of the peritoneum. It hangs from the lower edge of the stomach, covering and protecting abdominal organs, and is a common and clinically significant site for ovarian cancer metastasis [53]. Lipids from omental adipocytes and cytokines released by omental fibroblasts and adipose mesenchymal stem cells promote the growth of ovarian tumor implants on the omentum [54].
Tumor-derived exosomes exhibit the capacity to reprogram omental macrophages. Relevant studies have shown that ETS1 enhances the translocation of tumor cell-derived exosomes to omental macrophages through the interaction of exosomal laminin with macrophage integrin αvβ5, laying the foundation for the establishment of PMN. Exosomes originating from ETS1-overexpressing ovarian cancer cells induce M2 polarization and upregulate CXCL5 and CCL2 expression in macrophages, fostering the pro-tumorigenic effects of omental macrophages and ultimately promoting ovarian cancer metastasis to the omentum. Subsequent analyses revealed that ETS1 drives ovarian cancer cells to release exosomes with higher levels of laminin, thus accelerating the pro-metastatic effects through the integrin αvβ5/AKT/Sp1 pathway [55].
As the main component of omental stromal cells, carcinoma-associated fibroblast (CAF) plays a key role in the omental metastasis of OC cells [56]. Tumor cells induce the activation of CAFs, which in turn promote tumor cell metastasis by secreting exosomes [57]. The transformation from normal fibroblasts (NFs) to CAFs results in a reduction of miR-29c-3p. Low miR-29c-3p levels in CAFs-derived exosomes contribute to the de-repression of MMP2, promoting an aggressive phenotype in OC cells and thus the spread of ovarian cancer in the peritoneal cavity [58].
The omentum, rich in adipose tissue, contains adipose-derived stem cells (ADSCs), a significant subpopulation of mesenchymal stem cells (MSCs) capable of secreting EVs [59]. While there are conflicting reports on the role of ADSC-EVs in tumor progression, some studies suggest that they can promote migration and proliferation of human breast cancer cells by activating the Wnt/β-catenin signaling pathway [60]. Conversely, other studies indicate that ADSC-EVs may act as tumor suppressors. For example, prostate cancer growth was notably inhibited in hormonal mice after treatment with ADSC-EVs [61]. In ovarian cancer, researchers discovered that human omental adipose-derived mesenchymal stem cell (HO-ADSC) potentiates ovarian cancer cell proliferation, migration, and invasion via the exosome-mediated FOXM1 signaling pathway, as demonstrated in co-culture and mechanistic studies. It was also speculated that HO-ADSC exosomes may facilitate ovarian cancer growth and metastasis by being secreted into ascites. Experiments in which ovarian cancer cell lines were treated with ascites from HGSOC patients showed enhanced cell proliferation, migration, and invasion, partially verifying the hypothesis. This finding offers a novel possible explanation for the clinical phenomenon that EOC patients frequently present with ascites and often spread to the greater omental visceral adipose tissue [62].
Angiogenesis
Tumor tissues exhibit highly vascularized characteristics, with the formation of new blood vessels being a crucial step in tumor growth and metastasis. As angiogenesis progresses, there is an escalation in the nutrient supply to the tumor tissues, leading to an accelerated rate of tumor cell division and proliferation. Excessive angiogenic activity can also promote the infiltration and distant metastasis of solid tumors [63]. During these processes, the upregulation of vascular endothelial growth factor (VEGF) expression serves as a primary influencing factor, facilitating rapid angiogenesis and promoting tumor growth [64]. VEGF exerts its specific effects on vascular endothelial cells by inducing proliferation, migration, and lumen formation, while also increasing vascular permeability [65]. EVs promote VEGF expression in endothelial cells. Feng et al. reported that EVs derived from breast cancer cells promote sustained activation of VEGF in endothelial cells, thereby facilitating tumor angiogenesis [66]. Similarly, Liu et al. elucidated that exosomal miR-21 can raise VEGF levels in recipient cells and induce angiogenesis and malignant transformation in human bronchial epithelial cells [67]. In ovarian cancer, researchers isolated exosomes derived from OVACAR-3 cells and treated endothelial cells with these exosomes. The results indicated that exosomes extracted from ovarian cancer cells upregulated the expression of VEGF in endothelial cells and promoted its secretion, thereby accelerating endothelial cell proliferation and migration [68].
Exosomes containing miRNAs secreted by ovarian cancer cells can be picked up by recipient cells and play an important role in tumor angiogenesis to promote tumor growth and metastasis. The exosome miR-205 secreted by ovarian cancer cells promotes metastasis by inducing angiogenesis in vivo and in vitro via the PTEN/AKT pathway, positioning it as a potential therapeutic target for ovarian cancer [69]. Furthermore, another study has identified sEV miR-141-3p, secreted by epithelial ovarian cancer cells, as an important mediator of intercellular communication and may upregulate VEGFR-2 expression in endothelial cells. Also, sEV miR-141-3p promoted endothelial cell migration and angiogenesis through activating JAK/STAT3 and NF-κB signaling pathways [70]. In addition, soluble epithelial cadherin (sE-cadherin) on the surface of exosomes can also regulate tumor angiogenesis and has similar VEGF efficiency. sE-cadherin is highly expressed in malignant ascites of ovarian cancer patients, triggering angiogenesis and promoting tumor progression through β-catenin and activation of NF-κB signaling cascade [71].
There have been inconsistent reports regarding the effects of EVs from MSCs on tumor angiogenesis, possibly due to variations in the types of MSCs from which the EVs originate. Exosomes derived from bone marrow mesenchymal stem cells were found to inhibit the secretion and expression of VEGF in breast cancer cells, thus suppressing angiogenesis [72]. Conversely, Liu et al. demonstrated that exosomes from human exfoliated deciduous teeth stem cells exerted inhibitory effects on tumor progression through angiogenesis suppression [73]. Recent research highlights a beneficial role of MSC-derived EVs in ovarian cancer. These EVs facilitate the delivery of miR-424, a miRNA implicated in suppressing tumor invasion and migration [74], thereby downregulating VEGF expression. Consequently, they inhibit the proliferation, migration, and tube formation of human umbilical vein endothelial cells, further restraining ovarian cancer angiogenesis and metastasis [75].
Anti-angiogenic drugs hold value in the frontline treatment, platinum-sensitive recurrence, and platinum-resistant recurrence of ovarian cancer. However, clinical trials such as GOG-218 and ICON-7 have demonstrated that while bevacizumab therapy shows some efficacy in ovarian cancer, it falls short of ideal outcomes, with a median progression-free survival extension of only about 3 months [76, 77]. Therefore, the quest for new and viable anti-angiogenic targets holds significant research value for ovarian cancer. EVs can mediate ovarian cancer metastasis by influencing angiogenesis, and EVs derived from stem cells can suppress tumor growth by inhibiting angiogenesis. This discovery offers a fresh perspective and new possibilities for tumor treatment and metastasis research, potentially paving the way for developments in clinical therapy. Further research in this area is anticipated.
Immune regulation
Tumor immune escape refers to the phenomenon whereby tumor cells evade recognition and attack by the host immune system through various mechanisms, allowing them to survive and proliferate in the body [78]. It is a crucial factor in the development of cancer and resistance to treatment. The mechanisms by which tumor cells undergo immune escape can be categorized into intrinsic and extrinsic factors. On one hand, tumor cells avoid immune recognition and clearance by downregulating MHC molecules, suppressing tumor-specific antigens, and employing other strategies. On the other hand, an immunosuppressive microenvironment is established. EVs can assist tumor cells in immune escape, thereby promoting the growth and metastasis of ovarian cancer.
EVs can promote tumor immune escape by inhibiting the function of T cells. Kelleher et al. reported that EVs in ovarian cancer ascites can suppress the activity of T cells in the ascites. This inhibition may be induced by phosphatidylserine (PS) on the surface of EVs, blocking T cell receptor signaling [79]. Another study elucidated that sEV transport arginase-1 from tumor cells to antigen-presenting cells in secondary lymphoid organs, leading to the suppression of antigen-specific T-cell proliferation and activation. This establishes an immunosuppressive microenvironment, dampening anti-tumor immune responses and ultimately promoting ovarian cancer progression [80]. TAMs are prone to polarize into the immunosuppressive M2-like subtype, initiating an immunosuppressive microenvironment through the secretion of EVs. NEAT1, a long non-coding RNA, has been reported as an oncogene in various malignancies and exhibits oncogenic properties in ovarian cancer as well [81]. Studies have shown that NEAT1 is highly expressed in EVs derived from M2-TAMs. Further mechanistic studies revealed that NEAT1 upregulates the expression of ZEB1 and PD-L1 through miR-101-3p, promoting CD8+ T cell apoptosis [82].
Th17 cells, a subset of CD4+ T cells, are pro-inflammatory Th cells that secrete cytokines such as IL-17 to mediate pro-inflammatory and pro-rejection responses. Regulatory T cells (Tregs), another subset of CD4+ T cells, play a crucial role in mediating peripheral immune tolerance, exerting immunosuppressive effects, and are dubbed the “accomplices” of tumor immune escape [83]. Tregs can inhibit the inflammatory response induced by Th17 cells and functionally counterbalance Th17 cells. An imbalance between Tregs and Th17 cells promotes the progression of EOC. The researchers found that M2 macrophages altered the Treg/Th17 balance when cocultured with T cells owing to release of exosomes. Further molecular mechanistic studies have shown that TAM-derived exosomes transport miRNAs targeting STAT3 to T cells and regulate the polarization of T cell subpopulations, leading to an imbalance between Treg and Th17 cells, creating an immunosuppressive microenvironment, which promotes the progression and metastasis of EOC [84].
As one of the most promising targets in the field of tumor immunotherapy, CD47 plays a key role in the self-recognition of the immune system, and tumors frequently take advantage of CD47 overexpression to achieve immune escape [85]. CD47 was found to be expressed on exosomes and is a key regulator of macrophage immune evasion during ovarian cancer progression. Inhibiting the secretion and uptake of exosomes can enhance the phagocytosis of cancer cells by M1 macrophages, weaken immune escape capabilities, and thus inhibit peritoneal dissemination. Targeting exosomal CD47 is speculated to be beneficial for the treatment of ovarian cancer. However, researchers have only conducted in vitro phagocytosis experiments using M1 macrophages, indicating the need for further investigation into the effects of exosomal CD47 on M2 macrophages [86].
The above studies indicate that EVs are involved in immune escape and metastasis of ovarian cancer cells. However, Luo et al. recently reported that exosomes derived from expanded natural killer cells can activate NK cells from the immunosuppressive microenvironment, reversing immune suppression and enhancing anti-ovarian cancer effects [87]. Therefore, despite facing significant challenges, the potential of targeting EVs in ovarian cancer immunotherapy should not be overlooked as more immunological features of EVs are revealed.
EMT
Epithelial-mesenchymal transition (EMT) is the process by which epithelial cells lose their epithelial phenotype and transform into cells with more mesenchymal characteristics. In this process, epithelial cells lose cell polarity and cell adhesion, and gain migration and invasiveness [88]. In recent years, EMT has been considered a necessary initial step in tumor cell invasion and metastasis, and thus has been intensively studied. Increasing evidence suggests that EVs carry functional molecular cargo, coordinating the EMT process in different cancers.
Ascites-derived exosomes (ADEs) play an important role in the progression of ovarian cancer, and ADEs promote ovarian cancer metastasis not only in vitro but also in vivo. By Bioinformatics analysis, it was found that miR-6780b-5p might be an important miRNA to promote ovarian cancer metastasis in ADEs, and the correlation between miR-6780b-5p and tumor metastasis was verified in ascites of ovarian cancer patients. To further investigate the function of miR-6780b-5p in EMT, researchers assessed tumor cell migration and EMT markers after regulating miR-6780b-5p expression with agomir or antagomir transfection. It was discovered that ADEs promote ovarian cancer metastasis by transferring miR-6780b-5p into ovarian cancer cells to facilitate EMT [89].
EVs can suppress the EMT in ovarian cancer cells. Many studies have elucidated the pro-tumorigenic effects of EVs derived from CAFs. For instance, CAFs-derived EVs carrying nucleic acid messages and proteins can promote EMT progression in colorectal cancer, as well as proliferation and metastasis in breast cancer [90]. Interestingly, Chen et al. co-cultured OVCA cells with EVs derived from CAFs and found that EMT in ovarian cancer cells was inhibited. And after using the exosome secretion inhibitor GW4869, the motility and invasiveness of OVCA cells were greatly enhanced. Further studies have shown that CAFs-derived exosomes are transferred between CAFs and OVCA cells, hinder EMT in tumor cells, and deliver circIFNGR2 and inhibit malignant progression of OVCA through the circIFNGR2/miR-378/ST5 axis [91]. Table 1 provides a comprehensive summary of the mechanisms by which EVs derived from various cell sources contribute to OC metastasis.
In summary, EVs enhance ovarian cancer cells’ migratory and invasive abilities, facilitating their distant metastasis via peritoneal fluid, vasculature, and lymphatic pathways. At metastatic sites, EVs actively remodel stromal and immune cells within the microenvironment, fostering cancer cell adhesion, colonization, and proliferation, thereby expediting the metastatic process (Fig. 3).
The composition of EVs and their roles in ovarian cancer metastasis. The surface of EVs is composed of a phospholipid bilayer adorned with a variety of membrane proteins, including transmembrane proteins, transporters, antigen-presenting proteins, and receptors. Within these EVs are various bioactive cargos, such as lipids, proteins, DNA, and a range of RNA molecules, both coding and non-coding, like lncRNA, circRNA, and miRNA, all of which are essential for intercellular communication. The transfer of these EV components is crucial in the metastatic process of ovarian cancer, where they enhance the dissemination potential of primary tumor cells, support the formation of PMN, and orchestrate multiple pathophysiological processes such as immune regulation, angiogenesis, and EMT
Over the past few years, research on EVs has surged exponentially, including significant contributions in the field of ovarian cancer. Technological advancements, particularly in the methods of EV isolation and analysis, have enabled deeper investigations into their biogenesis, surface markers, and internal composition. Furthermore, interdisciplinary collaborations among fields such as cell biology, molecular biology, biomedical engineering, and clinical medicine have provided strong momentum for advancing EV research [92].
Investigation of the molecular mechanisms by which EVs drive tumor metastasis is essential for advancing from laboratory research to clinical application. Preclinical investigations have validated the crucial role of EVs as mediators of intercellular communication in tumor progression. A thorough examination of the role of EVs in ovarian cancer metastasis provides novel insights into the assessment and prevention of tumor spread. As potential biomarkers, EVs could facilitate the early detection of metastasis or serve as tools for prognostic evaluation [93, 94]. Moreover, the diverse roles of EVs in the tumor microenvironment lay the groundwork for the development of targeted therapies, where disrupting EV biogenesis, release, and uptake may offer a novel approach to limiting ovarian cancer metastasis.
However, the complexity and heterogeneity of EVs present significant challenges in studying their involvement in ovarian cancer metastasis. EVs released from different cell types under varying physiological and pathological conditions exhibit substantial differences in both quantity and cargo composition [95]. This heterogeneity complicates the analysis of EV cargo, which is typically explored using transcriptomics, proteomics, and metabolomics to elucidate their role in cancer. However, single-omics approaches are limited in their capacity to fully capture the multi-dimension information among RNA, proteins, and metabolites, highlighting the importance of integrative multi-omics research for a comprehensive understanding of EV function and application. Compared to colorectal, prostate and breast cancers, ovarian cancer remains underexplored in multi-omics studies of EVs, leading to an incomplete understanding of their involvement in ovarian cancer progression and highlighting the need for more extensive and in-depth research [96,97,98].
Additionally, the heterogeneity of EVs has driven a growing interest in single-vesicle analysis. Recently, He R. et al. developed an innovative sEV heterogeneity tracking algorithm, SEVtras, capable of specifically capturing sEVs within single-cell sequencing data and analyzing the secretion activity of different cell types. This method offers an efficient approach for analyzing sEV secretion patterns at the single-cell level, bridging sEV biology with single-cell transcriptomics, and advancing the high-throughput tracking of sEV heterogeneity [99]. These technological advancements are expected to provide critical insights into the heterogeneity and biological functions of EVs, aiding in the precise elucidation of their roles in cancer development and progression.
While research on EVs in ovarian cancer remains in its nascent stages, the future directions of this field are becoming clearer. Advanced multi-omics approaches and bioinformatics techniques are expected to elucidate the heterogeneity of EVs, identifying distinct subpopulations and their molecular signatures from various cellular origins. These findings are indispensable for deepening our comprehension of the role of EVs in cancer metastasis and for establishing the foundation for future clinical translation.
What are the advancements and future directions of EVs in ovarian cancer drug resistance?
The overall survival rate for ovarian cancer is low, partly due to resistance to drugs such as cisplatin. Paclitaxel combined with platinum-based chemotherapy regimen is the first line of treatment for patients with advanced ovarian cancer after initial tumor cytoreduction. Although most of the patients are sensitive to platinum, they will be treated with multiple chemotherapy regimens due to recurrence, and will go from platinum-sensitive to platinum-resistant, and the survival outcome of platinum-resistant EOC is very poor [100]. Currently, new treatments for ovarian cancer resistance are in clinical trials, mainly including: Anti-angiogenic therapy, immunotherapy targeting PD-1/PD-L1, poly-ADP ribose polymerase inhibitors (PARPi) Olaparib, cell-cycle targeting CHK1/2 or WEE-1 modulators, epigenetic modulators such as next-generation hypomethylating agent guadecitabine [101,102,103,104,105,106]. Despite the promise of these approaches, some clinical trials have yielded negative results, underscoring the urgent need for additional therapeutic strategies for drug-resistant ovarian cancer.
Emerging research has highlighted the indispensable role of extracellular vesicles (EVs) in regulating tumor drug resistance. EVs not only mediate drug resistance mechanisms but also hold potential as drug delivery vehicles in cancer therapy (Table 2). Therefore, in-depth studies of EV-mediated tumor drug resistance contribute to the systematic revelation of the molecular mechanisms of drug resistance in ovarian cancer, and correspondingly, understanding the current status of EVs in the study of drug-resistant treatment of ovarian cancer can provide valuable insights for guiding the direction of future research on the clinical translation of EVs.
Mediate drug resistance
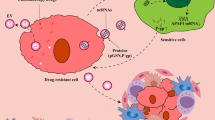
Tumor cell-derived exosomes are involved in drug resistance by transporting drug resistance-associated nucleic acids such as microRNA and mRNA. miR-1246 is a circulating non-coding RNA in a variety of tumors, and a clinical study found that circulating miR-1246 may be a clinical diagnostic biomarker for HGSOC [107]. Researchers observed high expression of miR-1246 was expressed in OC exosomes, and the expression of Cav1, a direct target of exosomal miR-1246, was negatively correlated with the expression of multi drug resistance protein. Moreover, the genetic material in exosomes was taken up by pro-tumorigenic cells infiltrated in the tumor microenvironment; therefore, the release of large amounts of miR-1246 into the tumor microenvironment via exosomes may facilitate chemoresistance in OC patients. This study provides new possibilities for the clinical application of exosomes, on the one hand, exosomal miR-1246 may be able to be used as a serum biomarker to predict chemoresistance, and on the other hand, to overcome chemoresistance in OC patients through exosomal miR-1246. However, the mechanism by which exosomal miR-1246 mediates chemoresistance has not yet been fully elucidated in this study [108]. Exosomal miR-429 released by SKOV3 cells enhances cisplatin resistance in recipient cells in vitro and in vivo via CASR/STAT3 after uptake by recipient cells, and A2780 cells co-cultured with SKOV3 pretreated with miR-429 antagomir showed sensitivity to cisplatin. This study confirms that the cisplatin resistance is produced by horizontal transfer of exosomal miR-429 [109]. EVs secreted by drug-resistant ovarian cancer cells increase resistance in sensitive cells. A study found that exosomes released by drug-resistant CP70 cells increase resistance in A2780 cells [110]. Another study isolated cisplatin-resistant SKOV3 cell-derived exosomes to treat SKOV3 cells and found that sensitization of SKOV3 cells to DDP was inhibited by exosomal miR-21-5p [111]. Apart from directly delivering drug resistance-associated miRNAs, exosomes can also regulate epigenetic factors by enriching and delivering transcripts. DNA methylation is an important expression of epigenetic modifications, and DNA Methyltransferase 1 (DNMT1) is a key enzyme responsible for the precise replication and maintenance of DNA methylation patterns [112]. The relative content of DNMT1 was significantly high in exosomes derived from ovarian cancer cell lines. In vitro and in vivo experiments revealed that endogenous DNMT1 \(\:\text{t}\text{r}\text{a}\text{n}\text{s}\text{c}\text{r}\text{i}\text{p}\text{t}\text{s}\)are highly specifically packaged into exosomes that are released into the extracellular milieu, thereby promoting DNMT1 expression in host cells. The resulting high expression of DNMT1 promotes cisplatin resistance in ovarian cancer cells through epigenetic alterations, and resistance is markedly suppressed in the presence of the exosome secretion inhibitor GW4869 [113].
Tumor-derived exosomes can also transmit drug resistance from cell to cell by carrying drug resistance-associated proteins, enzymes, and more. Plasma gelsolin (pGSN), a secreted isoform of GSN, is highly secreted in chemoresistant OVCA cells and is secreted and transported via exosomes. In patients with HGSOC, pGSN expression was significantly associated with poor chemotherapy responsiveness and shortened OS, making pGSN a poor prognostic marker [114]. Exo-pGSN upregulates pGSN expression in chemoresistant OVCA cells in an autocrine manner and confers cisplatin resistance in other chemoresistant OVCA cells [115].λAnother study reported an immunomodulatory role of sEV-pGSN in ovarian cancer chemoresistance. Under chemosensitive conditions, sEV-pGSN secretion is low, CD8+ T-cell function was optimal, and T-cell secretion of IFNγ was increased, which resulted in a reduction in the production of intracellular glutathione and sensitized chemosensitive cells to cisplatin-induced apoptosis. In contrast, in chemoresistance conditions, ovarian cancer cells secrete increased sEV-pGSN, which induces apoptosis in CD8+ T cells [116]. Cleft lip and palate transmembrane protein 1-like (CLPTM1L) is present in EVs of tumor culture supernatants and in patient sera, with levels increasing after chemotherapy. After treatment with ovarian cancer resistant cell line medium, cisplatin killing in sensitive cells was reduced, and treatment with 102-5 anti-CLPTM1L or exosome production inhibitor DMA restored cisplatin sensitivity. Also, tumor cell killing of carboplatin was reduced in EVs-treated ovarian cancer cells, and pretreatment of EVs with anti-CLPTM1L weakened carboplatin resistance and significantly enhanced killing. This confirmed that the exosome CLPTM1L from cisplatin-resistant ovarian cancer cells was able to transmit cisplatin resistance in drug-sensitive parental cells [117].
In addition, extracellular vesicles secreted by fibroblasts and immune-related cells in the tumor microenvironment also play an important role in ovarian cancer resistance. TAM derived exosomes can promote resistance in recipient cells by delivering microRNAs. miR-223 increase cisplatin resistance in gastric cancer by regulating the cell cycle and is a promising biomarker in recurrent ovarian cancer [118, 119]. Elevated levels of miR-223 were detected in serum exosomes of patients with recurrent EOC. The high levels of miR-223 observed in primary tumor homogenates may be due to exosomal transfer of miR-223 from surrounding TAMs rather than to high miR-223 expression in cancer cells. Metastatic TAM exosomal miR-223 is able to directly target PTEN and is involved in regulating EOC resistance. However, inhibiting miR-223 expression did not completely resist the promotion of drug resistance by TAM derived exosomes, and the specific reasons need to be further explored [120]. GATA3 encapsulated by TAM-derived EVs promotes chemoresistance in ovarian cancer by upregulating the CD24/ siglece − 10 axis. TAM-derived EVs releases large amounts of GATA3, which plays an important role in immunomodulation as a transcription factor that regulates T cell differentiation and development.GATA3 upregulates CD24 in OC cells, which binds to Siglecl-10 molecules on macrophages and reduces phagocytosis of the cells, thereby accelerating the role of CD24 in immune escape and chemotherapy resistance [121]. CAFs promote cisplatin resistance in OC cells by transferring exosomal miR-98-5p. Nude mouse xenograft tumor experiments showed that cancer-associated fibroblast-derived exosomal microRNA-98-5p downregulated CDKN1A in nude mice. Then CDKN1A, the key cell cycle regulatory protein, promotes cisplatin resistance in ovarian cancer after its downregulation [122].
EVs are associated with the bystander effects, a phenomenon that allows stress cells to communicate with their neighbors, leading to a variety of effects such as DNA damage. It has been shown that chemotherapeutic treatment of ovarian tumor cells leads to the release of EVs, which affects the phenotype of neighboring tumor cells. In the cisplatin-induced stress response, the release of EVs promotes the exchange of stressed cells and neighboring naive cells, and when these EVs are taken up by bystander ovarian cancer cells, they are able to induce enhanced invasiveness and drug resistance [123].
Platinum resistance in ovarian cancer stems from reduced intracellular platinum uptake, increased platinum efflux, intracellular platinum inactivation, and enhanced DNA damage repair [124]. EVs can promote intracellular chemotherapeutic agent efflux, particularly under hypoxic conditions, leading to increased cisplatin excretion by ovarian cancer cells [125]. Moreover, EV-mediated drug efflux is modulated by other factors. O-GlcNAcylation, an important post-translational protein modification, was found to be down-regulated to promote exosome secretion. This increased exosome-mediated cisplatin efflux from ovarian cancer cells, leading to chemoresistance. Blocking the transfer of EVs between cells may be a way to address chemoresistance in ovarian cancer, but more in vivo and in vitro experiments are needed in the future [126].
The exploration of EVs in drug resistance in ovarian cancer is still at an early stage. The above studies have initially revealed the mechanism of EVs inducing chemoresistance in ovarian cancer, but they have focused more on the influence of EVs on the cellular drug resistance phenotype. Many of these pathways and intricate mechanisms involved in this phenomenon remain incompletely understood, and no systematic findings have been formed yet, leaving more research to be conducted in the future.
Enhance drug delivery and reverse drug resistance
Effective targeted drug delivery is currently a bottleneck in disease treatment. Over the past decades, various nanocarrier drug delivery systems have been developed and synthesized in order to improve the therapeutic efficacy of drugs [127]. Tumor-targeted drug delivery systems can specifically reach tumor tissues, tumor cells and organelles to improve therapeutic efficacy [128]. Among nanoparticles, liposomes have been the most successful carriers, with the highest number of clinical approvals. However, the clinical application of lipid nanocarriers has experienced many difficulties, such as low bioavailability, easy removal from the bloodstream or stimulation of innate immune responses [129]. Recently, EVs, nature’s lipid nanoparticles, have received great attention as drug delivery vehicles. EVs offer high stability, low immunogenicity, excellent biocompatibility, and efficient penetration through biofilms, making them promising alternatives to lipid nanoparticles for drug delivery [130]. Moreover, there is a growing interest in nucleic acid drugs. EVs as potential carriers to stabilize nucleic acids and overcome their instability and cell permeability barriers offer new possibilities for the development and application of nucleic acid drugs.
EVs and liposomes each possess unique advantages and limitations. Recently, membrane fusion technology has been utilized to fuse EVs with liposomes to form hybrid particles, which have the advantages of both EVs and liposomes, and show great potential for targeted cancer therapy [131]. Overexpression of miR497 overcomes OC chemoresistance by inhibiting the mTOR pathway, and tretinoin (TP) also has a killing effect on cisplatin-resistant cells partly by inhibiting the mTOR pathway. Combined application of miR497 and TP was envisioned to further overcome OC chemoresistance by cooperatively inhibiting the mTOR signaling pathway. However, the low transcriptional efficiency and unstable chemically nature of mir497, as well as the severe systemic toxicity and weak aqueous solubility of TP, were obstacles to this concept. To overcome these challenges, researchers constructed hybrid nanoparticles formed by fusion of tumor exosomes and cRGD-modified liposomes to co-deliver miR497 and TP, named miR497/TP-HENPs. The miR497/TP-HENPs were able to encapsulate drugs and protect nucleic acids efficiently and release TP and miR497 stably and continuously, successfully overcoming OC resistance. Such nanoparticle encapsulation provides a new solution and translational strategy to overcome OC cisplatin resistance [132]. In another study, modification of exosomes by RGD was found to improve tumor targeting. RGD-modified exosomes efficiently targeted delivery of miR-484, improved vascular normalization, increased chemosensitivity of ovarian cancer, and prolonged the survival time of homozygous mice after chemotherapy, offering a new avenue for improving chemosensitivity in ovarian cancer.
Cisplatin resistance is primarily mediated by two mechanisms: reduced transport dependent on the plasma membrane transporter hCTR1 and sequestration within endosomes. Since the target of cisplatin is DNA in the nucleus, cisplatin isolated by endosomes will not be able to access the target [133]. Extracellular vesicles from milk show potential in drug delivery, milk exosomes can encapsulate cisplatin and transport the drug to cisplatin-resistant ovarian cancers, augmenting the anticancer effects of cisplatin [134]. By using inhibitors and knocking down key molecules, milk exosomes were found to deliver cisplatin mainly through clathrin-independent endocytosis and macropinocytosis, avoiding cisplatin capture by endosomes and enhancing the drug’s effect against ovarian cancer. However, it is not clear whether milk exosomes can effectively deliver other chemotherapeutic drugs and whether they can play a role in drug resistance in other cancers. As well as the study did not compare with other drug delivery systems such as liposomes. In addition, the safety of milk exosomes needs to be further verified before clinical application, particularly with regard to immune response [135].
MSC-derived EVs have emerged as key players in antitumor therapy [136]. In vitro and in vivo experiments revealed that hMSC-EVs enhanced sensitivity to cisplatin and inhibited the tumorigenic capacity of OC by transferring miR-18-5p [137]. Human umbilical cord is one of the sources of MSC and exhibits faster self-renewal compared to bone marrow-derived MSC [138]. Human umbilical cord MSCs release the exosome miR-146a, which reduces LAMC2 expression through the PI3K/Akt signaling pathway, thereby sensitizing OC cells to docetaxel and taxane [139]. Compared to RAW 264.7 macrophages, umbilical cord blood macrophages have the advantages of low immunogenicity and low toxicity. In contrast to peripheral blood macrophages, umbilical cord blood macrophages exhibit slower aging. It was demonstrated that M1 macrophage-derived exosomes reduced the IC50 of cisplatin in ovarian cancer and was more pronounced in drug-resistant cell lines, allowing M1 exosomes to be a potential new tool for delivering cisplatin. However, the lack of in vivo experiments, uncertainties regarding safety and antitumor effects in animals, and the need for further elucidation of the mechanism underlying resistance reversal underscore the necessity for additional research in this area [140].
In conclusion, drug resistance remains the greatest challenge in ovarian cancer treatment. The recent findings and research progress on the role of EVs in overcoming drug resistance in ovarian cancer are summarized and discussed, with an emphasis on their potential in clinical applications to combat chemotherapy resistance. This promising avenue may significantly enhance the prognosis of ovarian cancer patients. However, several challenges need to be addressed. Firstly, obtaining high-purity and high-yield EVs is an essential cornerstone for the clinical translation of EVs. Current isolation techniques have limitations, including low extraction efficiency and purity, as well as high time and economic costs, hindering the application of EVs in drug-resistant ovarian cancer treatment. Therefore, there is a need to develop new standardized isolation and purification strategies. Secondly, how to make EVs effective for drug loading and targeted delivery is also one of the technical challenges that require urgent solution. Currently, researchers have introduced genes, which fuse homing proteins and exosomal transmembrane proteins, into parental cells by means of genetic engineering, enabling specific proteins to be displayed on the surface of exosomal membranes to improve targeting. Yet targeting molecules for cancer cells are still lacking [141]. In addition, most of the current studies are conducted in vitro, requiring more rigorous in vivo experiments to validate biosafety as well as therapeutic efficacy. Clinical application studies are also scarce, insufficiently in-depth and of poor reliability. Future studies must address the aforementioned issues, with the prospect of broader application of EVs in overcoming chemoresistance in ovarian cancer being particularly promising.
Conclusions and future perspectives
To conclude, research on EVs has made significant strides in understanding OC metastasis and resistance mechanisms. Additionally, research on EVs has opened up numerous new avenues for the clinical treatment and management of cancer.
Firstly, EVs are widely present in various body fluids, offering unique advantages as an innovative liquid biopsy technique in vitro diagnostics. Currently, circulating tumor cells (CTC) and circulating tumor DNA (ctDNA) are the most commonly used in liquid biopsy [142]. While EVs cannot fully replace CTCs and ctDNA due to their lower DNA content and current technological limitations, their high stability and ease of collection provide distinct advantages. Recently, there has been a surge in in-depth studies on EVs as biomarkers, employing multi-omics and machine learning to identify EV biomarkers for disease diagnosis and prognosis. A multi-cohort, multi-stage study involving 2,141 patients found that EV-expressed GClnc1 could serve as a circulating biomarker for early detection and monitoring of gastric cancer progression [143]. Further studies revealed that circulating EV-derived lncRNA-GC1 could predict neoadjuvant chemotherapy response and exhibit earlier dynamic changes than traditional gastrointestinal biomarkers [144]. Another multicenter cohort study found that a panel of 13 miRNAs in plasma can be used for early detection of pancreatic ductal adenocarcinoma, demonstrating very high diagnostic performance [145]. In the context of OC, several studies have been initiated. Li et al. have developed a serum sEV miRNA model for the identification of EOC, which has shown superior performance compared to CA125 in distinguishing stage I EOC patients from those with benign conditions [146]. Another study has also developed a combination of serum sEV proteins (CA125, HE4, and C5a) for the early diagnosis of EOC [147].
Secondly, EVs are emerging as innovative tools in cancer therapeutics. On one hand, EVs can serve as effective delivery systems for targeted cancer therapy, enhancing drug efficacy and specificity while reducing side effects. On the other hand, EVs play a role in cancer immunotherapy. A recent study reported that exosomes derived from CAR-NK cells can enhance clinical benefits in the treatment of HER2-positive breast cancer brain metastases [148]. Numerous clinical trials are underway. For instance, a phase I trial at Anderson Cancer Center (NCT03608631) uses MSC-Exos containing siRNA targeting oncogenic KrasG12D mutation for pancreatic cancer treatment. Another phase II trial (NCT01159288) employs dendritic cell-derived exosomes as vaccines combined with cyclophosphamide therapy for unresectable non-small cell lung cancer, with results yet to be published.
In summary, while significant advancements have been achieved in the research of EVs in OC, numerous mechanisms and challenges remain to be elucidated. Future research should focus on: thoroughly investigating the biological roles of EVs in OC; developing more accurate and dependable EV biomarkers for the early diagnosis and prognosis of OC; and exploring innovative EV-based therapeutic strategies for OC. Additionally, reinforcing clinical validation studies of EVs as potential therapeutic targets will provide more robust evidence for clinical applications. With ongoing technological advancements, deeper research, and proactive clinical validation, EVs are anticipated to drive further breakthroughs and innovations in the diagnosis and treatment of OC, ultimately paving the way for more personalized and precision oncology.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- OC:
-
ovarian cancer
- EVs:
-
extracellular vesicles
- EOC:
-
epithelial ovarian cancers
- PARPis:
-
poly ADP-ribose polymerase inhibitors
- LRP1:
-
lipoprotein receptor-related protein 1
- TAMs:
-
tumor-associated macrophages
- CDKN1B:
-
cyclin-dependent kinase inhibitor 1B
- TNF:
-
tumor necrosis factor
- TMs:
-
TWEAK-stimulated macrophages
- PMN:
-
pre-metastatic niche
- M1:
-
classically activated macrophages
- M2:
-
alternatively activated macrophages
- HPMCs:
-
human peritoneal mesothelial cells
- MMT:
-
mesothelial-mesenchymal transition
- ANXA2:
-
annexin A2
- CAF:
-
carcinoma-associated fibroblast
- NFs:
-
normal fibroblasts
- ADSCs:
-
adipose-derived stem cells
- MSCs:
-
mesenchymal stem cells
- HO-ADSC:
-
human omental Adipose-derived mesenchymal stem cell
- VEGF:
-
vascular endothelial growth factor
- sE-cadherin:
-
soluble epithelial calmodulin
- PS:
-
phosphatidylserine
- Tregs:
-
regulatory T cells
- EMT:
-
epithelial-mesenchymal transition
- ADEs:
-
ascites-derived exosomes
- DNMT1:
-
DNA Methyltransferase 1
- pGSN:
-
plasma gelsolin
- CLPTM1L:
-
cleft lip and palate transmembrane protein 1-like
- TP:
-
tretinoin
- CTC:
-
circulating tumor cells
- ctDNA:
-
circulating tumor DNA
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63.
Webb PM, Jordan SJ. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. 2024;21(5):389–400.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28.
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(2):191–226.
Schoutrop E, Moyano-Galceran L, Lheureux S, Mattsson J, Lehti K, Dahlstrand H, et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol. 2022;86(Pt 3):207–23.
Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in patients with newly diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379(26):2495–505.
Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1721–31.
González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed Advanced Ovarian Cancer. N Engl J Med. 2019;381(25):2391–402.
Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus Bevacizumab as First-Line maintenance in Ovarian Cancer. N Engl J Med. 2019;381(25):2416–28.
Hardesty MM, Krivak TC, Wright GS, Hamilton E, Fleming EL, Belotte J, et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. 2022;166(2):219–29.
Moore KN, Angelergues A, Konecny GE, García Y, Banerjee S, Lorusso D, et al. Mirvetuximab Soravtansine in FRα-Positive, platinum-resistant ovarian Cancer. N Engl J Med. 2023;389(23):2162–74.
Harter P, Trillsch F, Okamoto A, Reuss A, Kim J-W, Rubio-Pérez MJ, et al. Durvalumab with paclitaxel/carboplatin (PC) and bevacizumab (bev), followed by maintenance durvalumab, bev, and olaparib in patients (pts) with newly diagnosed advanced ovarian cancer (AOC) without a tumor BRCA1/2 mutation (non-tBRCAm): results from the randomized, placebo (pbo)-controlled phase III DUO-O trial. J Clin Oncol. 2023;41(17suppl):LBA5506–LBA.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18(1):75–87.
Armstrong DK, Alvarez RD, Backes FJ, Bakkum-Gamez JN, Barroilhet L, Behbakht K, et al. NCCN Guidelines® insights: ovarian Cancer, Version 3.2022. J Natl Compr Canc Netw. 2022;20(9):972–80.
Feng JL, Dixon-Suen SC, Jordan SJ, Webb PM. Statin use and survival among women with ovarian cancer: an Australian national data-linkage study. Br J Cancer. 2021;125(5):766–71.
Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25(1):24–38.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404.
Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, et al. A brief history of nearly EV-erything - the rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10(14):e12144.
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585–606.
Hade MD, Suire CN, Mossell J, Suo Z. Extracellular vesicles: emerging frontiers in wound healing. Med Res Rev. 2022;42(6):2102–25.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–38.
Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–77.
Kipps E, Tan DS, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer. 2013;13(4):273–82.
Rauch JN, Luna G, Guzman E, Audouard M, Challis C, Sibih YE, et al. LRP1 is a master regulator of tau uptake and spread. Nature. 2020;580(7803):381–5.
Zhou W, Ma J, Zhao H, Wang Q, Guo X, Chen L, et al. Serum exosomes from epithelial ovarian Cancer patients contain LRP1, which promotes the Migration of Epithelial Ovarian Cancer Cell. Mol Cell Proteom. 2023;22(4):100520.
Wang X, Zhao X, Wang K, Wu L, Duan T. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci. 2013;104(4):516–23.
Li X, Tang M. Exosomes released from M2 macrophages transfer mir-221-3p contributed to EOC progression through targeting CDKN1B. Cancer Med. 2020;9(16):5976–88.
Zheng Y, Zhu K, Wang G. miR-106a-5p carried by tumor-derived extracellular vesicles promotes the invasion and metastasis of ovarian cancer by targeting KLF6. Clin Exp Metastasis. 2022;39(4):603–21.
Li J, Hu C, Chao H, Zhang Y, Li Y, Hou J, et al. Exosomal transfer of miR-106a-5p contributes to cisplatin resistance and tumorigenesis in nasopharyngeal carcinoma. J Cell Mol Med. 2021;25(19):9183–98.
Hu J, Xie C, Xu S, Pu Q, Liu H, Yang L, et al. Liver fibrosis-derived exosomal miR-106a-5p facilitates the malignancy by targeting SAMD12 and CADM2 in hepatocellular carcinoma. PLoS ONE. 2023;18(5):e0286017.
Xing L, Tang X, Wu K, Huang X, Yi Y, Huan J. LncRNA HAND2-AS1 suppressed the growth of triple negative breast cancer via reducing secretion of MSCs derived exosomal miR-106a-5p. Aging. 2020;13(1):424–36.
Shen X, Wang C, Zhu H, Wang Y, Wang X, Cheng X, et al. Exosome-mediated transfer of CD44 from high-metastatic ovarian cancer cells promotes migration and invasion of low-metastatic ovarian cancer cells. J Ovarian Res. 2021;14(1):38.
Kaduka Y, Takeda K, Nakayama M, Kinoshita K, Yagita H, Okumura K. TWEAK mediates anti-tumor effect of tumor-infiltrating macrophage. Biochem Biophys Res Commun. 2005;331(2):384–90.
Hu Y, Li D, Wu A, Qiu X, Di W, Huang L, et al. TWEAK-stimulated macrophages inhibit metastasis of epithelial ovarian cancer via exosomal shuttling of microRNA. Cancer Lett. 2017;393:60–7.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30(5):668–81.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6(1):75.
Chen D, Zhang X, Li Z, Zhu B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics. 2021;11(3):1016–30.
Wang F, Niu Y, Chen K, Yuan X, Qin Y, Zheng F, et al. Extracellular vesicle-packaged circATP2B4 mediates M2 macrophage polarization via miR-532-3p/SREBF1 Axis to promote epithelial ovarian Cancer metastasis. Cancer Immunol Res. 2023;11(2):199–216.
Yang S, Zhao H, Xiao W, Shao L, Zhao C, Sun P. Extracellular vesicle-packaged miR-181c-5p from epithelial ovarian cancer cells promotes M2 polarization of tumor-associated macrophages via the KAT2B/HOXA10 axis. J Gene Med. 2022;24(10):e3446.
Mo Y, Leung LL, Mak CSL, Wang X, Chan WS, Hui LMN, et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer. 2023;22(1):4.
Cui Y, Wang D, Xie M. Tumor-derived extracellular vesicles promote activation of Carcinoma-Associated fibroblasts and facilitate Invasion and Metastasis of Ovarian Cancer by carrying miR-630. Front Cell Dev Biol. 2021;9:652322.
Pradeep S, Kim SW, Wu SY, Nishimura M, Chaluvally-Raghavan P, Miyake T, et al. Hematogenous metastasis of ovarian cancer: rethinking mode of spread. Cancer Cell. 2014;26(1):77–91.
Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17(5):302–17.
Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T, et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat Commun. 2017;8:14470.
Li J, Guo T. Role of peritoneal mesothelial cells in the progression of peritoneal metastases. Cancers (Basel). 2022;14(12).
Wang C, Wang J, Shen X, Li M, Yue Y, Cheng X, et al. LncRNA SPOCD1-AS from ovarian cancer extracellular vesicles remodels mesothelial cells to promote peritoneal metastasis via interacting with G3BP1. J Exp Clin Cancer Res. 2021;40(1):101.
Gao L, Nie X, Gou R, Hu Y, Dong H, Li X, et al. Exosomal ANXA2 derived from ovarian cancer cells regulates epithelial-mesenchymal plasticity of human peritoneal mesothelial cells. J Cell Mol Med. 2021;25(23):10916–29.
Guan X, Zong ZH, Liu Y, Chen S, Wang LL, Zhao Y. circPUM1 promotes tumorigenesis and progression of ovarian Cancer by sponging mir-615-5p and miR-6753-5p. Mol Ther Nucleic Acids. 2019;18:882–92.
Li X, Tang M, Zhu Q, Wang X, Lin Y, Wang X. The exosomal integrin α5β1/AEP complex derived from epithelial ovarian cancer cells promotes peritoneal metastasis through regulating mesothelial cell proliferation and migration. Cell Oncol (Dordr). 2020;43(2):263–77.
Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, et al. Exosomes promote Ovarian Cancer Cell Invasion through transfer of CD44 to peritoneal mesothelial cells. Mol Cancer Res. 2017;15(1):78–92.
Yoshimura A, Sawada K, Nakamura K, Kinose Y, Nakatsuka E, Kobayashi M, et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC Cancer. 2018;18(1):1065.
Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503.
Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94.
Li H, Zeng C, Shu C, Cao Y, Shao W, Zhang M, et al. Laminins in tumor-derived exosomes upregulated by ETS1 reprogram omental macrophages to promote omental metastasis of ovarian cancer. Cell Death Dis. 2022;13(12):1028.
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol Cancer. 2019;18(1):91.
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86.
Han Q, Tan S, Gong L, Li G, Wu Q, Chen L, et al. Omental cancer-associated fibroblast-derived exosomes with low microRNA-29c-3p promote ovarian cancer peritoneal metastasis. Cancer Sci. 2023;114(5):1929–42.
Zhang Y, Dong W, Wang J, Cai J, Wang Z. Human omental adipose-derived mesenchymal stem cell-conditioned medium alters the proteomic profile of epithelial ovarian cancer cell lines in vitro. Onco Targets Ther. 2017;10:1655–63.
Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383(1–2):13–20.
Takahara K, Ii M, Inamoto T, Nakagawa T, Ibuki N, Yoshikawa Y, et al. microRNA-145 mediates the Inhibitory Effect of Adipose tissue-derived stromal cells on prostate Cancer. Stem Cells Dev. 2016;25(17):1290–8.
Qu Q, Liu L, Cui Y, Chen Y, Wang Y, Wang Y. Exosomes from human omental adipose-derived mesenchymal stem cells secreted into Ascites promote peritoneal metastasis of epithelial ovarian Cancer. Cells. 2022;11:21.
Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22(1):15–36.
Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular mechanisms and future implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29(1):30–9.
Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176(6):1248–64.
Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017;8:14450.
Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X, et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–35.
Ghorbanian M, Babashah S, Ataei F. The effects of ovarian cancer cell-derived exosomes on vascular endothelial growth factor expression in endothelial cells. Excli j. 2019;18:899–907.
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–20.
Masoumi-Dehghi S, Babashah S, Sadeghizadeh M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J Cell Commun Signal. 2020;14(2):233–44.
Tang MKS, Yue PYK, Ip PP, Huang RL, Lai HC, Cheung ANY, et al. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9(1):2270.
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr). 2017;40(5):457–70.
Liu P, Zhang Q, Mi J, Wang S, Xu Q, Zhuang D, et al. Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of mir-100-5p and miR-1246. Stem Cell Res Ther. 2022;13(1):89.
Li T, Li Y, Gan Y, Tian R, Wu Q, Shu G, et al. Methylation-mediated repression of MiR-424/503 cluster promotes proliferation and migration of ovarian cancer cells through targeting the hub gene KIF23. Cell Cycle. 2019;18(14):1601–18.
Li P, Xin H, Lu L. Extracellular vesicle-encapsulated microRNA-424 exerts inhibitory function in ovarian cancer by targeting MYB. J Transl Med. 2021;19(1):4.
Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final overall survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J Clin Oncol. 2019;37(26):2317–28.
Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–36.
Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. 2023;8(1):104.
Kelleher RJ Jr., Balu-Iyer S, Loyall J, Sacca AJ, Shenoy GN, Peng P, et al. Extracellular vesicles Present in Human ovarian tumor microenvironments induce a phosphatidylserine-dependent arrest in the T-cell Signaling Cascade. Cancer Immunol Res. 2015;3(11):1269–78.
Czystowska-Kuzmicz M, Sosnowska A, Nowis D, Ramji K, Szajnik M, Chlebowska-Tuz J, et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat Commun. 2019;10(1):3000.
Yin L, Wang Y. Long non-coding RNA NEAT1 facilitates the growth, migration, and invasion of ovarian cancer cells via the let-7 g/MEST/ATGL axis. Cancer Cell Int. 2021;21(1):437.
Yin L, Wang Y. Extracellular vesicles derived from M2-polarized tumor-associated macrophages promote immune escape in ovarian cancer through NEAT1/miR-101-3p/ZEB1/PD-L1 axis. Cancer Immunol Immunother. 2023;72(3):743–58.
Hinshaw DC, Benavides GA, Metge BJ, Swain CA, Kammerud SC, Alsheikh HA, et al. Hedgehog signaling regulates Treg to Th17 Conversion through metabolic rewiring in breast Cancer. Cancer Immunol Res. 2023;11(5):687–702.
Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, et al. Exosomes released from Tumor-Associated macrophages transfer miRNAs that induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol Res. 2018;6(12):1578–92.
Tang L, Yin Y, Cao Y, Fu C, Liu H, Feng J, et al. Extracellular vesicles-derived hybrid nanoplatforms for amplified CD47 blockade-based Cancer Immunotherapy. Adv Mater. 2023;35(35):e2303835.
Shimizu A, Sawada K, Kobayashi M, Yamamoto M, Yagi T, Kinose Y, et al. Exosomal CD47 plays an essential role in Immune Evasion in Ovarian Cancer. Mol Cancer Res. 2021;19(9):1583–95.
Luo H, Zhou Y, Zhang J, Zhang Y, Long S, Lin X, et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front Immunol. 2022;13:1087689.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Cai J, Gong L, Li G, Guo J, Yi X, Wang Z. Exosomes in ovarian cancer ascites promote epithelial-mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021;12(2):210.
Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20(1):154.
Chen X, Ren X, Zhou EJ, Bian Y. Exosome-transmitted circIFNGR2 modulates ovarian Cancer metastasis via miR-378/ST5 Axis. Mol Cell Biol. 2023;43(1):22–42.
Shin H, Choi BH, Shim O, Kim J, Park Y, Cho SK, et al. Single test-based diagnosis of multiple cancer types using Exosome-SERS-AI for early stage cancers. Nat Commun. 2023;14(1):1644.
de Miguel-Perez D, Ak M, Mamindla P, Russo A, Zenkin S, Ak N, et al. Validation of a multiomic model of plasma extracellular vesicle PD-L1 and radiomics for prediction of response to immunotherapy in NSCLC. J Experimental Clin Cancer Research: CR. 2024;43(1):81.
Ricklefs FL, Wollmann K, Salviano-Silva A, Drexler R, Maire CL, Kaul MG, et al. Circulating extracellular vesicles as biomarker for diagnosis, prognosis, and monitoring in glioblastoma patients. Neuro Oncol. 2024;26(7):1280–91.
Burbidge K, Zwikelmaier V, Cook B, Long MM, Balva B, Lonigro M, et al. Cargo and cell-specific differences in extracellular vesicle populations identified by multiplexed immunofluorescent analysis. J Extracell Vesicles. 2020;9(1):1789326.
Eylem CC, Yilmaz M, Derkus B, Nemutlu E, Camci CB, Yilmaz E, et al. Untargeted multi-omic analysis of colorectal cancer-specific exosomes reveals joint pathways of colorectal cancer in both clinical samples and cell culture. Cancer Lett. 2020;469:186–94.
Casanova-Salas I, Aguilar D, Cordoba-Terreros S, Agundez L, Brandariz J, Herranz N et al. Circulating tumor extracellular vesicles to monitor metastatic prostate cancer genomics and transcriptomic evolution. Cancer Cell. 2024;42(7).
Shen L, Huang H, Wei Z, Chen W, Li J, Yao Y et al. Integrated transcriptomics, proteomics, and functional analysis to characterize the tissue-specific small extracellular vesicle network of breast cancer. MedComm (2020). 2023;4(6):e433.
He R, Zhu J, Ji P, Zhao F. SEVtras delineates small extracellular vesicles at droplet resolution from single-cell transcriptomes. Nat Methods. 2024;21(2):259–66.
Ghoneum A, Almousa S, Warren B, Abdulfattah AY, Shu J, Abouelfadl H, et al. Exploring the clinical value of tumor microenvironment in platinum-resistant ovarian cancer. Semin Cancer Biol. 2021;77:83–98.
Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(12):1731–8.
Lan CY, Wang Y, Xiong Y, Li JD, Shen JX, Li YF, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol. 2018;19(9):1239–46.
Biagi JJ, Oza AM, Chalchal HI, Grimshaw R, Ellard SL, Lee U, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC clinical trials Group Study. Ann Oncol. 2011;22(2):335–40.
Lee JM, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol. 2018;19(2):207–15.
Matulonis UA, Oza AM, Secord AA, Roman LD, Blagden SP, Banerjee SN, et al. Epigenetic resensitization to platinum in recurrent, platinum-resistant ovarian cancer (OC) using guadecitabine (SGI-110), a novel hypomethylating agent (HMA): results of a randomized phase II study. J Clin Oncol. 2016;34(15suppl):5547.
Lheureux S, Cabanero M, Cristea MC, Mantia-Smaldone G, Olawaiye A, Ellard S, et al. A randomized double-blind placebo-controlled phase II trial comparing gemcitabine monotherapy to gemcitabine in combination with adavosertib in women with recurrent, platinum resistant epithelial ovarian cancer: a trial of the Princess Margaret, California, Chicago and Mayo Phase II Consortia. J Clin Oncol. 2019;37(15suppl):5518.
Todeschini P, Salviato E, Paracchini L, Ferracin M, Petrillo M, Zanotti L, et al. Circulating miRNA landscape identifies miR-1246 as promising diagnostic biomarker in high-grade serous ovarian carcinoma: a validation across two independent cohorts. Cancer Lett. 2017;388:320–7.
Kanlikilicer P, Bayraktar R, Denizli M, Rashed MH, Ivan C, Aslan B, et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018;38:100–12.
Li T, Lin L, Liu Q, Gao W, Chen L, Sha C, et al. Exosomal transfer of miR-429 confers chemoresistance in epithelial ovarian cancer. Am J Cancer Res. 2021;11(5):2124–41.
Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DR. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol Oncol. 2015;137(1):143–51.
Zhuang L, Zhang B, Liu X, Lin L, Wang L, Hong Z, et al. Exosomal mir-21-5p derived from cisplatin-resistant SKOV3 ovarian cancer cells promotes glycolysis and inhibits chemosensitivity of its progenitor SKOV3 cells by targeting PDHA1. Cell Biol Int. 2021;45(10):2140–9.
Wu C, Liu Y, Liu W, Zou T, Lu S, Zhu C, et al. NNMT-DNMT1 Axis is essential for maintaining Cancer Cell sensitivity to oxidative phosphorylation inhibition. Adv Sci (Weinh). 2022;10(1):e2202642.
Cao YL, Zhuang T, Xing BH, Li N, Li Q. Exosomal DNMT1 mediates cisplatin resistance in ovarian cancer. Cell Biochem Funct. 2017;35(6):296–303.
Asare-Werehene M, Communal L, Carmona E, Le T, Provencher D, Mes-Masson AM, et al. Pre-operative circulating plasma Gelsolin predicts residual Disease and detects early stage ovarian Cancer. Sci Rep. 2019;9(1):13924.
Asare-Werehene M, Nakka K, Reunov A, Chiu CT, Lee WT, Abedini MR, et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene. 2020;39(7):1600–16.
Asare-Werehene M, Communal L, Carmona E, Han Y, Song YS, Burger D, et al. Plasma Gelsolin inhibits CD8(+) T-cell function and regulates glutathione production to Confer Chemoresistance in Ovarian Cancer. Cancer Res. 2020;80(18):3959–71.
Parashar D, Geethadevi A, McAllister D, Ebben J, Peterson FC, Jensen DR, et al. Targeted biologic inhibition of both tumor cell-intrinsic and intercellular CLPTM1L/CRR9-mediated chemotherapeutic drug resistance. NPJ Precis Oncol. 2021;5(1):16.
Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34(1):28.
Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35.
Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81.
Chen C, Zhang L, Ruan Z. GATA3 encapsulated by Tumor-Associated macrophage-derived extracellular vesicles promotes Immune escape and Chemotherapy Resistance of Ovarian Cancer cells by upregulating the CD24/Siglec-10 Axis. Mol Pharm. 2023;20(2):971–86.
Guo H, Ha C, Dong H, Yang Z, Ma Y, Ding Y. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell Int. 2019;19:347.
Samuel P, Mulcahy LA, Furlong F, McCarthy HO, Brooks SA, Fabbri M et al. Cisplatin induces the release of extracellular vesicles from ovarian cancer cells that can induce invasiveness and drug resistance in bystander cells. Philos Trans R Soc Lond B Biol Sci. 2018;373(1737).
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–83.
Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA, et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806–21.
Qian L, Yang X, Li S, Zhao H, Gao Y, Zhao S, et al. Reduced O-GlcNAcylation of SNAP-23 promotes cisplatin resistance by inducing exosome secretion in ovarian cancer. Cell Death Discov. 2021;7(1):112.
Su C, Liu Y, Li R, Wu W, Fawcett JP, Gu J. Absorption, distribution, metabolism and excretion of the biomaterials used in Nanocarrier drug delivery systems. Adv Drug Deliv Rev. 2019;143:97–114.
Fan D, Cao Y, Cao M, Wang Y, Cao Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023;8(1):293.
Zong Y, Lin Y, Wei T, Cheng Q. Lipid nanoparticle (LNP) enables mRNA delivery for Cancer Therapy. Adv Mater. 2023;35(51):e2303261.
Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. ExosomesNature’s lipid nanoparticles, a rising star in Drug Delivery and Diagnostics. ACS Nano. 2022;16(11):17802–46.
Zhang W, Ngo L, Tsao SC, Liu D, Wang Y. Engineered Cancer-Derived Small Extracellular vesicle-liposome hybrid delivery system for targeted treatment of breast Cancer. ACS Appl Mater Interfaces. 2023;15(13):16420–33.
Li L, He D, Guo Q, Zhang Z, Ru D, Wang L, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. 2022;20(1):50.
Zhitomirsky B, Assaraf YG. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat. 2016;24:23–33.
Somiya M, Yoshioka Y, Ochiya T. Biocompatibility of highly purified bovine milk-derived extracellular vesicles. J Extracell Vesicles. 2018;7(1):1440132.
Zhou G, Gu Y, Zhu Z, Zhang H, Liu W, Xu B, et al. Exosome mediated cytosolic cisplatin delivery through clathrin-independent endocytosis and enhanced anti-cancer effect via avoiding endosome trapping in cisplatin-resistant ovarian Cancer. Front Med (Lausanne). 2022;9:810761.
Yuan Z, Kolluri KK, Gowers KH, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles. 2017;6(1):1265291.
Wang X, Jiang L, Liu Q. miR-18a-5p derived from mesenchymal stem cells-extracellular vesicles inhibits ovarian cancer cell proliferation, migration, invasion, and chemotherapy resistance. J Transl Med. 2022;20(1):258.
Hoang DM, Pham PT, Bach TQ, Ngo ATL, Nguyen QT, Phan TTK, et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 2022;7(1):272.
Qiu L, Wang J, Chen M, Chen F, Tu W. Exosomal microRNA–146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2–mediated PI3K/Akt axis. Int J Mol Med. 2020;46(2):609–20.
Zhang X, Liu L, Tang M, Li H, Guo X, Yang X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev Ind Pharm. 2020;46(7):1150–62.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Amato O, Aftimos P, Ignatiadis M. Liquid biopsy accelerates precision medicine. Ann Oncol. 2023;34(4):333–5.
Guo X, Peng Y, Song Q, Wei J, Wang X, Ru Y, et al. A Liquid Biopsy Signature for the early detection of gastric Cancer in patients. Gastroenterology. 2023;165(2):402–e1313.
Guo X, Gao Y, Song Q, Wei J, Wu J, Dong J, et al. Early assessment of circulating exosomal lncRNA-GC1 for monitoring neoadjuvant chemotherapy response in gastric cancer. Int J Surg. 2023;109(5):1094–104.
Nakamura K, Zhu Z, Roy S, Jun E, Han H, Munoz RM, et al. An exosome-based Transcriptomic Signature for Noninvasive, early detection of patients with pancreatic ductal adenocarcinoma: a Multicenter Cohort Study. Gastroenterology. 2022;163(5):1252–e662.
Li L, Zhang F, Zhang J, Shi X, Wu H, Chao X, et al. Identifying serum small extracellular vesicle MicroRNA as a Noninvasive Diagnostic and Prognostic Biomarker for Ovarian Cancer. ACS Nano. 2023;17(19):19197–210.
Li P, Bai Y, Shan B, Zhang W, Liu Z, Zhu Y, et al. Exploration of potential diagnostic value of protein content in serum small extracellular vesicles for early-stage epithelial ovarian carcinoma. Front Oncol. 2021;11:707658.
Tao B, Du R, Zhang X, Jia B, Gao Y, Zhao Y, et al. Engineering CAR-NK cell derived exosome disguised nano-bombs for enhanced HER2 positive breast cancer brain metastasis therapy. J Control Release. 2023;363:692–706.
Acknowledgements
Figures in the paper were drawn using Figdraw platform(www.fgdraw. com).
Funding
The study was supported by National Natural Science Foundation of China (Grant No. 82173220).
Author information
Authors and Affiliations
Contributions
WD and TC were responsible for the conception and design of the study. WD drafted and edited the manuscript. TC and JWZ reviewed and made revisions to the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, W., Zhou, J. & Chen, T. Unraveling the extracellular vesicle network: insights into ovarian cancer metastasis and chemoresistance. Mol Cancer 23, 201 (2024). https://doi.org/10.1186/s12943-024-02103-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-024-02103-x