Abstract
Background
To determine the impact of metabolic syndrome (MetS) and/or metabolic dysfunction-associated fatty liver disease (MAFLD), which are pathophysiologically similar and include insulin resistance, on the development of new-onset cardiovascular disease with and without type 2 diabetes and according to sex.
Methods
This study included 570,426 individuals without a history of cardiovascular disease who were enrolled in a nationwide claims database from 2008 to 2016 and were classified by the presence or absence of MetS and/or MAFLD stratified by the presence or absence of type 2 diabetes and sex. The fatty liver index was used to determine the presence or absence of fatty liver that required a diagnosis of MAFLD. Risks of developing coronary artery disease (CAD) and cerebrovascular disease (CVD) in each category were analyzed using a multivariate Cox proportional hazard model.
Results
During a median follow-up of 5.2 years, 2252 CAD and 3128 CVD events occurred. Without type 2 diabetes the hazard ratio (HR) (95% CI) for CAD/CVD compared with neither MAFLD nor MetS was 1.32 (1.17–1.50)/1.41(1.28–1.57) for MAFLD only (without MetS), 1.78 (1.22–2.58)/1.66 (1.34–2.06) for MetS only (without MAFLD), and 2.10 (1.84–2.39)/1.73 (1.54–1.95) for MAFLD + MetS. For those with type 2 diabetes, the HR for CAD for MAFLD only (compared with neither MAFLD nor MetS) was 1.29 (1.06–1.58), for MetS only 1.34 (0.84–2.13), and for MAFLD + MetS 1.22 (1.02–1.47). For CVD, there was a significant increase in HR only in MAFLD + MetS [1.44 (1.18–1.76)]. The results of the analysis stratified by sex showed that MAFLD had a greater impact in men, and MetS had a greater impact in women regarding the development of CAD.
Conclusions
Distinguishing between MetS and/or MAFLD in the presence or absence of type 2 diabetes and according to sex may aid in accurately identifying patients at high risk of cardiovascular disease.
Similar content being viewed by others
Introduction
The state in which the risk of cardiovascular disease is increased by the accumulation of cardiovascular risk factors such as insulin resistance (IR), glucose intolerance, obesity, hypertension, and dyslipidemia has been long known as metabolic syndrome (MetS) [1]. In recent years, fatty liver disease, including non-alcoholic fatty liver disease (NAFLD), has been gaining attention as a condition with underlying IR similar to MetS [2, 3]. The prevalence of NAFLD has been increasing, particularly in Asia [4]. In addition to IR, in NAFLD as in MetS, there are multiple risk factors such as hypertension, dyslipidemia, and glucose intolerance [5]. Also, as in MetS [6], the major cause of death in patients with NAFLD is cardiovascular disease [7]. However, opinions as to whether NAFLD is a predictor of cardiovascular disease independent of comorbid metabolic abnormalities vary among studies [8, 9]. Furthermore, NAFLD is diagnosed by excluding secondary fatty liver due to significant alcohol consumption or other factors, such as viral liver disease, drug-induced liver injury, etc., while coexisting metabolic abnormalities are not considered in that diagnosis [10]. In 2020, the concept of metabolic dysfunction-associated fatty liver disease (MAFLD) was proposed to obtain an inclusive diagnosis for these patients and recognize the presence of MAFLD (i.e., a metabolic component of liver disease) that can exist despite the presence of other liver disease [11]. The concept of MAFLD more accurately reflects the underlying pathophysiology than the previously used NAFLD and is expected to contribute to improved patient care [12].
It was reported that MAFLD increases the risk of cardiovascular disease as does MetS [13] since the concept of MAFLD is similar to that of MetS except for the presence of fatty liver is essential to its diagnosis [14]. However, no study has examined in the same cohort whether MetS or MAFLD is the more likely to lead to the development of cardiovascular disease. Moreover, we previously reported that MetS was less predictive of cardiovascular disease in diabetic patients than in non-diabetic patients [15]. Similarly, in MAFLD, the presence or absence of type 2 diabetes may have an impact on its predictive ability for cardiovascular disease. It was reported that MAFLD was slightly less predictive of cardiovascular disease in patients with than without type 2 diabetes [13]. In addition, the risk of MAFLD complicated with MetS has not yet been examined. Although there are large sex differences in the prevalence, severity, and risk factors for any of the metabolic abnormalities that are related to fatty liver, cardiovascular disease, and MetS [16], to our knowledge sex differences have not been evaluated in relation to the value of MAFLD to predict cardiovascular disease.
This study aimed to investigate the degree of concordance between the diagnosis of MetS and MAFLD in clinical practice and examine whether a diagnosis of MetS and/or MAFLD would be predictive of cardiovascular disease in Asians using a nationwide claims database and to clarify whether there is a difference between those with and without type 2 diabetes and according to sex.
Materials and methods
Study participants
We retrospectively analyzed a large nationwide claims-based database that included claims data for 805,592 employees who purchased health insurance for themselves and their dependents at their companies. Details of the database were reported previously [17, 18]. Persons aged 18 to 72 years who could be monitored for at least 3 years between April 1, 2008 and July 31, 2016 were included in this analysis and when possible were continued to be followed until August 31, 2019. Those with coronary artery disease (CAD) or cerebrovascular disease (CVD) at baseline, with type 1 diabetes, or with missing medical examination data required for analysis, including blood chemistry data required for diagnosis of MetS and MAFLD, were excluded. Ultimately, 570,426 cases (334,401 men and 236,025 women) without a history of either CAD or CVD were included as study participants.
This study was reviewed and approved by the Ethics Committee of Niigata University.
Definitions
The diagnostic criteria for MetS were based on criteria proposed in a joint statement in 2009 by the International Diabetes Federation (IDF), National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity [1]. MetS was diagnosed based on at least three of the following: 1. Waist circumference (WC) ≥ 90 cm for men and ≥ 80 cm for women, which is the Asian standard proposed by the IDF and World Health Organization, to indicate MetS. 2. Fasting glucose ≥ 5.6 mmol/l (100 mg/dl) or receiving drug treatment for elevated glucose. In addition to the fasting glucose level, hemoglobin A1c (HbA1c) ≥ 38 mmol/mol (5.7%) [11], which is also included in the criteria for MAFLD, was used as a diagnostic criterion for MetS. 3. Triglycerides (TG) ≥ 1.7 mmol/l (150 mg/dl) or receiving drug treatment for elevated TG. 4. Systolic blood pressure (SBP) ≥ 130 and/or diastolic blood pressure (DBP) ≥ 85 mmHg, or receiving antihypertensive drug treatment. 5. High density lipoprotein cholesterol (HDL-C) < 1.0 mmol/l (40 mg/dl) in men, < 1.3 mmol/l (50 mg/dl) in women, or receiving drug treatment for reduced HDL-C.
The fatty liver index (FLI), a widely used clinical indicator of the presence of fatty liver, was used to diagnose fatty liver in this study [13, 19]. An FLI ≥ 60 was indicated as the criterion for the presence of fatty liver in studies of Westerners [19, 20]. Asians were considered to have fatty liver at lower values (e.g., FLI ≥ 30) than Westerners [13]. In a recent large-scale clinical study conducted in South Korea of approximately 3 million people [21], an FLI ≥ 37.09 was an independent cardiovascular disease risk factor regardless of the presence or absence of type 2 diabetes or independent of sex. Therefore, an FLI ≥ 37 was used as the cutoff value in this study. The formula for the FLI is as follows: (e0.953*loge (TG) + 0.139*BMI + 0.718*loge (gamma−glutamyl transferase: γ−GTP) + 0.053*WC − 15.745)/(1 + e0.953*loge(TG) + 0.139*BMI + 0.718*loge (γ−GTP) + 0.053*WC − 15.745) * 100.
The definition of MAFLD was based on that proposed by the International Consensus Panel in 2020 [11]. Specifically, among participants with an FLI ≥ 37 who (1) had a body mass index (BMI) ≥ 23 (using Asian criteria) and type 2 diabetes or (2) a BMI < 23 without type 2 diabetes, MAFLD was diagnosed based on at least two of the following: WC ≥ 90 cm for men and ≥ 80 cm for women, using Asian criteria; BP ≥ 135/85 mmHg or use of antihypertensive medication; serum TG ≥ 1.7 mmol/l (150 mg/dl) or receiving specific drug treatment; serum HDL-C < 1.0 mmol/l (40 mg/dl) for men and < 1.3 mmol/l (50 mg/dl) for women or receiving specific drug treatment; prediabetes [fasting plasma glucose (FPG) 5.6–6.9 mmol/l (100-125 mg/dl) or HbA1c 38–46 mmol/mol (5.7–6.4%)]; and visceral adiposity index (VAI) ≥ 2.54 [22].
Data on serum high-sensitivity C-reactive protein (CRP) and glucose tolerance were not available in the database used in this study. In addition, since HOMA-IR could not be calculated, VAI was used as an alternative index of IR. VAI was calculated as follows: men [WC/ (39.68 + (1.88 × BMI))] × [TG/1.03] × [1.31/HDL-C] and women [WC/ (36.58 + (1.89 × BMI))] × [TG/0.81] × [1.52/HDL-C] [23].
Type 2 diabetes was diagnosed if FPG ≥ 7.0 mmol/l (126 mg/dl) or HbA1c ≥ 47 mmol/mol (6.5%), or both, or if antidiabetic medication was prescribed regardless of FPG or HbA1c levels. CAD and CVD events were identified using a combination of the diagnostic procedure combination (DPC), International Classification of Diseases, Tenth Revision (ICD-10) codes, prescribed medications, and medical procedures performed. Details are as described previously [24, 25].
Statistical analysis
Categorical variables were indicated by numbers and percentages, and intergroup comparisons were made by Pearson’s chi-square test. Continuous variables in each group of study participants were classified and tested for normality with the Kolmogorov–Smirnov test to investigate whether a diagnosis of MetS and/or MAFLD would be predictive of cardiovascular disease and whether this varies according to sex or the presence or absence of type 2 diabetes. Since the results did not show a normal distribution, the Kruskal–Wallis test was used for intergroup comparisons, and when significant results were examined by the Bonferroni-Dunn multiple comparisons post hoc test. The impact of MetS and MAFLD to predict the development of CAD and CVD was examined by multivariate Cox proportional hazard analysis. Covariates used were age, sex, current smoking status, low-density lipoprotein cholesterol, and use of statins, which are considered risk factors for cardiovascular events or affect the liver fibrosis [26, 27], in addition to the components of MetS and MAFLD. The presence or absence of type 2 diabetes was used as a covariate in the gender-specific analyses only. Analyses were performed using SPSS (version 28.0, IBM, Chicago, IL, USA). Statistical significance was considered for P < 0.05.
Results
Table 1 shows the baseline characteristics of study participants according to the presence or absence of MAFLD and/or MetS.
The median follow-up period was 5.2 years, and the prevalence of type 2 diabetes among all participants was about 6.5%. In the MAFLD only group (without MetS) indices reflecting IR such as VAI were significantly higher than in the MetS-only (without MAFLD) group as was the proportion of smokers. On the other hand, the MetS-only group had a higher proportion of older patients and patients with hypertension and type 2 diabetes as well as significantly higher rates of the use of statins and significantly higher systolic blood pressure (SBP) and pulse pressure values compared to the MAFLD-only group. The above results in the comparison between the MAFLD only group and the MetS only group were similar whether they were compared only between non-diabetic participants or between diabetic participants (Additional file 1: Tables S1, S2). They were also similar when compared by sex (Additional file 1: Tables S3, S4). In addition, the distribution and prevalence of MetS and MAFLD differed greatly between men and women (Fig. 1). Specifically, in women, prevalences of MetS and MAFLD were similar, and the coexistence of MetS and MAFLD was not high. On the other hand, in men, the prevalence of MAFLD was much higher than in women, and male patients with the diagnosis of MetS had a more than 80% chance of also having MAFLD. At the same time, the proportion of MAFLD patients also fulfilling the diagnosis of MetS was not high.
Distribution of participants according to the presence or absence of metabolic dysfunction-associated fatty liver disease (MAFLD), metabolic syndrome (MetS), and type 2 diabetes (DM). A Distribution of total participant population according to the presence or absence of MAFLD, MetS, and DM. B Distribution of women according to the presence or absence of MAFLD, MetS, and DM. C Distribution of men according to the presence or absence of MAFLD, MetS, and DM
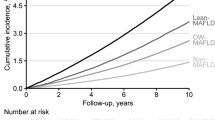
The results of analyses of the impact of MAFLD and MetS on the incidence of CAD and CVD in all participants and on participants when stratified by the presence or absence of type 2 diabetes using the Cox proportional hazard model are shown in Fig. 2 and Table 2. In the analysis of overall participants, the risk of developing CAD [multivariable-adjusted hazard ratio (HR)] was significantly increased in the group with either MAFLD only or MetS only compared with groups having neither MAFLD, MetS, nor type 2 diabetes. There was a further increase in the HR for CAD with the coexistence of both MAFLD and MetS, but it was not as high as the HR with type 2 diabetes only (Fig. 2). The risk of developing CVD was significantly increased in the MAFLD-only and MetS-only groups, and the HRs for MetS and MetS + MAFLD were similar to that for type 2 diabetes only (Fig. 2). In addition, the HRs for MetS was slightly higher than that for MAFLD for both CAD and CVD.
Impact of metabolic dysfunction-associated fatty liver disease (MAFLD), metabolic syndrome (MetS), and type 2 diabetes (DM) on cardiovascular disease. A Impact of MAFLD, MetS, and DM on coronary artery disease (CAD). B Impact of MAFLD, MetS, and DM on cerebrovascular disease (CVD). Analysis was performed using a Cox proportional hazards model adjusted for age, sex, current smoking, LDL-C and use of statin. *P < 0.001 vs. Group with neither MAFLD, MetS nor DM. **P < 0.01 vs. Group with neither MAFLD, MetS nor DM. MAFLD, metabolic dysfunction-associated fatty liver disease; MetS, metabolic syndrome; DM, type 2 diabetes; HR, hazard ratio
In the analysis of the subpopulation classified by the presence or absence of type 2 diabetes, the trend for both CAD and CVD in the group without type 2 diabetes was similar to that in the overall analysis (Table 2). However, in the type 2 diabetes group, there was no significant increase in the risk of developing CAD with MetS only but there was a significant increase in risk with MAFLD only. On the other hand, with neither MAFLD only nor MetS only was there a significant increase in the risk of CVD, but such an increase was evident only when these conditions coexisted.
The results of the analysis stratified by sex are shown in Table 3. In men, MetS only did not increase the risk of CAD, although MAFLD only (and MAFLD + MetS) did. In contrast, in women MAFLD only did not increase the risk of CAD, but MetS only (and MetS + MAFLD) did. On the other hand, there was a significant increase in the risk of CVD in both men and women for either MAFLD or MetS only, with a slightly greater increase in risk for MetS only than for MAFLD only in both men and women.
Discussion
This historical cohort study aimed to clarify the association between the presence of MetS and MAFLD, which share the common pathophysiological background of IR, and the risk of developing cardiovascular disease. Results showed that, overall, MetS had slightly superior predictive ability for the development of cardiovascular disease than MAFLD. However, the results differed greatly depending on sex and the presence or absence of type 2 diabetes as well as between CAD and CVD.
As in our previous report, MetS as a predictor of cardiovascular disease was reduced in type 2 diabetes patients who were already at high risk for atherosclerotic disease [15]. However, in predicting the development of CAD but not CVD, MAFLD was shown to be useful even in patients with type 2 diabetes in our study. MAFLD was associated with more severe IR as indicated by higher values for VAI, BMI, WC, and FLI than for MetS only. This result can be interpreted as indicating that the presence of more severe hyperinsulinemia is an additional risk factor for CAD, even in those with type 2 diabetes.
Neither the presence of MetS only or MAFLD only was useful in predicting the development of CVD in persons with type 2 diabetes. However, if both were present, the CVD risk increased even in persons with type 2 diabetes. The severity of fatty liver and the progression of liver fibrosis have been significantly correlated with the risk of stroke [28]. In this study, the FLI was significantly higher in the group with both MAFLD and MetS compared with the other groups, even in groups with diabetic patients. An increased FLI reflects the progression of liver fibrosis to some extent [29].
Patients with type 2 diabetes are more likely to develop advanced MAFLD (steatohepatitis, fibrosis, cirrhosis, etc.) than those without type 2 diabetes [30, 31]. Also, there is a close bidirectional relationship between cardiovascular disease and advanced MAFLD [32, 33]. MAFLD may cause difficulty in achieving adequate glycemic control in patient with diabetes [34]. Indeed, in the current study the presence of MetS only (without MAFLD) did not significantly increase the risk of CAD in people with type 2 diabetes. Those findings imply that clinicians should pay close attention to persons with MAFLD and type 2 diabetes as being at high risk for cardiovascular disease [33].
As to sex differences in the impact of MetS and MAFLD on the development of cardiovascular disease, MetS had a strong impact on the development of CAD in women. Even when women have risk factors for CAD, premenopausal women are less likely to develop fatty liver. Estrogen has been reported to have a strong protective effect against fatty liver in premenopausal women [16, 35, 36]. Unfortunately, information on menopause was not available for this study, but considering that 72% of female participants were younger than 50 years it can be inferred that the majority of female participants were premenopausal.
MAFLD did have a strong impact on the development of CAD in men. In men, unlike in women, MetS was almost always combined with MAFLD, and a MetS diagnosis captured only a small proportion of those having concomitant MAFLD. Thus, many patients with MAFLD are overlooked only by a diagnosis of MetS. Thus, for assessment of risk of CAD, in men it is more appropriate to use a diagnosis of MAFLD. In addition to the influence of sex hormones, a wide variety of mechanisms were reported to be responsible for these sex differences in both men and women [37, 38]. Further research is expected to elucidate these mechanisms.
A strength of the current study is that it is the first large-scale clinical study to directly compare the impact of MetS and MAFLD on the risk of developing cardiovascular disease, stratified by the presence or absence of type 2 diabetes and by sex, using real-world data. It is known that the impact of MetS and MAFLD on cardiovascular events is strongly influenced by the presence or absence of type 2 diabetes and by sex [16, 39], but there are insufficient detailed studies stratified by these factors [16]. The combination of DPC, ICD-10 codes, contents of prescribed medications, and medical procedures performed enabled accurate identification of CAD and CVD events [24, 25].
This study had the following limitations. First, we used the FLI in the identification of fatty liver, because no imaging or histopathological information was available. However, the FLI has been considered appropriate and is widely used to identify fatty liver in large-scale clinical studies [11, 13]. In addition, the currently proposed diagnostic criteria for MAFLD allowed the use of a biomarker-based index such as the FLI to identify fatty liver [11]. Nevertheless, it is difficult to distinguish which components of the FLI (BMI, WC, TG levels and γ-GPT levels [40]) or hepatic fat that were responsible for the increased risk. Thus, our findings should be confirmed with imaging or histopathological information. Second, since the database used is for Japanese company employees and their dependents, few elderly people were included. Also, no information on menopause was available, which may have played a role in the results. Third, the diagnostic definition of MAFLD, unlike NAFLD, includes either the presence or absence of liver diseases, and the impact of liver diseases such as viral hepatitis could not be considered due to lack of information. Fourth, data on high-sensitivity CRP, HOMA-IR, and the glucose tolerance test, which are diagnostic criteria for MAFLD, were not available in this database, making it possible that MAFLD was underdiagnosed. However, we calculated and used VAI as an alternative index for HOMA-IR, which is considered to reflect IR without being inferior to HOMA-IR [22]. Fifth, liver fibrosis is associated with the development of cardiovascular disease [41], but the database had no information on liver fibrosis (including platelet count). Finally, although we considered a large number of risk factors that might have influenced the development of cardiovascular disease, we did not have information on renal function, atrial fibrillation, etc., that may present a risk of cardiovascular disease.
Conclusion
Although MAFLD and MetS are closely related and refer to very similar pathological conditions, their coexistence is not necessarily high. In addition, when the diagnoses of MetS and MAFLD are considered to be predictive of cardiovascular disease, both are useful, but their usefulness differs depending on sex and the presence or absence of type 2 diabetes. The use of the respective diagnoses of MetS and MAFLD for this purpose should account for sex and the presence or absence of type 2 diabetes. This strategy may help to accurately identify patients at risk of developing cardiovascular disease and to prevent its progression through early and active interventions. It is essential to conduct further studies aimed at investigating the differences in pathophysiology in each patient group when using the presence, absence, or combination of MetS and MAFLD as predictors of cardiovascular disease.
Availability of data and materials
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations
- MetS:
-
Metabolic syndrome
- MAFLD:
-
Metabolic dysfunction associated fatty liver disease
- CAD:
-
Coronary artery disease
- CVD:
-
Cerebrovascular disease
- IR:
-
Insulin resistance
- NAFLD:
-
Non-alcoholic fatty liver disease
- WC:
-
Waist circumference
- TG:
-
Triglycerides
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- HDL-C:
-
HDL cholesterol
- LDL-C:
-
LDL cholesterol
- γ-GTP:
-
Gamma-glutamyl transferase
- FLI:
-
Fatty liver index
- FPG:
-
Fasting plasma glucose
- VAI:
-
Visceral adiposity index
- CRP:
-
C-reactive protein
- DPC:
-
Diagnostic procedure combination
- ICD-10:
-
International classification of disease, tenth revision
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5.
Rhee EJ, Lee WY, Cho YK, Kim BI, Sung KC. Hyperinsulinemia and the development of nonalcoholic fatty liver disease in nondiabetic adults. Am J Med. 2011;124(1):69–76.
Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59(2):713–23.
Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–98.
Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47-64.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32.
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389-397.e310.
Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, et al. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367: l5367.
Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, Sarin SK, Fabrellas N, Zelber-Sagi S, Fan JG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–90.
Lee H, Lee YH, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: a nationwide cohort study. Clin Gastroenterol Hepatol. 2020;19(10):2138–47.
Lim S, Kim JW, Targher G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2021;32(7):500–14.
Sone H, Mizuno S, Fujii H, Yoshimura Y, Yamasaki Y, Ishibashi S, Katayama S, Saito Y, Ito H, Ohashi Y, et al. Is the diagnosis of metabolic syndrome useful for predicting cardiovascular disease in Asian diabetic patients? Analysis from the Japan Diabetes Complications Study. Diabetes Care. 2005;28(6):1463–71.
Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–69.
Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20(5):413–9.
Yamada-Harada M, Fujihara K, Osawa T, Yamamoto M, Kaneko M, Kitazawa M, Matsubayashi Y, Yamada T, Yamanaka N, Seida H, et al. Relationship between number of multiple risk factors and coronary artery disease risk with and without diabetes mellitus. J Clin Endocrinol Metab. 2019;104(11):5084–90.
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33.
Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, Fan J, Goh KL, Hamaguchi M, Hashimoto E, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85.
Kim JH, Moon JS, Byun SJ, Lee JH, Kang DR, Sung KC, Kim JY, Huh JH. Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study. Cardiovasc Diabetol. 2020;19(1):51.
Kim B, Choi HY, Kim W, Ahn C, Lee J, Kim JG, Kim J, Shin H, Yu JM, Moon S. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLoS ONE. 2018;13(11): e0206994.
Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Fujihara K, Yamada-Harada M, Matsubayashi Y, Kitazawa M, Yamamoto M, Yaguchi Y, Seida H, Kodama S, Akazawa K, Sone H. Accuracy of Japanese claims data in identifying diabetes-related complications. Pharmacoepidemiol Drug Saf. 2021;30(5):594–601.
Yamada MH, Fujihara K, Kodama S, Sato T, Osawa T, Yaguchi Y, Yamamoto M, Kitazawa M, Matsubayashi Y, Yamada T, et al. Associations of systolic blood pressure and diastolic blood pressure with the incidence of coronary artery disease or cerebrovascular disease according to glucose status. Diabetes Care. 2021;44(9):2121–31.
Ciardullo S, Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. 2021;121:154752.
Ou H, Fu Y, Liao W, Zheng C, Wu X. Association between smoking and liver fibrosis among patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2019;2019:6028952.
Xu J, Dai L, Zhang Y, Wang A, Li H, Wang Y, Meng X, Wu S, Wang Y. Severity of nonalcoholic fatty liver disease and risk of future ischemic stroke events. Stroke. 2021;52(1):103–10.
Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–22.
Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, Shu SS, Chan AW, Yeung MW, Chan JC, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65(8):1359–68.
Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care. 2015;38(7):1347–55.
Zhou YY, Zhou XD, Wu SJ, Hu XQ, Tang B, Poucke SV, Pan XY, Wu WJ, Gu XM, Fu SW, et al. Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2018;30(6):631–6.
Eslam M, Ahmed A, Després JP, Jha V, Halford JCG, Wei Chieh JT, Harris DCH, Nangaku M, Colagiuri S, Targher G, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;6(9):743–53.
Afolabi BI, Ibitoye BO, Ikem RT, Omisore AD, Idowu BM, Soyoye DO. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J Natl Med Assoc. 2018;110(3):256–64.
Tobari M, Hashimoto E. Characteristic features of nonalcoholic fatty liver disease in japan with a focus on the roles of age, sex and body mass index. Gut Liver. 2020;14(5):537–45.
Balakrishnan M, Patel P, Dunn-Valadez S, Dao C, Khan V, Ali H, El-Serag L, Hernaez R, Sisson A, Thrift AP, et al. Women Have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19(1):61-71.e15.
Goossens GH, Jocken JWE, Blaak EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. 2021;17(1):47–66.
Lefebvre P, Staels B. Hepatic sexual dimorphism—implications for non-alcoholic fatty liver disease. Nat Rev Endocrinol. 2021;17:662–70.
Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. 2019;25(11):1657–66.
Neuman MG, Malnick S, Chertin L. Gamma glutamyl transferase - an underestimated marker for cardiovascular disease and the metabolic syndrome. J Pharm Pharm Sci. 2020;23(1):65–74.
Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, Ferro D, Violi F, Sanyal AJ, Del Ben M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18(10):2324-2331.e2324.
Acknowledgements
The authors thank JMDC Inc. for providing technical support for database construction.
Funding
This work is supported by the Japan Society for Promotion of Science (JSPS) KAKENHI Grant Numbers JP 20K19706, 19H04028, 21K11569, the Ministry of Health, Labour and Welfare, and The Kanae Foundation for the Promotion of Medical Science. The sponsor had no role in the design and conduct of this study.
Author information
Authors and Affiliations
Contributions
YM developed the study design, researched the data, contributed to discussions, wrote the manuscript and edited the manuscript. KF and HS planned and supervised this research, researched the data, contributed to discussions, and edited the manuscript. MY, Yu M, TS, YY, TO, Mas Y, MK, TY, and SK revised the manuscript for important intellectual content. All authors read and approved the final manuscript. YM is the guarantor of this work and, as such, had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of Niigata University.
Consent for publication
Not applicable.
Competing interests
There were no conflicts of interest relevant to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Baseline characteristics of participants without type 2 diabetes according to the classification by the presence or absence of MAFLD or Mets. Table S2. Baseline characteristics of participants with type 2 diabetes according to the classification by the presence or absence of MAFLD or Mets. Table S3. Baseline characteristics of women classified by the presence or absence of MAFLD or MetS. Table S4. Baseline characteristics of men classified by the presence or absence of MAFLD or MetS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Matsubayashi, Y., Fujihara, K., Yamada-Harada, M. et al. Impact of metabolic syndrome and metabolic dysfunction-associated fatty liver disease on cardiovascular risk by the presence or absence of type 2 diabetes and according to sex. Cardiovasc Diabetol 21, 90 (2022). https://doi.org/10.1186/s12933-022-01518-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01518-4