Abstract
Exosomes have emerged as a novel approach for the treatment and diagnosis of cancer after RNA content was discovered in exosomes in 2007. As important meditators of intercellular communication, exosomes have become a strong focus of investigation for researchers in the past decade, as witnessed through the exponential increase of research on exosomes. The capability of exosomes to transfer functionally active cargo highlights their importance as promising biomarkers and diagnostic molecules, as well as prospective drug delivery systems. The accessibility of exosomes in nearly all biofluids additionally alludes to its unprecedented ability in various types of cancers due to its extensive impact on tumor formation and progression. This review analyzes the role of exosomal long RNA species, which is comprised of mRNA, lncRNA, and circRNA, in tumor formation and progression, with an emphasis on their potential as future diagnostic biomarkers and treatment vectors in cancer biology. Their alignment with the development of exosomal databases is further examined in this review, in view of the accumulation of studies published on exosomes in the past decade.
Similar content being viewed by others
Background
Exosomes are currently best defined as small membranous vesicles that are released into the cell exterior upon fusion of the multi-vesicular body (MVB) with the cytoplasmic membrane. These vesicles are distinguished from other extracellular vesicles (EVs) by their size of 40-100 nm in diameter and specific surface molecular characteristics, namely the presence of tetraspanins such as CD9 and CD63 [1, 2]. EV cargo is comprised of specific contents that depend on the original cell type and condition from which they originated [3, 4]. Exosomal composition can further vary due to the selective sorting of cargo into exosomes [5]. In addition to multiple proteins, nucleic acids, and lipids that have been detected in exosomes, exosomes also contain messenger RNAs (mRNAs) and non-coding RNAs—such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) [6,7,8,9]. In the recent years, a large number of researchers have focused on evaluating exosomal miRNA content and characterizing its effect on various diseases. However, the lack in quantity and expression specificity greatly limit the value of miRNAs as diagnostic molecules. Other types of RNA, such as the long RNA species [10, 11], may play an equal or perhaps an even more significant role in cell-cell communication by altering biological signaling pathways that affect disease progression (Fig. 1). In this review, the long RNA species are defined as mRNAs, lncRNAs, and circRNAs larger than 200 nucleotides.
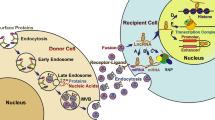
The biogenesis of exosomes. Beginning with endocytosis, the biogenesis of exosomes initially leads to the formation of endosomes. Further invagination of the endosomal membrane results in the incorporation of cytosolic protein or RNA within the endosome. The resulting multi-vesicular bodies (MVBs) then fuse with the plasma membrane and release the exosomes into the extracellular space, allowing the exosomes to interact with the recipient cells via endocytosis, direct fusion, or the binding of surface proteins. Once inside the recipient cells, RNA content, such as lncRNAs, can target proteins or epigenetic marks—affecting protein function and controlling the state of gene expression
Exosomes are readily accessible in nearly all types of human biofluids—including saliva, breast milk, cerebrospinal fluid, ascites, urine, and semen—and are important conveyors of the immune response due to its widespread presence in the human body [12,13,14,15,16,17,18]. Its detection in many body fluids is also evidence of its stability in a variety of adverse environments. Subsequently, the function of exosomes has been extensively studied. Accumulating evidence has shown that exosomes are important molecules for cell-to-cell communication and are involved in many physiological and pathological processes, including cell migration, angiogenesis, immune response, and tumor cell growth [19, 20]. Exosomes were initially assumed to be released by cells as waste cargo when they were first discovered [21, 22]. However, they are now recognized as important sources of diagnostic biomarkers and carriers of information flow. Through their involvement in cell-to-cell communication, exosomes have been shown to transfer biologically active molecules to its recipient cell, thus altering the content and behavior of the recipient cell [4, 23]. As exosomes transfer functionally active cargo, the possibility of future treatments enlisting the help of exosomes to deliver therapeutic drugs to cancer cells is a topic of much discussion [24,25,26,27,28,29,30,31].
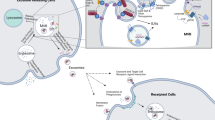
Comprehension of the mechanisms involved in the interaction of tumor cells with its environment is essential for the understanding of cancer biology. Tumor-derived exosomes have been reported to play an important role in the development and prognosis of tumors, and can be used to alter tumor microenvironment, mediate tumor cell proliferation, angiogenesis, metastasis, and drug resistance (Fig. 2) [32, 33]. Intriguingly, researchers found a significant increase in the level of exosomes released by tumor cells as well as a contrast in exosomal content from normal physiological conditions [34, 35]. Tumor cells also have a precise targeting mechanism for the contents of exosomes, suggesting that exosomes play an important role in tumor formation and progression [36]. Consistent with the fact that exosomes are present in many body fluids, these factors make exosomes an attractive target for the discovery of new cancer biomarkers and therapeutic targets.
Herein, we summarize the role of exosome-derived RNAs in cancer, focusing on mRNAs, lncRNAs, and circRNAs. While miRNAs are important exosomal cargos, we focus on the potential of other RNAs in exosomes. The study of mRNAs, lncRNAs, and circRNAs in exosomes are relatively new in comparison to miRNAs and serve as promising sources of new therapeutic targets. Moreover, we analyze the role of exosome-derived RNA in tumor formation and progression in the context of its development in the past decade, with an emphasis on their potential as future diagnostic biomarkers and treatment vectors in cancer biology.
Exosomes and exosome-derived RNA in cancer biology
Exosomes play a multifaceted role in the development and progression of tumors. In one aspect, exosomes have the ability to inhibit tumor cell growth. Normal cell-secreted exosomes can transfer tumor suppressor genes into cancerous cells and inhibit the proliferation of tumor cells by blocking the corresponding signaling pathways [37, 38]. Exosomes derived from tumor cells can induce specific, tumor resistance responses, such as the release of tumor-specific antigens by T cell exocytosis [39]. In another aspect, exosomes can promote the occurrence and development of tumors and play an important role in the tumor invasion and metastasis process. Such cancerous cell-derived exosomes can promote tumor growth and inhibit the cytotoxic effects of natural killer (NK) cells and T cells [40,41,42]. Tumor-derived exosomes can transfer pathologically expressed substances to normal cells in that the growth and differentiation of the latter are not inhibited [43, 44]. Due to its presence in various body fluids, exosomes can be also transported throughout the body via the same mechanism of action for achieving tumor metastasis [45]. In short, through its involvement in cell-to-cell transduction, tumor formation, and resistance to tumor progression, exosomes are a critical component of the tumor microenvironment.
Previous studies have reported that exosomes serve as protection against RNA degradation. Through a comparison of samples stored in different storage conditions, Hong et al. found that exosomes provide secure protection of its contents [46]. Exosomes contain and stabilize different amounts of RNA, which are transported to the target cells via endocytosis. Exosome-derived RNA is relatively stable throughout the process, affecting the phenotype and function of mRNA in the recipient cells. However, an important subject of consideration is whether the RNA molecules are intact or partially degraded [47]. Intact molecules can encode functional proteins and potentially retain original functions, whereas partially degraded molecules may acquire new functions—affecting cellular processes depending on their newly assumed functions.
The area of exosome-derived RNA has rapidly developed since it was initially reported in 2007, as reflected by the increase in publications in the past decade (Additional file 1). Previous studies have shown that the RNA profile of exosome-derived cells and its donor cells differs substantially [48, 49]. Total exosomal RNA typically lacks the 18S and 28S ribosomal RNA peaks compared to the total RNA of its donor cell. Comparison of the RNA profiles has demonstrated that exosome-derived RNA contains mRNAs, lnRNAs, and circRNAs [50,51,52]. Thus, detecting the specific components secreted by tumor-derived exosomes, such as non-coding RNA, may possibly lead to the discovery of new cancer biomarkers.
Increasing evidence has demonstrated that the long RNA species have extensive clinical applications (Table 1). Researchers have found an absence of human telomerase reverse transcriptase (hTERT) mRNA in serum-derived exosomes compared to normal controls, despite being detectable in patients of different cancers [53]. In addition, recent studies have shown that the number of three long-chain RNAs (two mRNAs, KRTAP5–4, MAGEA3 and one lncRNA, BCAR) in serum exosomes of patients with colorectal adenoma is significantly different from healthy subjects, with the area under the ROC curve (AUC) being 0.936. Similarly, the detection of plasma-derived exosomal lncRNA HOTTIP may be a potential biomarker for diagnosing gastric cancer [54]. Its expression levels were significantly correlated with invasion depth (P = 0.0298) and TNM stage (P < 0.001). The AUC for exosomal HOTTIP was 0.827, which demonstrated a higher diagnostic capability than current tumor biomarkers, such as CEA, CA 19–9 and CA72–4 (AUC = 0.653, 0.685, and 0.639, respectively) (P < 0.001). Furthermore, there have been reports on the presence of circRNAs in exosomes [8], as well as certain circRNAs with varying expression patterns in normal and cancerous tissues [55]. Therefore, the long RNA species show great potential as future biomarkers and therapeutic targets. Further research will not only trigger the discovery of novel circulating biomarkers, but will also help researchers identify more molecular signatures of exosomes with functional applications.
Messenger RNA
Messenger RNAs (mRNAs) have been identified as important exosomal cargos and functional regulators of cell behavior in cancer-derived exosomes. Intriguingly, the state in which exosomes are released has different effects on the recipient cells. Using chip technology analysis, Eldh et al. found that exosomes secreted by cells under oxidative stress can result in recipient cells producing anti-oxidant activity as compared to those produced under normal conditions [56]. Exposing exosomes to UV light furthered weakened the protective effect of exosomes in shielding recipient cells from oxidative stress, resulting in cell death. This indicates that exosomes have the ability to communicate a protective signal to its recipient host cell and is a critical component of cell communication.
Seminal research by Valadi et al. determined the presence of exosomal mRNA and miRNA in mouse and human mast cells (MC/9 and HMC-1, respectively) by microarray analysis [50]. Assessments revealed that the distribution of mRNA in exosomes did not match with those found in the cytoplasm of donor cells, suggesting that mRNA and miRNA molecules are selectively loaded into exosomes. The distribution of mRNA in exosomes is significantly different in distinct cell types and species [51, 57]. Researchers found that specific mRNAs involved in cell migration, angiogenesis, and proliferation were present in glioblastoma-derived exosomes, whereas mRNAs associated with protein synthesis and cell development were detected in mouse-specific mast cells (MC/9). In addition, after translocation of murine mRNA into human mast cells, new murine proteins were found in the recipient cells. This implies that the transferred exosomal mRNA is functional after relocation and can be translated into proteins. Many researchers refer to this type of RNA as “exosomal shuttle RNA” (esRNA).
Exosome-derived mRNAs in the cancer biology
Through network analyses, Hong et al. found that colorectal cancer (CRC) cell-derived exosomes are rich in cell cycle-related mRNAs that promote endothelial cell proliferation. Most of the EVs enriched with mRNAs were associated with M-phase activities, and were differentially regulated in CRC patients—suggesting that exosomes derived from cancerous cells may be involved in tumor growth and metastasis [46]. Additional studies have also identified the mRNA transport pathways of tumor-derived exosomes between different tumor cells [58, 59]. Tumor cell-derived exosomes were shown to be able to transfer mRNA to other types of tumor cells via clathrin-mediated endocytosis. This suggests that exosome delivery of mRNA may have therapeutic utility in diagnosing multiple organ tumorigenesis.
Exosome-derived mRNA can be additionally translated or transcribed into cDNA in recipient cells [60]. These features demonstrate an exosomal capability of affecting the expression of the target cell. Fluorescently labeled glioblastoma EVs were demonstrated to transfer Gaussia luciferase (Gluc) mRNA to human brain microvascular endothelial cells (HBMVECs), leading to the translation of proteins [6]. Tannous et al. used luciferase-encoding lentiviral vectors encoding Gluc to study the transport of RNA in exosomes [61]. Purified microbubbles containing Gluc mRNA were added to HBMVECs, and the activity of Gluc released into the medium was monitored over time. Gluc activity produced by the recipient cells continued to increase within 24 h, indicating that Gluc mRNA was undergoing translation. This shows that mRNAs incorporated into tumor EVs can produce functional proteins after being transported to recipient cells, facilitating a horizontal transfer of genetic information.
Lobb et al. demonstrated that exosomes derived from mesenchymal non-small cell lung cancer (NSCLC) cells have important functions in primary tumors by altering the phenotype of recipient cells [62]. The authors used a model of human bronchial epithelial cells (HBECs) in which donor cells were converted from the epithelium to the mesenchymal phenotype by introducing oncogenic changes commonly found in NSCLC. An increase of EMT-associated transcription factor ZEB1 mRNA was detected in the recipient cells, in addition to observing a transfer of chemoresistant phenotypes to donor epithelial HBECs through NSCLC exosomes. This work demonstrates for the first time that exosomes derived from oncogene-converted, mesenchymal lung cells may transfer mesenchymal and chemoresistant phenotypes into recipient cells through the transfer of ZEB1 mRNA.
Outlook of mRNA biomarkers
Skog et al. showed that the mRNA found in serum-derived exosomes of glioblastoma patients could be translated into the mutein EGFRvIII [6]. In glioma patients, about 50% of the tumor mRNA mutations occur in patients with plasma exosomal mRNA mutations. In fact, no exosome-derived mutant mRNA was detected in the serum of patients who underwent surgical tumor resection, implying that exosomal mutant mRNA is derived from tumor cells. March-Villalba et al. additionally found that plasma telomerase reverse transcriptase (hTERT) mRNA was upregulated in the peripheral blood and tumor tissues of prostate cancer patients [53]. The size of the tumors was associated with the degree of malignancy—suggesting its potential as a non-invasive tumor marker in the diagnosis of prostate cancer.
Recent studies have shown that the number of long-chain RNAs, mRNAs KRTAP5–4 and MAGEA3 in serum-derived exosomes of colorectal adenoma patients is significantly different from healthy controls (AUC = 0.936) [11]. This further suggests that mRNAs may be used for the early detection of cancer. Researchers found that certain mRNAs such as Annexin 2, Smad2, P27, MTAP, CIP4, and PEDF, are differentially expressed in the exosomes of osteosarcoma patients under different chemotherapeutic responses [63]. These data indicate that exosomal mRNAs are reliable biomarkers for the classification of osteosarcoma with different chemosensitivities.
Nabet et al. showed that breast cancer cells trigger the NOTCH-MYC signaling pathway in tumor fibroblasts to promote the exosomal release of unshielded RN7SL1 RNA [64]. After the transfer of exosomes to immune cells, breast cancer cells, bone marrow, and dendritic cells, unshielded RN7SL1 triggered inflammatory responses, enhanced tumor growth, metastasis, and therapeutic resistance, in addition to inducing expression of CD40, CD86, PDL1, and MHCII. Evidence from tumor and blood samples confirmed that the activation of stromal cells results in the coupling of RNA damage-associated molecular patterns (DAMPs) and unshielded RNA to promote the aggressive features of cancer. Assessments of the blood samples of breast cancer patients revealed the presence of unshielded RN7SL1 in exosomes. Thus, exosomal content can be utilized to identify patients with more aggressive cancers by detecting exosome-binding RN7SL1.
Long non-coding RNA
Long non-coding RNAs (lncRNAs) are RNA transcripts longer than 200 nucleotides that have limited or no protein-coding capacity. LncRNAs are involved in many important biological processes such as chromatin modification, gene expression, and nuclear transport, and are regulators of apoptosis, tumor migration, and drug resistance. Although lncRNA was once thought to be transcriptional noise, further investigation has demonstrated that lncRNAs play a functional role in carcinogenesis, tumor regulation, and gene expression [65, 66]. Its functional relevance in cancer also hints at the possibility of using lncRNAs as future diagnostic markers, drug delivery mediums, or targets for cancer treatments.
Exosome-derived lncRNAs have been detected in a wide range of bodily fluids due to active cellular secretion. Although ribonuclease is present in the blood, lncRNA nevertheless exists stably due to the protection of exosomes and microvesicles. Observation of the RNA content of exosomes, apoptotic bodies, and microvesicles in the blood showed that lncRNAs are mainly distributed in exosomes [11, 51]. This strongly implies that lncRNAs may be secreted into the bloodstream through this type of extracellular vesicle. Hewson et al. showed that certain exosome-related lncRNAs are involved in cellular metabolism, nucleosome structure, and cell signaling through interaction with L-lactate dehydrogenase B (LDHB), high-mobility group protein 17 (HMG-17), and CSF2RB [67]. These lncRNAs can bind to specific proteins, interact with candidate proteins, or be packaged into exosomes after being introduced into cells. Knowledge of the interactions of cellular pathways and exosome-associated non-coding transcripts can not only better clarify cell-to-cell interactions, but also provide a better understanding of the future of exosomal cell-targeted therapies.
Exosome-derived lncRNAs in cancer biology
Exosomes are considered important mechanisms for the crosstalk between various cells. They have been shown to function as transport vesicles for functional lncRNA, which may affect the phenotype of the recipient cells [68,69,70]. In the tumor microenvironment, tumor cells and tumor-associated macrophages (TAMs) are reported as the most significant sources of exosomes. Interaction of ovarian cancer cells with TAMs can enhance endothelial cells’ ability to promote the development of ovarian cancer. However, how epithelial ovarian cancer (EOC) cells regulate the interactions between TAM and endothelial cells is still not clear. Wu et al. showed that TAM-derived exosomes could inhibit the migration of endothelial cells by targeting the miR-146b-5p/TRAF6/NF-kB/MMP2 pathway [71]. Yet, the addition of EOC-derived exosomes into the co-culture system restored the migration of endothelial cells by transferring lncRNAs to remotely reverse this effect. This suggests that lncRNAs have a powerful role in the regulation of the tumor microenvironment.
Previous studies have demonstrated that exosome-derived lncRNA can affect tumor growth, metastasis, invasion, and prognosis by regulating the tumor microenvironment [72, 73]. By traveling to cells through exosomes, lncRNAs can create a microenvironment suitable for the metastasis of tumor cells. Conigliaro et al. showed that the exosomes secreted by CD90 and tumor stem cells can induce an angiogenic effect in human umbilical vein endothelial cells (HUVECs) [43]. Through its adhesion to CD90 cells and HUVECs, such exosomes can invade endothelial cells, deliver lncRNA H19 to its corresponding target cells, and stimulate angiogenesis by synthesizing and releasing vascular endothelial growth factors (VEGF). Moreover, Lang et al. found that U87MG cells can also promote angiogenesis by transporting lnc-CCAT into endothelial cells through exosomes [74]. Secretion of exosomes rich in linc-POU3F3 induced angiogenesis in glioma cells by increasing the expression of basic fibroblast growth factor (bFGF), basic fibroblast growth factor receptor (bFGFR), VEGFA, and Angio [75]. Further investigations have also confirmed that the overexpression of lnc-CCAT2 upregulates the expression of VEGFA and TGFβ in HUVECs—reducing apoptosis by promoting angiogenesis and Bcl-2 expression and inhibiting Bax and caspase-3 expression.
In a study by Takahashi and colleagues, TGF-β induced interstitial epithelial cells and promoted the invasion and metastasis of cancer cells [76]. TGF-β induced the upregulation of several lncRNAs in pancreatic cells, of which lncRNA-HULC (lnc-HULC) demonstrated the most prominent changes. After lnc-HULC knockdown, the survival, invasion, and migration abilities of the cells decreased, but the level of lnc-HULC in exosomes derived from TGF-β-induced pancreatic cells increased. LncRNA zfas1 was additionally found to be related to lymphatic metastasis in gastric cancer patients [77]. Its transmission through exosomes enhanced cell proliferation and migration of cancerous cells—strongly indicating that tumors can enhance cellular migration and invasion abilities through exosome-derived lncRNAs.
Exosome-derived lncRNA and chemoresistance
Drug resistance is often the main reason for the failure of clinical chemotherapy. Exosome-mediated translocation of non-coding RNAs have been shown to be an important mechanism of acquired drug resistance in some cancer cells [78, 79]. Long intergenic non-coding RNA ROR (LINC-ROR) was found to be an effector molecule of chemotherapeutic drug resistance in hepatocellular cancer cells (HCC) [80]. High expression of LINC-ROR was detected in HCC cells, but more specifically, they were enriched in the extracellular vesicles derived from tumor cells. An increased level of chemoresistance was observed in HCC cells treated with exosomes containing high levels of LINC-ROR. Similarly, the cells’ sensitivity to chemotherapy increased after LINC-ROR knockdown. As hepatocellular cancers are known to be highly resistant to chemotherapy, this suggests that cancerous cells may be utilizing exosome-derived lncRNAs to improve chemoresistance.
Another study found that HCC cells exposed to different chemotherapy drugs, such as sorafenib, camptothecin, and doxorubicin, increased the expression of lnc-VLDLR in cells and the exosomes secreted from these cells [81]. Expression profiling identified that lnc-VLDLR was significantly upregulated in cancerous cells. Recipient cells co-cultured with these exosomes reduced chemotherapy-induced cell death and increased lnc-VLDLR expression in the recipient cells. Takahashi et al. concluded that lnc-VLDLR was an lncRNA-rich extracellular vesicle that contributed to cellular stress response.
Recently, lncRNA activated in RCC with sunitinib resistance (lncARSR) was found to be an endogenous competing RNA that promotes sunitinib resistance in resistant renal tumor exosomes. In sunitinib-resistant renal tumors, overexpressed lncARSR competes with miR-34 and miR-449, promotes AXL and c-MET expression, reactivates RTKs, and mediates drug resistance [82]. Exosomes of tamoxifen-resistant LCC2 cell lines were additionally found to contain high levels of lncRNA-UCA1 [83]. Exosome-mediated transport of UCA1 significantly increased tamoxifen resistance in ER-positive MCF-7 cells. MCF-7 cells pretreated with ExoS/Lcc2 significantly increased cell viability, decreased the expression of cleaved caspase-3, and reduced apoptosis rates after treatment with tamoxifen. These findings provide new insight into the role of exosomal lncRNA and demonstrate the mediating ability of lncRNA in chemoresistance.
Exosome-derived lncRNAs as biomarkers
Exosomal lncRNAs have strong clinical application prospects as it can be used in many common experimental techniques such as real-time fluorescent quantitative polymerase chain reaction (RTFQ-PCR), gene chip analysis, and other sequencing tests [84, 85]. In addition, they can be conveniently drawn and are stable in nature [86, 87]. Although exosome-derived lncRNAs are pathophysiologically significant, the exact function of these lncRNAs remains unknown. However, these flaws in the understanding of its function or pathophysiological effect do not limit its possibility as a diagnostic biomarker.
With the increasing amount of studies on exosome-derived lncRNA, its application in other bodily fluids as a diagnostic clinical marker is of great interest. Isin et al. found that lncRNA-p21 expression is upregulated in benign prostate cancer compared to that of prostatic hyperplasia in exosomes extracted from the urine, suggesting that it can be used as a molecular marker for the differential diagnosis of prostate cancer [88, 89]. Similarly, researchers found that lncRNA HOX transcript antisense RNA (HOTAIR) was increased in urine-derived exosomes of urothelial bladder cancer (UBC) patients [90]. Specific knockdown of HOTAIR in vitro inhibited the migration and invasion abilities in UBC cell lines and altered the expression of genes involved in epithelial-mesenchymal transition (EMT), such as SNAI1, TWIST1, ZEB1, ZO1, MMP1, LAMB3, and LAMC2. These data indicate that urine-derived lncRNAs demonstrate a possibility as future therapeutic targets due to its extensive effect on the tumor microenvironment.
Furthermore, studies on exosome-derived lncRNAs in vaginal lavage specimens revealed that the expression of HOTAIR, metastasis associated lung adenocarcinoma transcript 1 (MALAT1), and maternally expressed 3 A (MEG3A) in cervical cancer patients and healthy samples were significantly different [91]. Similarly, LINC00152 was found to be highly expressed in the plasma-derived exosomes in gastric cancer patients. The expression level of LINC00152 in the plasma was not significantly different from that of plasma-derived exosomes—suggesting that LINC00152 is stably protected by exosomes. The diagnostic sensitivity of exosome-derived LINC00512 was 48.1%, specificity was 85.2%, and the AUC was 0.66—demonstrating a good diagnostic advantage [92]. In addition, the increase of lncRNA-CRNDE-h in the serum can be used to identify patients with colorectal cancer, benign colorectal disease, or healthy controls. The diagnostic sensitivity and specificity were 70.3% and 94.4%, respectively, and the AUC was 0.89 [93]. The applicability of lncRNAs in a wide range of cancers demonstrates its strong potential as convenient, noninvasive biomarkers for future cancer therapies.
Prospects for exosome-derived lncRNAs
Due to its length, lncRNA can bind to both mRNA and miRNA at the same time, enabling it to play a silencing role in gene expression. Current research shows that its biological mechanism is extremely complex and may include the encoding of proteins [94, 95]. As such, lncRNAs may be the bridge between other non-coding RNA interactions. In 2016, Ahadi et al. found that four types of exosomes derived from prostate cancer cells contained certain lncRNAs [96]. These lncRNAs by nature are enriched with miRNA seed sequences, including let-7 family members as well as miR-17, miR-18a, Mir-20a, miR-93, and miR-106b. These exosomal lncRNAs also contained binding sequences for RNA binding proteins (RBP)—the two most common motifs being ELAVL1 and RBMX. Given that exosomal lncRNAs are rich in miRNA seed sequences and RBP sites, their interaction may play an important role in the carcinogenesis of cancer.
Despite its large potential, the study of lncRNAs in exosomes is still in its infancy. Yet, lncRNAs are ideal biomarkers because of their: 1) Specificity: Exosomes have specific markers of the tissue or cell of origin [97, 98], and the contents of exosomes released vary under different physiological or pathological conditions. Research has shown that despite a low cellular expression of lncRNA, they are highly expressed in exosomes—suggesting a selective loading mechanism for lncRNAs [99]. 2) Stability: Due to protection from the lipid bilayer, enzymes cannot easily digest the contents of exosomes. RNA levels did not change significantly after exposure to a variety of cellular stress conditions—indicating that external conditions have little effect on the stability of lncRNAs [100, 101]. 3) Accessibility: Researchers have been able to ensure rapid and accurate isolation of exosomes widely distributed in various types of body fluids through techniques such as flow cytometry, Western Blotting, and real-time PCR. Finally, due to the relatively stable contents of exosomes, studies have been applied to gene therapy. Shtam et al. transfected siRNA into exosomes, and successfully achieved the silencing of RAD51 using exosomes as vectors [102]. Thus, the emerging field of exosomal lncRNAs is worth our time and effort.
Circular RNA
Circular RNAs (circRNAs) are a class of non-coding RNAs that have been recently found to be biologically functional in mammals [103,104,105,106]. Formed by backsplicing events where a downstream 5′ site binds to an upstream 3′ site, circRNAs lack 5′ and 3′ terminal ends [107]. As such, circRNAs are inherently resistant to the major enzymes of degradation, which work by targeting the 5′ and 3′ termini. This results in the high cellular stability and extended half-life often seen in circRNAs. CircRNAs were first reported in viruses as early as the 1970s, but have long been assumed to be RNA splicing errors [108, 109]. However, with the recent development of high-throughput sequencing, circRNAs have been shown to exist in large numbers of eukaryotic transcriptomes [105, 106, 110, 111]. Researchers detected more than 400 circRNAs in human saliva supernatants and found that the parental genes of these circRNAs are associated with inflammatory responses, cytoskeletal formation, cell motility, T cell polarity formation, and integrin-mediated signaling [112]. Despite this, the understanding of how cells regulate circRNAs is still limited. CircRNAs are largely reported as miRNA sponges that affect downstream target genes of miRNAs, regulate alternative splicing, and influence host gene transcription [105, 113, 114]. Further investigation is still needed to elucidate the precise function of circRNAs.
Exosome-derived circRNAs in cancer biology
Li et al. demonstrated that circRNAs exist in exosomes through RNA sequencing analyses of hepatic MHCC-LM3 cancer cells and cell-derived exosomes [8]. More than 90% percent of the analyzed circRNAs were composed of exons, demonstrated high stability, and were not susceptible to exonuclease cleavage—signs of a possible tumor diagnostic marker. In a comparison of healthy donors and CRC patients, 67 circRNAs were absent and 257 new circRNAs were detected in cancer patients. In addition, 1215 exosomal circRNAs were derived from the human serum, with some circRNAs demonstrating significant differences in exosome content between CRC patients and normal controls. Further overexpression of miR-7 in HEK293T cells and MCF-7 cells showed that circRNA CDR1as was significantly downregulated in exosomes, suggesting that the process of circRNAs entering exosomes may be regulated by intracellular miRNAs.
Dou et al. found that the circRNA of the KRAS mutant (DKO-1) and the combined mutant/wild type (DLD-1) expressed lower abundance levels compared to that of the wild type (DKs-8) [115]. This suggests that the oncogene mutation reduces the expression of circRNA in cells. Exosomes in seven of the most abundant circRNAs of the wild type (DKs-8) were also highly expressed, indicating that circRNAs can be transferred from cells to exosomes. Similarly, Beckler et al. also found that the composition of the exosome proteome was significantly affected by mutated KRAS [116]. A comprehensive proteomic analysis of exosomal content showed that exosomes from mutated KRAS cells contain many tumor-promoting proteins that can be transferred to recipient cells.
Currently there are two hypotheses about the function of circRNA in exosomes: cell-to-cell communication and circRNA clearance. As exosomes are widely accessible in the bodily fluids, exosomes containing circRNAs have been shown to transfer biological activity to recipient cells. Li et al. found that circRNAs retained biological activity after being translocated into recipient cells as exosomes containing CDR1as inhibited miR-7 induced growth suppression [8]. Yet, Lasda et al. proposed that the presence of circRNAs in exosomes and extracellular vesicles is the mechanism by which cells clear endogenous circRNA [117]. The authors analyzed the relative amounts of circRNA and linear RNA in secreted extracellular vesicles in Hela, 293 T, and U2OS cells and found that the ratio of circRNA and linear RNA significantly increased. The authors proposed that secretory vesicles, including exosomes, are the mechanism by which cells selectively release endogenous circRNA. Since other cells, such as macrophages, absorb secreted vesicles, these vesicles may also act as a means of cell-to-cell communication. Is it possible that the presence of exosomal exonucleases lead to a decreased amount of linear RNA and an increased amount of circRNA? According to Alhasan et al., the phenomenon of platelet-rich circRNA is the result of exonucleases [118]; therefore we cannot completely rule out this possibility with the data presented in this article. Nonetheless, both hypotheses are relatively new and are worthy of further verification.
Potential of circRNA in future treatments
Researchers report that circRNAs are not only located in cell-derived exosomes; in fact, a large number of complete and stable circRNAs are contained in tumor-derived exosomes—indicating that exosomal circRNA is highly anticipated as a future biomarker for tumor detection. A recent study showed that ciRS-7 expression has the capability to predict microvascular invasion (MVI) in HCC [119]. Xu et al. found that the expression levels of ciRS-7 are significantly correlated with the three clinicopatholgical parameters of HCC associated with the deterioration of the disease: age, serum levels, and hepatic MVI. This suggests that ciRS-7 may be a promising biomarker of hepatic MVI and a novel therapeutic target for restraining MVI in HCC.
CircRNAs have several advantages as potential therapeutic targets for future clinical applications. Tay et al. investigated the design and effects of different mRNA sponge expression vectors in malignant melanoma cell lines, and found that circular carriers can make mRNA sponges more durable and stable than linear ones [120]. Since circRNAs are not sensitive to nucleases, this may explain why circRNAs are more stable than linear RNAs. Because of its special circular structure, circular RNA sponges can add more specific miRNA binding sites to circRNAs, thus indefinitely enhancing their anti-miRNA ability [121]. Compared to linear RNA sponges, circRNA sponges may have a more potent inhibitory effect on the oncogenic activity of miRNAs because distinct circRNAs contain many specific miRNA-binding sites. In addition, they can indirectly regulate the expression of genes by affecting effector molecules in the miRNA pathway, thus acting as a miRNA sponge in different species [122, 123]. In the near future, it is believed that miRNA circular sponges are expected to become a new strategy for future RNA gene therapies targeted against cancer. As a stable and efficient miRNA inhibitor, artificial miRNA “sponge” technology may be a new alternative to gene knockdown. It can simultaneously inhibit the expression of other paralogous miRNAs, in addition to producing a more long-lasting inhibiting effect.
Currently, the understanding of the role of exosomal circRNA in malignancies, molecular mechanisms, and possible clinical applications are still limited and need further study. Databases for circRNAs have not yet been fully established, and the lack of an internationally accepted nomenclature for classification remains a problem to be solved. However, the establishment and development of exosomal databases, such as ExoCarta, ExAtlas, and exoRBase, will further facilitate these studies on the long RNA species.
Available databases for exosomal RNA
With the recent exponential increase of exosomal research in the past decade, many exosome databases have been established (Table 2). Vesiclepedia is one of these databases, where it features active involvement of EV researchers in creating a continuous and updated database. It not only includes molecular information on exosomes, but also provides information on other classes of EVs, such as microparticles, microvesicles, and apoptotic blebs. Vesiclepedia currently consists of a total of 35,264 protein, 18,718 mRNA, 1772 miRNA, and 342 lipid entries from 341 independently published studies [124, 125]. Researchers are able to browse and retrieve information based on organism, vesicle type, content type, and sample material to search for related datasets, genes, or published studies. EVpedia is another comprehensive database that integrates high-throughput datasets of vesicular components (proteins, mRNAs, miRNAs, and lipids) from both prokaryotic and eukaryotic extracellular vesicles. At present, it includes 443 high-throughput studies, 957 high-throughput datasets, and 592,870 molecules [126,127,128]. It focuses on providing analytical tools for comparative analyses, such as ortholog identification, Gene Ontology enrichment analyses, and network analyses.
Several databases have been developed solely for exosomes and extracellular biomarkers. For example, ExoCarta contains molecular information only on exosomes. The ExoCarta database is an open platform that contains more than 286 studies on proteins, mRNAs, miRNAs, and lipids in exosomes derived from different tissues and organs [129,130,131]. It features dynamic protein-protein interaction networks and biological pathways of exosomal proteins, which can be imported into FunRich, a tool for additional enrichment and analysis. ExRNA Atlas is another database that extracts exRNA profiles from human and mouse biofluids generated by the Extracellular RNA Communication Consortium (ERCC) [132,133,134]. It is an integrative software that analyzes and visualizes datasets in the context of specific biological pathways and networks, in addition to providing standardized exRNA protocols. Other more specialized databases, such as miRandola, can be used to study the biological functions of predicted extracellular biomarkers in its circulating non-coding RNA database [135, 136].
The establishment of exoRBase has recently drawn the attention of many researchers, as it focuses on the long RNA species specifically derived from human blood exosomes. It currently contains 18,333 mRNAs, 15,501 lncRNAs, and 58,330 circRNAs, in addition to providing its expression level, annotation, and possible tissues of origin [10]. It integrates and visualizes RNA expression profiles based on normalized RNA-sequence samples of healthy controls and patients of different diseases. With the development of these exosomal databases, it is certain that the functional implications and mechanisms of exosomes will be gradually elucidated—advancing the discovery of exosomal biomarkers and therapeutic targets.
Conclusions
Despite exosomes being regarded as waste cargo when initially discovered [21, 22], this perception changed when RNA content was detected in exosomes in 2007 [50]. From the discovery of RNA content in exosomes to the first studies published on exosomal RNA as biomarkers [6, 8, 9, 70], and finally to the creation of exosomal databases [10, 131], exosome-derived RNA have been the spotlight of exosomal research. A timeline of the significant events of exosomal RNA research is presented in Additional file 2. As we have witnessed in this past decade, exosomes are emerging as promising tools for the treatment and diagnosis of cancer. Their applicability, accessibility, and specificity are factors that make them attractive targets for researchers. However, despite the advances in exosome detection, there are some limitations in the study of exosomes. For instance, a standardized method for collecting, handling, and isolating exosome samples has yet to be established [137, 138]. The present methods of extracting exosomes are extremely time-consuming and not practical for routine diagnostics. The purity of tumor-derived exosome samples is another issue, as samples collected from the serum and plasma may include EVs released by cells other than tumor cells [139]. Improvements to the current strategies are needed to advance research on exosomes.
Successful treatment of cancer depends on the understanding of the complex mechanisms in the tumor microenvironment. Exosomes, as critical components of cellular communication, function as key facilitators in the exchange of information between cells. The accessibility of exosomes in nearly all biofluids indicates its unprecedented possibility in a wide range of cancers, as demonstrated by its extensive impact on the tumor microenvironment. In addition, their ability to mediate cell communication makes them strong targets for future therapeutic treatments as well as potential vectors for drug delivery. Exosomal contents, namely mRNAs, lncRNAs, and circRNAs, can reflect the progression of disease—highlighting their importance as promising, non-invasive biomarkers for diagnostic and prognostic purposes. Further studies on exosomes will not only have profound impacts on the treatment of cancer, but will also lay the foundation for novel treatment methods employing exosomes as biomarkers and therapeutic targets.
Abbreviations
- AUC:
-
Area under the ROC curve
- bFGF:
-
Basic fibroblast growth factor
- bFGFR:
-
Basic fibroblast growth factor receptor
- circRNA:
-
Circular RNA
- CRC:
-
Colorectal cancer
- DAMPs:
-
Damage-associated molecular patterns
- EMT:
-
Epithelial-mesenchymal transition
- EOC:
-
Epithelial ovarian cancer
- ERCC:
-
Extracellular RNA Communication Consortium
- esRNA:
-
Exosomal shuttle RNA
- EV:
-
Extraceullular vesicle
- Gluc:
-
Gaussia luciferase mRNA
- HBEC:
-
Human bronchial epithelial cell
- HBMVEC:
-
Human brain microvascular endothelial cells
- HCC:
-
Hepatocellular carcinoma
- HMG-17:
-
High-mobility group protein 17
- HOTAIR:
-
lncRNA HOX transcript antisense RNA
- hTERT:
-
Human telomerase reverse transcriptase
- HUVECs:
-
Human umbilical vein endothelial cells
- LDHB:
-
L-lactate dehydrogenase B
- LINC-ROR:
-
Long intergenic non-coding RNA ROR
- lncARSR:
-
lncRNA activated in RCC with sunitinib resistance
- lnc-HULC:
-
lncRNA-HULC
- lncRNA:
-
Long non-coding RNA
- MALAT1:
-
Metastasis associated lung adenocarcinoma transcript 1
- MEG3A:
-
Maternally expressed 3 A
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MVB:
-
Multi-vesicular body
- MVI:
-
Microvascular invasion
- NK:
-
Natural killer cells
- NSCLC:
-
Non-small cell lung cancer
- RBP:
-
RNA binding proteins
- RTFQ-PCR:
-
Real-time fluorescent quantitative polymerase chain reaction
- TAMs:
-
Tumor-associated macrophages
- UBC:
-
Urothelial bladder cancer
- VEGF:
-
Vascular endothelial growth factors
References
Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442.
Khushman M, Bhardwaj A, Patel GK, Laurini JA, Roveda K, Tan MC, Patton MC, Singh S, Taylor W, Singh AP. Exosomal Markers (CD63 and CD9) Expression Pattern Using Immunohistochemistry in Resected Malignant and Nonmalignant Pancreatic Specimens. Pancreas. 2017;46:782–8.
Sato-Kuwabara Y, Melo SA, Soares FA, Calin GA. The fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review). Int J Oncol. 2015;46:17–27.
van Niel G, D'Angelo G, Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018.
Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6.
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22.
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–4.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21.
Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y, Zheng Q, Li Y, Wang P, He X, Huang S. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106–12.
Dong L, Lin W, Qi P, Xu MD, Wu X, Ni S, Huang D, Weng WW, Tan C, Sheng W, et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol Biomark Prev. 2016;25:1158–66.
Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–78.
Dear JW, Street JM, Bailey MA. Urinary exosomes: a reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics. 2013;13:1572–80.
Kong FL, Wang XP, Li YN, Wang HX. The role of exosomes derived from cerebrospinal fluid of spinal cord injury in neuron proliferation in vitro. Artif Cells Nanomed Biotechnol. 2017;46(1):200–5
Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, Sjostrand M, Gabrielsson S, Lotvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9.
Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, Bailey-Wood R, Wilson K, Tabi Z, Mason MD, Adams M. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis. 2005;35:149–52.
Vojtech L, Woo S, Hughes S, Levy C, Ballweber L, Sauteraud RP, Strobl J, Westerberg K, Gottardo R, Tewari M, Hladik F. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290–304.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–8.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
M HR, Bayraktar E, G KH, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017;18(3):538.
Yu DD, Wu Y, Shen HY, Lv MM, Chen WX, Zhang XH, Zhong SL, Tang JH, Zhao JH. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106:959–64.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–96.
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754–63.
Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays. 2011;33:737–41.
Shahabipour F, Barati N, Johnston TP, Derosa G, Maffioli P, Sahebkar A. Exosomes: Nanoparticulate tools for RNA interference and drug delivery. J Cell Physiol. 2017;232:1660–8.
Wang J, Zheng Y, Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front Pharmacol. 2016;7:533.
Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–22.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503.
Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016;74:103–41.
Suchorska WM, Lach MS. The role of exosomes in tumor progression and metastasis (Review). Oncol Rep. 2016;35:1237–44.
Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21.
Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer. 2014;50:1025–34.
Rana S, Yue S, Stadel D, Zoller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–84.
Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250–60.
Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70.
Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–42.
Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013;41:245–51.
Shenoy GN, Loyall J, Maguire O, Iyer V, Kelleher RJ, Jr., Minderman H, Wallace PK, Odunsi K, Balu-Iyer SV, Bankert RB. Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses. Cancer Immunol Res. 2018;6(2):236–47.
Kimura K, Hohjoh H, Fukuoka M, Sato W, Oki S, Tomi C, Yamaguchi H, Kondo T, Takahashi R, Yamamura T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat Commun. 2018;9:17.
Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155.
Whiteside TL. Exosomes in Cancer: Another Mechanism of Tumor-Induced Immune Suppression. Adv Exp Med Biol. 2017;1036:81–9.
Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28:1551–8.
Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, Kim JH, Choi DS, Kim YK, Hwang D, Gho YS. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556.
Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017;8.
Trams EG, Lauter CJ, Salem N, Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70.
Perez-Boza J, Lion M, Struman I: Exploring the RNA landscape of endothelial exosomes. RNA. 2017;24(3):423–35.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
Chen J, Wang S, Jia S, Ding G, Jiang G, Cao L. Integrated Analysis of Long Non-Coding RNA and mRNA Expression Profile in Pancreatic Cancer Derived Exosomes Treated Dendritic Cells by Microarray Analysis. J Cancer. 2018;9:21–31.
Zhang SJ, Chen X, Li CP, Li XM, Liu C, Liu BH, Shan K, Jiang Q, Zhao C, Yan B. Identification and Characterization of Circular RNAs as a New Class of Putative Biomarkers in Diabetes Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:6500–9.
March-Villalba JA, Martínez-Jabaloyas JM, Herrero MJ, Santamaria J, Aliño SF, Dasí F. Cell-Free Circulating Plasma hTERT mRNA Is a Useful Marker for Prostate Cancer Diagnosis and Is Associated with Poor Prognosis Tumor Characteristics. PLoS One. 2012;7:e43470.
Zhao R, Zhang Y, Zhang X, Yang Y, Zheng X, Li X, Liu Y, Zhang Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol Cancer. 2018;17:68.
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215.
Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, Lotvall J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353.
Redzic JS, Balaj L, van der Vos KE, Breakefield XO. Extracellular RNA mediates and marks cancer progression. Semin Cancer Biol. 2014;28:14–23.
Jiang H, Li Z, Li X, Xia J. Intercellular transfer of messenger RNAs in multiorgan tumorigenesis by tumor cell-derived exosomes. Mol Med Rep. 2015;11:4657–63.
Kapoor NR, Chadha R, Kumar S, Choedon T, Reddy VS, Kumar V. The HBx gene of hepatitis B virus can influence hepatic microenvironment via exosomes by transferring its mRNA and protein. Virus Res. 2017;240:166–74.
Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adhes Migr. 2007;1:156–8.
Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–43.
Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Moller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. 2017;141:614–20.
Xu JF, Wang YP, Zhang SJ, Chen Y, Gu HF, Dou XF, Xia B, Bi Q, Fan SW. Exosomes containing differential expression of microRNA and mRNA in osteosarcoma that can predict response to chemotherapy. Oncotarget. 2017;8:75968–78.
Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, Minn AJ. Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell. 2017;170:352–366.e313.
Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–37.
Ma P, Pan Y, Li W, Sun C, Liu J, Xu T, Shu Y. Extracellular vesicles-mediated noncoding RNAs transfer in cancer. J Hematol Oncol. 2017;10:57.
Hewson C, Capraro D, Burdach J, Whitaker N, Morris KV. Extracellular vesicle associated long non-coding RNAs functionally enhance cell viability. Noncoding RNA Res. 2016;1:3–11.
Kadota T, Fujita Y, Yoshioka Y, Araya J, Kuwano K, Ochiya T: Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol Aspects Med. 2017;60:92–103.
Quesenberry PJ, Goldberg LR, Aliotta JM, Dooner MS, Pereira MG, Wen S, Camussi G. Cellular phenotype and extracellular vesicles: basic and clinical considerations. Stem Cells Dev. 2014;23:1429–36.
Song J, Kim D, Han J, Kim Y, Lee M, Jin EJ. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med. 2015;15:121–6.
Wu Q, Wu X, Ying X, Zhu Q, Wang X, Jiang L, Chen X, Wu Y, Wang X. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int. 2017;17:62.
Qian Z, Shen Q, Yang X, Qiu Y, Zhang W. The Role of Extracellular Vesicles: An Epigenetic View of the Cancer Microenvironment. Biomed Res Int. 2015;2015:649161.
Wang Z, Chen JQ, Liu JL, Tian L. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med. 2016;14:297.
Lang HL, Hu GW, Zhang B, Kuang W, Chen Y, Wu L, Xu GH. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38:785–98.
Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM, Wu L, Xu GH. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21:959–72.
Takahashi K, Ota Y, Suzuki Y, Iwamoto H, Yamakita K, Kitano Y, Sudo R, Tamaki Y, Okada M, Aso K, et al. Sa1818 Extracellular Vesicle-Encapsulated Long Non-Coding RNA HULC Modulates Epithelial-Mesenchymal Transition in Human Pancreatic Cancer. Gastroenterology. 2015;148:S-340–1.
Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991–1004.
Giallombardo M, Taverna S, Alessandro R, Hong D, Rolfo C. Exosome-mediated drug resistance in cancer: the near future is here. Ther Adv Med Oncol. 2016;8:320–2.
Martinez VG, O'Neill S, Salimu J, Breslin S, Clayton A, Crown J, O'Driscoll L. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology. 2017;6:e1362530.
Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–67.
Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12:1377–87.
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–68.
Xu CG, Yang MF, Ren YQ, Wu CH, Wang LQ. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:4362–8.
Arita T, Ichikawa D, Konishi H, Komatsu S, Shiozaki A, Shoda K, Kawaguchi T, Hirajima S, Nagata H, Kubota T, et al. Circulating long non-coding RNAs in plasma of patients with gastric cancer. Anticancer Res. 2013;33:3185–93.
Huang JL, Zheng L, Hu YW, Wang Q. Characteristics of long non-coding RNA and its relation to hepatocellular carcinoma. Carcinogenesis. 2014;35:507–14.
Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine--a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182–6. discussion 187
Isin M, Ozgur E, Cetin G, Erten N, Aktan M, Gezer U, Dalay N. Investigation of circulating lncRNAs in B-cell neoplasms. Clin Chim Acta. 2014;431:255–9.
Isin M, Uysaler E, Ozgur E, Koseoglu H, Sanli O, Yucel OB, Gezer U, Dalay N. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168.
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–19.
Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, Fucile C, Richheimer S, Beckham CJ. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One. 2016;11:e0147236.
Zhang J, Liu SC, Luo XH, Tao GX, Guan M, Yuan H, Hu DK. Exosomal Long Noncoding RNAs are Differentially Expressed in the Cervicovaginal Lavage Samples of Cervical Cancer Patients. J Clin Lab Anal. 2016;30:1116–21.
Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36:2007–12.
Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–63.
Bazzini AA, Johnstone TG, Christiano R, Mackowiak SD, Obermayer B, Fleming ES, Vejnar CE, Lee MT, Rajewsky N, Walther TC, Giraldez AJ. Identification of small ORFs in vertebrates using ribosome footprinting and evolutionary conservation. EMBO J. 2014;33:981–93.
Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, Mitchell A, Ma J, Dubrulle J, Reyon D, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636.
Ahadi A, Brennan S, Kennedy PJ, Hutvagner G, Tran N. Long non-coding RNAs harboring miRNA seed regions are enriched in prostate cancer exosomes. Sci Rep. 2016;6:24922.
Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–45.
Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81.
Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int. 2014;38:1076–9.
de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1.
Yang Y, Cai Y, Wu G, Chen X, Liu Y, Wang X, Yu J, Li C, Chen X, Jose PA, et al. Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci (Lond). 2015;129:675–85.
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57.
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8.
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8.
Bao C, Lyu D, Huang S. Circular RNA expands its territory. Mol Cell Oncol. 2016;3:e1084443.
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–6.
Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–40.
Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–70.
Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409.
Bahn JH, Zhang Q, Li F, Chan TM, Lin X, Kim Y, Wong DT, Xiao X. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61:221–30.
He J, Xie Q, Xu H, Li J, Li Y. Circular RNAs and cancer. Cancer Lett. 2017;396:138–44.
Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, Sun W, Dou K, Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–8.
Dou Y, Cha DJ, Franklin JL, Higginbotham JN, Jeppesen DK, Weaver AM, Prasad N, Levy S, Coffey RJ, Patton JG, Zhang B. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci Rep. 2016;6:37982.
Demory Beckler M, Higginbotham JN, Franklin JL, Ham A-J, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic Analysis of Exosomes from Mutant KRAS Colon Cancer Cells Identifies Intercellular Transfer of Mutant KRAS. Mol Cell Proteomics. 2013;12:343–55.
Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. PLoS One. 2016;11:e0148407.
Alhasan AA, Izuogu OG, Al-Balool HH, Steyn JS, Evans A, Colzani M, Ghevaert C, Mountford JC, Marenah L, Elliott DJ, et al. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood. 2016;127:e1–e11.
Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143:17–27.
Tay FC, Lim JK, Zhu H, Hin LC, Wang S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Adv Drug Deliv Rev. 2015;81:117–27.
Bak RO, Hollensen AK, Mikkelsen JG. Managing microRNAs with vector-encoded decoy-type inhibitors. Mol Ther. 2013;21:1478–85.
Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–8.
Liu J, Liu T, Wang X, He A. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58.
Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borras FE, Breakefield X, Budnik V, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450.
Dong Y. Introducing Vesiclepedia. Nature Methods. 2013;10:194.
Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015;31:933–9.
Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, Go G, Yoon YJ, Kim JH, Jang SC, et al: EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2.
Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013;13:1554–71.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688–92.
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–4.
Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000.
Cheung KH, Keerthikumar S, Roncaglia P, Subramanian SL, Roth ME, Samuel M, Anand S, Gangoda L, Gould S, Alexander R, et al. Extending gene ontology in the context of extracellular RNA and vesicle communication. J Biomed Semantics. 2016;7:19.
Ainsztein AM, Brooks PJ, Dugan VG, Ganguly A, Guo M, Howcroft TK, Kelley CA, Kuo LS, Labosky PA, Lenzi R, et al. The NIH Extracellular RNA Communication Consortium. J Extracell Vesicles. 2015;4:27493.
Subramanian SL, Kitchen RR, Alexander R, Carter BS, Cheung KH, Laurent LC, Pico A, Roberts LR, Roth ME, Rozowsky JS, et al. Integration of extracellular RNA profiling data using metadata, biomedical ontologies and Linked Data technologies. J Extracell Vesicles. 2015;4:27497.
Russo F, Di Bella S, Vannini F, Berti G, Scoyni F, Cook HV, Santos A, Nigita G, Bonnici V, Laganà A, et al. miRandola 2017: a curated knowledge base of non-invasive biomarkers. Nucleic Acids Res. 2018;46:D354–9.
Russo F, Di Bella S, Nigita G, Macca V, Lagana A, Giugno R, Pulvirenti A, Ferro A. miRandola: extracellular circulating microRNAs database. PLoS One. 2012;7:e47786.
Maas SL, de Vrij J, van der Vlist EJ, Geragousian B, van Bloois L, Mastrobattista E, Schiffelers RM, Wauben MH, Broekman ML, Nolte-‘t Hoen EN. Possibilities and limitations of current technologies for quantification of biological extracellular vesicles and synthetic mimics. J Control Release. 2015;200:87–96.
Boriachek K, Islam MN, Moller A, Salomon C, Nguyen NT, Hossain MSA, Yamauchi Y, Shiddiky MJA. Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles. Small. 2017;14(6).
Thery C, Amigorena S, Raposo G, Clayton A: Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. Chapter 3. Unit 3–22.
Acknowledgements
We would like to thank Sujuan Duan, Xiaona Gong, Wenwen Cui, and Xing Liu for their helpful discussions and for supporting our work.
Funding
This study was partially sponsored by grants from the National Natural Science Foundation of China (No. 81560452 and 81672866 to XBL and No. 81260147 and 81560158 to GFH); the Natural Science Foundation of Jiangxi Province (No. 20161BAB205192 and 20171ACB21073 to XBL and No. 20122BAB205066 to GFH); Excellent Youth Foundation of Jiangxi Scientific Committee (No. 20162BCB23001 to XBL); Science and Research Fund of Jiangxi Health and Family Planning Commission (No. 20164002 to XBL); The Foundation of Nanchang Science and Technology Bureau (No. 2016 ZSCX 009 to XBL); and The Yuanhang Project of Jiangxi Province to XBL.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
RHZ, KKC, and JTZ were the major contributors in writing and revising the manuscript. BFX and ZHH performed the literature search. CJ, JS, and FFZ discussed and revised the manuscript. XBL and GFH participated in the design of the review and helped to finalize the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Exosomal RNA articles published per year. Graph showing the number of articles published relating to exosome-derived RNA per year since 2007. (DOCX 58 kb)
Additional file 2:
Timeline. A timeline of the important discoveries in exosome research, focusing on the breakthroughs in exosomal RNA research. (PPTX 90 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, R., Chen, K.K., Zhang, J. et al. The decade of exosomal long RNA species: an emerging cancer antagonist. Mol Cancer 17, 75 (2018). https://doi.org/10.1186/s12943-018-0823-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-018-0823-z