Abstract
Background
Medical cannabinoids differ in their pharmacology and may have different treatment effects. We aimed to conduct a pharmacology-based systematic review (SR) and meta-analyses of medical cannabinoids for efficacy, retention and adverse events.
Methods
We systematically reviewed (registered at PROSPERO: CRD42021229932) eight databases for randomized controlled trials (RCTs) of dronabinol, nabilone, cannabidiol and nabiximols for chronic pain, spasticity, nausea /vomiting, appetite, ALS, irritable bowel syndrome, MS, Chorea Huntington, epilepsy, dystonia, Parkinsonism, glaucoma, ADHD, anorexia nervosa, anxiety, dementia, depression, schizophrenia, PTSD, sleeping disorders, SUD and Tourette. Main outcomes and measures included patient-relevant/disease-specific outcomes, retention and adverse events. Data were calculated as standardized mean difference (SMD) and ORs with confidence intervals (CI) via random effects. Evidence quality was assessed by the Cochrane Risk of Bias and GRADE tools.
Results
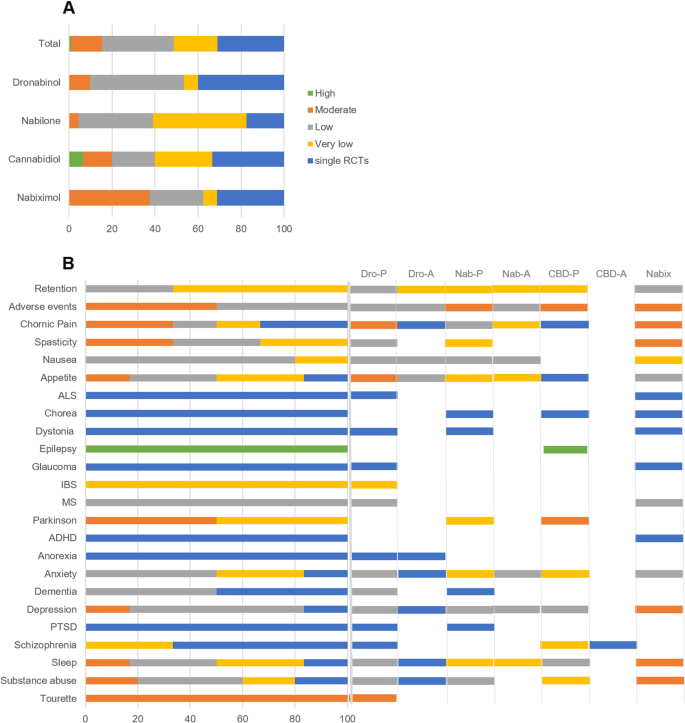
In total, 152 RCTs (12,123 participants) were analysed according to the type of the cannabinoid, outcome and comparator used, resulting in 84 comparisons. Significant therapeutic effects of medical cannabinoids show a large variability in the grade of evidence that depends on the type of cannabinoid. CBD has a significant therapeutic effect for epilepsy (SMD − 0.5[CI − 0.62, − 0.38] high grade) and Parkinsonism (− 0.41[CI − 0.75, − 0.08] moderate grade). There is moderate evidence for dronabinol for chronic pain (− 0.31[CI − 0.46, − 0.15]), appetite (− 0.51[CI − 0.87, − 0.15]) and Tourette (− 1.01[CI − 1.58, − 0.44]) and moderate evidence for nabiximols on chronic pain (− 0.25[− 0.37, − 0.14]), spasticity (− 0.36[CI − 0.54, − 0.19]), sleep (− 0.24[CI − 0.35, − 0.14]) and SUDs (− 0.48[CI − 0.92, − 0.04]). All other significant therapeutic effects have either low, very low, or even no grade of evidence. Cannabinoids produce different adverse events, and there is low to moderate grade of evidence for this conclusion depending on the type of cannabinoid.
Conclusions
Cannabinoids are effective therapeutics for several medical indications if their specific pharmacological properties are considered. We suggest that future systematic studies in the cannabinoid field should be based upon their specific pharmacology.
Similar content being viewed by others
Background
There is a worldwide growing interest and investments in using medical cannabinoids for the treatment of numerous diseases. Furthermore, in 2020, the United Nations (UN) finally recognized the medical value of cannabinoids and removed cannabis from Schedule IV of the 1961 Single Convention on Narcotic Drugs. This allows, in a less restricted manner, the use of medical cannabinoids. It is therefore of critical importance to thoroughly review the grade of evidence of the effectiveness of medical cannabinoids to inform policy and clinical decisions.
Previous systematic reviews have been limited in their coverage of all relevant diseases, but most importantly primarily ignored the fact that medical cannabinoid products—a term that encompasses all plant-derived and synthetic derivatives—differ in their pharmacology [1,2,3,4,5]. The synthetic cannabinoids dronabinol, which is ( −)-trans-Δ9-tetrahydrocannabinol (THC) (Marinol® and Syndros®), and nabilone—a synthetic cannabinoid with structural similarities to THC (Cesamet®), are partial agonists at the cannabinoid receptor 1 (CB1) and with somehow lower affinity at CB2 receptors [6]. Both cannabinoids have indications as appetite stimulants, antiemetics, cannabis addiction, sleep apnea and analgesics and are approved by the FDA for HIV/AIDS-induced loss of appetite and chemotherapy-induced nausea and vomiting. Cannabidiol (CBD; Epidolex®) acts as a negative allosteric modulator at CB1 receptors [7] and also acts at several other receptors, such as CB2 receptors, serotonin 1A receptors, opioid receptors and several ligand-gated ion channels [8]; it represents the only CBD formulation approved by both USA and Europe for the treatment of seizures associated with Dravet syndrome, Lennox-Gastaut syndrome or tuberous sclerosis complex. Nabiximols, a cannabis-derived extract that contains equal quantities of THC and CBD (Sativex®), was approved in 2010 in the UK for symptoms associated to MS, and exported to more than 28 countries from Asia, Africa, the Middle East, Europe (Spain, Czech Republic, Germany, Denmark, Sweden, Italy, Austria, France, Poland) and Canada. Moreover, plant-derived medical cannabis contains almost 150 phytocannabinoids, though most of them have neither been isolated nor pharmacologically characterized [9]. THC and CBD can vary largely in concentrations across different medical cannabis products and can thereby differ in their pharmacological properties. Therefore, a systematic review (SR) that does not consider the different pharmacological properties of medical cannabinoids can be misleading.
The aim of this SR and meta-analysis is to examine possible therapeutic differences for medical cannabinoids in all relevant medical conditions.
Results
Our 32 searches identified 6308 abstracts. Figure 1 shows a flow diagram depicting our selection procedure for the SR and meta-analysis resulting in 53 (dronabinol), 35 (nabilone), 27 (CBD) and 37 (nabiximols) selected RCTs (see Additional file 2). The list of indications by cannabinoid and characteristics of the studies are shown in Tables 1 and 2 and the full description is presented in Additional file 2: Tables S2-5 [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160]. The summary of findings from the 152 RCTs analysed resulting in 84 comparisons (23 outcomes, 12,123 participants) is shown in Table 3 and the GRADE summary in Fig. 2. Low risk of bias was judged in 26, 6, 26 and 19% and high risk of bias was found in 5, 9, 1 and 2 studies of the dronabinol, nabilone, CBD and nabiximols trials, respectively (Additional file 3: Figs. S1-8, for references see Tables S2-5). The complete risk of bias assessment for each RCT can be found in Additional file 3: Table S6 (for references see Tables S2-5).
Primary outcomes
Chronic pain
The meta-analysis (Fig. 3) showed the beneficial effect of cannabinoids on chronic pain (SMD − 0.26, 95% CI − 0.35 to − 0.17; P < 0.00001). Further subgroup analyses indicated that compared to placebo, dronabinol [10, 11, 95, 106, 139, 150, 161, 12, 23, 34, 45, 56, 72, 73, 84] and nabiximols [10, 33,34,35,36,37,38,39,40,41,42,43,44, 46,47,48,49,50,51,52] were associated with significant improvements and moderate evidence (Fig. 2B) in conditions causing chronic pain (dronabinol SMD − 0.31; nabiximols SMD − 0.25, P < 0.0001). Trials using nabilone vs placebo [114, 115, 118, 119, 122, 124,125,126, 162] (but not vs active [120, 121, 123]) also reported a significant effect (SMD − 0.41, P = 0.02), but the evidence on this effect was low (Fig. 2B). The to date single RCTs with CBD vs placebo [153] and dronabinol vs active drug [69] reported no effect.
Spasticity with MS and paraplegia
When all RCTs were pooled (Fig. 4), a significant effect favouring cannabinoids was found (SMD − 0.31, 95% CI − 0.45 to − 0.16; P < 0.0001). Yet, subgroup analyses indicated that only nabiximols [38, 40,41,42,43,44, 46, 47, 49, 53,54,55, 57, 58] were associated with improvements in spasticity (SMD − 0.36, 95% CI − 0.54 to − 0.19; P < 0.0001), and the limited number of studies found with dronabinol [12, 67, 70,71,72, 150] /nabilone [126, 127] did not provide enough evidence.
Nausea and vomiting
The meta-analysis of nausea and vomiting (Additional file 4: Fig. S9) including all studies showed a general efficacy of cannabinoids (SMD − 0.29, 95% CI − 0.39 to − 0.18; P < 0.00001). Confidence on the results from earlier trials reporting improvements in nausea and vomiting versus an active comparator (dronabinol [77, 80, 83, 87]: SMD − 0.28, P = 0.003; nabilone [129,130,131,132,133,134,135,136,137,138, 141]: SMD − 0.44, P < 0.00001) is low due to the lack of methodical rigor. Dronabinol [10, 76, 79, 81, 82, 85, 88, 91], nabilone [115, 140, 142, 162] and nabiximols [10, 59, 60, 62, 63, 85]) were not better than placebo.
Appetite
The meta-analysis (Fig. 5) showed the efficacy of cannabinoids for increasing appetite scores compared to the control arms (SMD − 0.26, P = 0.005). Only the combination of dronabinol-placebo [10, 74,75,76, 82, 85, 88,89,90, 92] (but not vs active [78, 83, 86]) retained the stimulating effect on appetite (SMD − 0.51, 95% CI − 0.87 to − 0.15; P = 0.006). Low/very low evidence and a lack of significance was found for nabilone (vs placebo [114, 115, 143, 162]: SMD − 044, P = 0.12; vs active [129, 137, 138]: SMD 0.24), CBD [154] (SMD 0.10, P = 0.80) or nabiximols [10, 40, 61,62,63, 85] (SMD − 0.25, P = 0.16).
Amyotrophic lateral sclerosis
To date, only one cross-over RCT with dronabinol [75] and one parallel RCT with nabiximols [40] have been carried out in patients suffering from ALS (Additional file 4: Fig. S10). The two trials did not report any improvement in ALS scores and the pooled effect indicated an almost significant effect favouring placebo (SMD 0.31, P = 0.07).
Chorea Huntington
The meta-analysis of the three included studies (Additional file 4: Fig. S11) showed a tendency towards favouring cannabinoids with significant subgroup differences (P = 0.03). That is, the calculated SMD from a single study with nabilone [144] (SMD − 0.45, 95% CI − 0.79 to − 0.11; P = 0.009) but not with CBD [155] or nabiximols [64] (SMD 0.18, P = 0.48 / SMD 0.17, P = 0.4) was significant.
Dystonia
Results with the three small cross-over studies (Additional file 4: Fig. S12) showed a potential benefit of nabilone [145] (SMD − 0.49, P = 0.09) and a lack of effect of nabiximols [64] (SMD 0) and dronabinol [73] (SMD 0.05).
Epilepsy
First reported by an early small study [156] and recently by a series of publications from a large international clinical trial [13, 157,158,159,160], CBD was associated with a significant decrease in seizure frequencies (Fig. 6, SMD − 0.50, 95% CI − 0.62 to 0.38; P < 0.00001).
Glaucoma
Only a very small cross-over trial [94] tested the effects of dronabinol and nabiximols on ocular hypertension (Additional file 4: Fig. S13). Dronabinol produced a transient benefit (SMD − 1.28, 95% CI − 2.36 to − 0.20; P = 0.02), while nabiximols (CBD combined with small amounts of dronabinol) resulted in a transient worsening (SMD − 0.82, P = 0.08).
Irritable bowel syndrome
Two (one parallel [106] and one cross-over [96]) studies tested the effect of acute dronabinol administration on colonic and visceral symptoms (Additional file 4: Fig. S14). Individual results favoured dronabinol and placebo, respectively, resulting in an overall no effect (SMD 0) with a very low evidence.
Multiple sclerosis
Nabiximols [41, 43, 49, 58, 65] or/and dronabinol [97, 117] did not improve symptoms associated with MS (Additional file 4: Fig. S15, overall SMD − 0.13, 95% CI − 0.31 to 0.05; P = 0.15), and none of the subgroups achieved significant improvements (dronabinol SMD − 0.15, P = 0.43; nabiximols SMD − 0.14, P = 0.28).
Parkinson’s disease
Meta-analysis from all studies (Fig. 7) favoured cannabinoids (SMD − 0.41, 95% CI − 0.69 to − 0.13; P = 0.004), and subgroup analyses indicated that CBD [14,15,16] (SMD − 0.41, 95% CI − 0.75 to − 0.08; P = 0.02) but not nabilone [125, 146] (SMD − 0.38; P = 0.27) was associated with a significant improvement in parkinsonian symptoms.
Parkinson’ disease forest plot, stratified according to cannabinoid type and comparator used. The horizontal lines indicate 95% CIs. The diamond markers represent the subtotal and overall weighed standardized mean difference (SMD) mean difference and 95% CI. The vertical line shows the line of no effect
ADHD
One small parallel RCT [66] comparing nabiximols with placebo in ADHD found significant differences in scores of hyperactivity and impulsivity (SMD − 0.83, 95% CI − 1.58 to − 0.09; P = 0.03).
Anorexia nervosa
Two small cross-over RCTs with dronabinol [98, 100] (Additional file 4: Fig. S16) found an increase in body weight when compared with placebo (SMD − 0.47; P = 0.03), but not with diazepam (SMD − 0.06, P = 0.84).
Anxiety
Measurements of anxiety were included in dronabinol vs placebo trials in 4 RCTs [23, 45, 92, 102] and vs prochlorperazine in one study [86]; nabilone in comparison with placebo trials in 6 RCTs [118, 119, 125, 143, 148, 151] and versus active comparators in two RCTs [121, 123]; in 11 RCTs [15,16,17,18, 20,21,22, 24, 25, 153, 163] comparing CBD to placebo and in six nabiximols trials [48, 53, 61,62,63,64]. The meta-analysis including all studies (Additional file 4: Fig. S17) showed that cannabinoids attenuate anxiety levels (SMD − 0.19, 95% CI − 0.37 to − 0.00; P = 0.05), but none of the subgroup analysis showed a significant improvement in anxiety. The quality of evidence of these results was low or very low (Fig. 2B).
Dementia
Disturbed, agitated behaviour in dementia was assessed in 4 RCTs (Additional file 4: Fig. S18), with an overall significant effect (SMD − 0.37, 95% CI − 0.61 to − 0.13; P = 0.002); however, the evidence for specific cannabinoids is low or missing (Fig. 2B). While the three studies with dronabinol [74, 105, 107] collectively did not reach significance (SMD − 0.27, P = 0.09), a single study with nabilone [114] reported a significant reduction (SMD − 0.53, 95% CI − 0.87 to − 0.19; P = 0.002).
Depression
Symptoms of depression caused by diverse medical conditions were evaluated with dronabinol in seven RCTs versus placebo [12, 23, 45, 75, 92, 102, 117] and in one study versus prochlorperazine [86]; with nabilone, three studies comparing placebo [118, 125, 151] and two comparing an active drug [121, 123] were carried out; placebo was compared with CBD in 6 RCTs [15, 19, 22, 24, 153, 154] and with nabiximols in 7 RCTs [48, 49, 53, 61,62,63,64]. The overall meta-analysis (Additional file 4: Fig. S19, SMD − 0.04, P = 0.53) was consistent with the results found in all subgroups reporting minor or no attenuations of depressive symptoms. CBD and nabilone did not modify depressive symptoms, and dronabinol and nabiximols showed a minor improvement compared with placebo (dronabinol: SMD − 0.15, P = 0.39; nabiximols: SMD − 0.12, P = 0.35), but the evidence was moderate only for nabiximols (Fig. 2B).
PTSD
Two small studies with dronabinol [104] and nabilone [152] (Additional file 4: Fig. S20) found significant improvements compared with placebo (dronabinol: SMD − 0.63, 95% CI − 1.22 to − 0.03; P = 0.04; nabilone: SMD − 0.88, 95% CI − 1.65 to − 0.11; P = 0.03).
Schizophrenia and psychosis
The trials evaluating PANNS symptoms (Additional file 4: Fig. S21) showed no effect of cannabinoids (SMD 0.04, P = 0.89) but with subgroup differences (P = 0.03). Thus, a study with dronabinol [108] found a deterioration (SMD 0.89, 95% CI 0.25 to 1.53; P = 0.007), whereas CBD [25, 26, 28, 164] had no effect but the grade of evidence was very low (Fig. 2B).
Sleep
Several trials included within their outcomes sleep measurements (Fig. 8). From the studies with dronabinol, seven [10, 12, 34, 75, 92, 109, 150] were compared to placebo and one cross-over [100] with diazepam; with nabilone, 6 trials [118, 125, 143, 149, 152, 162] used placebo and two trials [120, 123] used active comparators; and eight CBD [13, 15, 19, 22, 153, 157,158,159] and 23 nabiximols [10, 33,34,35, 37, 38, 40, 41, 43, 44, 46,47,48,49,50,51,52, 57, 58, 61,62,63] used placebo. The overall meta-analysis showed a clear improvement in sleep scores (SMD − 0.20, 95% CI − 0.29 to − 0.11; P < 0.0001), but also significant subgroup differences (P = 0.005). Significant effects favouring cannabinoids were restricted to trials comparing nabilone and nabiximols with placebo. Although nabiximols demonstrated the highest efficacy (SMD − 0.24, 95% CI − 0.35 to − 0.14; P < 0.00001) and a moderate quality evidence (Fig. 2B), meta-regression did not indicate a significant superiority versus nabilone (additional file 5, Q = 1.96, P = 0.1618).
Substance abuse
The overall analysis (Fig. 9) indicates that cannabinoids have a beneficial effect in the treatment of drug dependence (SMD − 0.41, 95% CI − 0.63 to − 0.19; P = 0.0003), an effect seen in all subgroup analyses except for CBD [19, 20, 22, 24, 30,31,32]. Although dronabinol [92, 110, 111] showed the highest efficacy (vs placebo: SMD − 0.47, P = 0.0006; vs. active [101]: SMD − 0.85; P = 0.003), followed by nabilone [143, 149, 151] (SMD − 0.55, 95% CI − 0.93 to − 0.18; P = 0.003), confidence on those results was low and the moderate evidence on the effect estimate was provided only by nabiximols [61,62,63, 68] (SMD − 0.48, 95% CI − 0.92 to − 0.04; P = 0.03) (Fig. 2B). Further meta-regression analysis indicated that the differences in the effect sizes were not related to the cannabinoid type (Additional file 5).
Tourette
The two studies [103, 112] reporting the superiority of dronabinol over placebo in attenuating tics severity suggest that dronabinol may be beneficial for Tourette syndrome with a moderate grade of evidence (Fig. 2B) (Fig. 10, SMD − 1.01, 95% CI − 1.58 to − 0.44; P = 0.0005).
Secondary outcomes
Dropouts and adverse events were analysed in 45 trials with dronabinol (37 vs placebo [10,11,12, 23, 34, 45, 70,71,72,73,74,75,76, 79, 81, 83,84,85, 88, 89, 91, 92, 94,95,96, 98, 102, 105,106,107,108,109,110, 112, 139, 150, 161] and 8 vs active comparators [69, 78, 80, 82, 86, 87, 100, 101]), 29 with nabilone (16 vs placebo [114, 118, 119, 124,125,126,127, 140, 142, 144,145,146,147,148, 151, 152] and 13 vs active drugs [120, 121, 123, 129, 130, 132,133,134,135,136,137,138, 141]) and in 22 and 33 with CBD [13,14,15,16,17,18,19,20,21,22, 26, 29, 30, 32, 153,154,155,156,157,158,159,160] and nabiximols [10, 33,34,35,36,37,38, 40,41,42,43,44, 46,47,48,49,50,51,52,53,54,55, 57,58,59,60, 64,65,66, 68, 85, 94], respectively vs placebo (Additional file 6).
Retention
Overall retention (Additional file 6: Fig. S22) for all cannabinoids was better in control arms, although not significantly different (OR 1.12, P = 0.1). After subgroup analyses, this result remained in CBD-containing medications versus placebo (OR 1.38, 95% CI 0.77 to 2.47 and OR 1.17, 95% CI 0.92 to 1.49) while dronabinol/nabilone subgroups had an almost identical proportion of dropouts in each treatment arm, regardless of the comparator used. The low/very low evidence of these results (Fig. 2B) suggests that retention may be influenced by other or additional factors than the treatment.
Adverse events
Despite the fact that the dropout rate in cannabinoid-treated patients does not differ from placebo or active comparators, all cannabinoids produce significant adverse events (Additional file 6: Fig. S23). The evidence was low for dronabinol versus placebo (OR 2.16, 95% CI 1.59 to 2.94; P < 0.00001) also in trials using active comparators (OR 2.75, 95% CI 1.43 to 5.26; P = 0.002), but nabiximols and nabilone were associated with a high number of participants reporting adverse events in comparison to placebo (nabiximols OR 1.97, 95% CI 1.48 to 2.64; nabilone OR 3.12, 95% CI 1.52 to 6.42). Though nabiximols showed the highest significance (P < 0.00001) and CBD the lowest (OR 1.82, 95% CI 1.08 to 3.07; P = 0.02), meta-regression analysis did not indicate significant differences (Additional file 5: Q = 0.04, P = 0.8424). It is also important to consider the severity and the adverse event-related dropouts. That is, severe or serious adverse events were reported only by 4.5% of the CBD trials followed by dronabinol and nabilone (5.4% and 6.3%), dronabinol versus active comparators (12.5%), nabiximols (15.2%) and nabilone versus active comparators with 23.1%; lowest adverse event-related dropouts were found with dronabinol and CBD (24.3% and 27.3%), followed by nabilone (vs placebo 43.8%, vs active comparator 53.8%), nabiximols (54.5%) and dronabinol vs active comparators (62.5%).
Discussion
Previous SRs and meta-analyses on cannabinoids [1,2,3,4,5] (and many others) did not consider, or only considered via sensitivity analysis, that medical cannabinoids and medical plant-derived cannabis products differ largely in their pharmacological mode of action [6,7,8,9] and pharmacokinetics [165]. For the first time, we provide pharmacology-based comparative systematic results for dronabinol, nabilone, CBD and nabiximols for all relevant medical indications. As shown in Fig. 2A, the confidence on the effect estimate strongly differs for these four medications. That is, high quality of evidence is seen only with CBD (6.7% of all CBD trials), and moderate quality of evidence is higher with CBD-containing (CBD 13.3%, nabiximols 37.5%) cannabinoids than with THC-containing (dronabinol 10%, nabilone 4.3%) medications. Notably, these differences are not directly related to a better efficacy, as the proportion of the 152 trials reporting positive results on their primary outcomes did not differ between cannabinoids (dronabinol 52%, nabilone 70%, CBD 52% and nabiximols 57%), resulting in an overall positive effect (data not shown, SMD − 0.33, 95% CI − 0.40 to 0.26; P = 0.0004). Although further meta-regression analyses did not show any specific impact of the cannabinoid type, we still found other differences for the four medications. First, CBD shows with a high grade of evidence effectiveness in the treatment of epilepsy (in particular for Dravet syndrome and Lennox-Gastaut syndrome). Second, there is an overall significant effect of cannabinoids on the improvement of chronic pain, but only dronabinol and nabiximols had moderate evidence. Third, although we found an overall significant effect of cannabinoids on appetite stimulation (especially in HIV/AIDS patients), this effect might be driven by dronabinol with a moderate grade of evidence. Fourth, although the overall effect in Parkinson favoured cannabinoids, only CBD seems to have an effect. Fifth, there was an overall significant effect of cannabinoids on improvement in sleep quality and disturbances and this effect was mainly driven by nabiximols. CBD does not improve sleep but the evidence for this is low. Therefore, it is unclear whether the THC or CBD component of nabiximols (because of low or very low evidence) induces this therapeutic effect. Finally, dronabinol and nabilone improves with a low grade of evidence nausea and vomiting due to chemotherapy. However, this effect is only significant in comparison to active comparators such as prochlorperazine that is not well tolerated by patients undergoing chemotherapy [166] and thus speaks against the use of THC-containing medications for the treatment of nausea and vomiting.
A dichotomy of THC vs. CBD-containing medications is also seen with respect to alterations of physiological functions such as appetite in all medical indications. A recent meta-analysis shows that pharmaceutical THC (dronabinol, nabilone) has no negative effect on appetite, whereas CBD decreases appetite (OR = 2.46 [1.74:4.01] with moderate evidence) [167].
In summary, all medical cannabinoid medications differ in their pharmacology, in their therapeutic profile, and in their profile of adverse events.
The strengths of our study are that we performed for the first time a pharmacology-based comparative systematic analysis of medical cannabinoids. Whole plant-derived cannabis products were excluded from our analysis, as those products have a complex and undefined pharmacology. Thus, we also excluded cannabinoid products with undefined mixtures and other non-approved synthetic cannabinoids in order to reduce heterogeneity. We also excluded studies on healthy individuals and studies with no RCT design to reduce heterogeneity and increase the grade of evidence of our interpretations. Finally, data analysis using SMD allowed the inclusion of a large variety of measurements in the evaluation of the outcomes and allowed us to include many more RCTs for all relevant medical indications than in a previous extensive meta-analysis [3].
There are also limitations. One limitation is the exclusion of an important number of studies (15% of all studies, 31% of all comparisons) that were unable to be graded as they are single RCTs for ALS, Chorea Huntington, dystonia, glaucoma, ADHD, anorexia and PTSD, and therefore could not be included in our conclusions (Fig. 2). Due to missing trials, which was especially the case with CBD for many indications, a second limitation is that we were often unable to directly compare all cannabinoid types, which strongly restricted our conclusions. A third limitation is the inclusion of several RCTs with small study sizes. Small study sizes are of particular concern as it has been previously demonstrated that effects are larger in small studies using cannabinoids [2, 168]. Differences in sample characteristics, durations of the trials and doses or route of administration contributed to heterogeneity in some comparisons, thus limiting the confidence on the findings and the meta-analyses results. In this regard, a systematic meta-regression approach adding those variables as covariates was not possible due to the small number of studies.
In conclusion, medical cannabinoids have an overall positive therapeutic effect for epilepsy, chronic pain, spasticity, appetite, Parkinson’s disease, sleep, SUDs and Tourette. Cannabinoids produce significant adverse events and there is low to moderate grade of evidence for this conclusion depending on the type of cannabinoid. Adverse events produced by cannabinoids do not influence retention in clinical trials, as the dropout rate in cannabinoid-treated patients does not differ from placebo or active comparators. CBD trials reported less adverse events than trials with other medical cannabinoids, but regression analysis did not show any significant differences between these medications; noteworthy, CBD trials reported the lowest percentage of serious adverse events (4.5% of all trials compared with 23% of all nabilone trials).
Most importantly, significant therapeutic effects of medical cannabinoids underlie a large variability in the grade of evidence that depends on the type of cannabinoid. Thus, CBD has a significant therapeutic effect for epilepsy and Parkinson’s disease. The grade of evidence for the treatment of CBD for these conditions is high/moderate. There is moderate evidence for dronabinol for the treatment of chronic pain, appetite and Tourette. Moderate evidence is obtained for nabiximols for having significant therapeutic effects on chronic pain, spasticity, sleep and SUDs. All other significant therapeutic effects of medical cannabinoids have either low, very low or even no grade of evidence, which is the case of single RCTs. In conclusion, dronabinol, nabilone, CBD and nabiximols not only differ in their pharmacology but also in their therapeutic profile. Therefore, future SRs and meta-analyses should consider the pharmacology of cannabinoids.
Conclusions
Cannabinoids are effective therapeutics for several medical indications if their specific pharmacological properties are considered. We suggest that future systematic studies in the cannabinoid field should be based upon their specific pharmacology.
Methods
Methodological details are provided in Additional file 1 [169,170,171,172].
Study design
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [173] and was registered at PROSPERO (CRD42021229932).
Search strategy and selection criteria
We searched in eight databases using Medical Subject Heading (MeSH) terms on all literature published until May 2021 (updated in October 2021) separately for dronabinol, nabilone, cannabidiol and nabiximols (Fig. 1, Additional file 2: Table S1) [12, 59, 94, 174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263]. Studies identified by our search that fulfilled the inclusion criteria given below were reviewed by both authors and disagreements were solved through discussion or by consulting colleagues with long-standing expertise in the field of medical cannabinoids. The inclusion criteria were as follows:
-
Type of studies: randomized controlled parallel and cross-over trials (RCTs) with allocation concealment that was blinded (single or double blinded) which examined the study objective. We excluded all other study designs, including cohort studies, case control studies, outcome research, case studies, case series, expert opinion and conference abstracts.
-
Type of participants: humans of any age or sex, with a medical condition or health problem of any type.
-
Types of interventions: four medical cannabinoids: dronabinol, nabilone, cannabidiol and nabiximols for the treatment of any medical condition. We excluded natural cannabis-based formulations (i.e. smoked marijuana). If a study compared one type of cannabinoid to another or one type of cannabinoid with another active drug, we included both arms. The following indications were included: chronic pain; spasticity with multiple sclerosis and paraplegia; nausea, vomiting or loss of appetite; gastroenterological, neurodegenerative and other neurological diseases including: amyotrophic lateral sclerosis, irritable bowel syndrome, multiple sclerosis (tremor and bladder dysfunction), Chorea Huntington, epilepsy, dystonia, Parkinson and glaucoma, and psychiatric disorders including ADHD, anorexia nervosa, anxiety disorders, dementia, depression, psychotic disorders and schizophrenia, PTSD, sleeping disorders, substance abuse disorders and Tourette.
-
Types of outcomes measures: Eligible outcomes were patient-important and disease-specific outcomes (primary outcomes), retention and adverse events (secondary outcomes).
Data were extracted based on the PICO (Population, Intervention, Comparator and Outcome) format. Risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions [169] and contained in Review Manager (RevMan) version 5.4.1. (The Cochrane Collaboration, 2020). Grading of evidence was assessed using GRADEpro [170]. Both assessments were completed independently by both reviewer authors.
Data synthesis and statistical analysis
All analyses were conducted using Review Manager (RevMan) version 5.4.1. (The Cochrane Collaboration, 2020). Dichotomous and continuous outcomes were pooled as odds ratios (ORs) and standardized mean difference (SMD), respectively using random effects. For cross-over trials, SMD and SE were calculated with the correlation coefficient estimated at 0.5, according to the Becker-Balagtas marginal method [171]. Heterogeneity was assessed using the I2 statistic. Analyses were stratified by outcome and conducted with subgroup analyses by cannabinoid type and comparator. For direct comparisons between two subgroups, meta-regression was performed using type of cannabinoid as covariate.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author (AB) on reasonable request.
Abbreviations
- ADHD:
-
Attention deficit and hyperactivity disorder
- AIDS:
-
Acquired immunodeficiency syndrome
- ALS:
-
Amyotrophic lateral sclerosis
- CB1:
-
Cannabinoid receptor type 1
- CB2:
-
Cannabinoid receptor type 2
- CBD:
-
Cannabidiol
- CI:
-
Confidence interval
- HIV:
-
Human immunodeficiency virus
- MeSH:
-
Medical Subject Heading
- OR:
-
Odds ratio
- PANNS:
-
Psychiatric Assessments Psychotic symptoms
- PICO:
-
Population, Intervention, Comparator and Outcome
- PTSD:
-
Post-traumatic stress disorder
- RCT:
-
Randomized controlled trial
- SE:
-
Standard error
- SMD:
-
Standardized mean difference
- SR:
-
Systematic review
- SUD:
-
Substance use disorder
- THC:
-
( −)-trans-Δ9-Tetrahydrocannabinol
References
Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry. 2020;20(1):24.
Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995–1010.
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA - J Am Med Assoc. 2015;313(24):2456–73 (on=viewrecord&id=L605276146&from=export).
Ben AM. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105(1–2):1–25.
Hoch E, Niemann D, von Keller R, Schneider M, Friemel CM, Preuss UW, et al. How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269(1):87–105.
Mechoulam R, Hanuš LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–64.
Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805.
Pacher P, Kogan NM, Mechoulam R. Beyond THC and Endocannabinoids. Annu Rev Pharmacol Toxicol. 2020;60:637–59.
Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–92.
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39(2):167–79.
Noyes RJ, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18(1):84–9.
Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet (London, England). 2003;362(9395):1517–26.
Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: a placebo-controlled randomized clinical trial. JAMA Neurol. 2021;78(3):285–92.
Chagas MHN, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28(11):1088–98.
de Almeida CMO, Brito MMC, Bosaipo NB, Pimentel A V, Tumas V, Zuardi AW, et al. Cannabidiol for rapid eye movement sleep behavior disorder. Mov Disord. 2021;36(7):1711–5.
de Faria SM, de Morais FD, Tumas V, Castro PC, Ponti MA, Hallak JEC, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34(2):189–96.
Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–26.
Crippa JAS, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FLS, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25(1):121–30.
Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865–74.
Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911–22.
Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol. 2019;10:2466.
Meneses-Gaya C, Crippa JA, Hallak JE, Miguel AQ, Laranjeira R, Bressan RA, et al. Cannabidiol for the treatment of crack-cocaine craving: an exploratory double-blind study. Rev Bras Psiquiatr. 2021;43(5):467–76.
Malik Z, Bayman L, Valestin J, Rizvi-Toner A, Hashmi S, Schey R. Dronabinol increases pain threshold in patients with functional chest pain: a pilot double-blind placebo-controlled trial. Dis esophagus Off J Int Soc Dis Esophagus. 2017;30(2):1–8.
Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433–6.
O’Neill A, Wilson R, Blest-Hopley G, Annibale L, Colizzi M, Brammer M, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2021;51(4):596–606.
Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235(7):1923–32.
Hallak JEC, Machado-de-Sousa JP, Crippa JAS, Sanches RF, Trzesniak C, Chaves C, et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56–61.
Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–31.
Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41(8):1974–82.
Hindocha C, Freeman T, Grabski M, Stroud J, Crudgington H, Davies A, et al. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Biol Psychiatry. 2018;83(9):S235.
Mongeau-Pérusse V, Brissette S, Bruneau J, Conrod P, Dubreucq S, Gazil G, et al. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: a randomized placebo-controlled trial. Addiction. 2021.
Fallon MT, Albert Lux E, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. Br J Pain. 2017;11(3):119–33.
Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299–306.
Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, et al. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage. 2018;55(2):179-188.e1.
Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47(1):166–73.
Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J pain. 2012;13(5):438–49.
Fairhurst C, Kumar R, Checketts D, Tayo B, Turner S. Efficacy and safety of nabiximols cannabinoid medicine for paediatric spasticity in cerebral palsy or traumatic brain injury: a randomized controlled trial. Dev Med Child Neurol. 2020;62(9):1031–9.
Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care. 2010;33(1):128–30.
Riva N, Mora G, Sorarù G, Lunetta C, Ferraro OE, Falzone Y, et al. Safety and efficacy of nabiximols on spasticity symptoms in patients with motor neuron disease (CANALS): a multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2019;18(2):155–64.
Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32(5):451–9.
Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, et al. Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. Eur J Pain. 2009;13(5):472–7.
Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260(4):984–97.
Leocani L, Nuara A, Houdayer E, Schiavetti I, Del Carro U, Amadio S, et al. Sativex(®) and clinical-neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol. 2015;262(11):2520–7.
Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J pain. 2008;9(3):254–64.
Markovà J, Essner U, Akmaz B, Marinelli M, Trompke C, Lentschat A, et al. Sativex(®) as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019;129(2):119–28.
Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®) ), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122–31.
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812–9.
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–41.
Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133(1–3):210–20.
Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18(7):999–1012.
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45(1):50–2.
Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, et al. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple clerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol. 2009;32(1):41–7.
Collin C, Davies P, Mutiboko IK, Ratcliffe S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14(3):290–6.
De Blasiis P, Siani MF, Fullin A, Sansone M, Melone MAB, Sampaolo S, et al. Short and long term effects of Nabiximols on balance and walking assessed by 3D-gait analysis in people with Multiple Sclerosis and spasticity. Mult Scler Relat Disord. 2021;51:102805.
Weizman L, Dayan L, Brill S, Nahman-Averbuch H, Hendler T, Jacob G, et al. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. 2018;91(14):e1285–94.
Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex®(nabiximols). Mult Scler. 2012;18(2):219–28.
Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al. Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Mult Scler. 2004;10(4):417–24.
Duran M, Pérez E, Abanades S, Vidal X, Saura C, Majem M, et al. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br J Clin Pharmacol. 2010;70(5):656–63.
Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31(11):1553–60.
Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiat. 2014;71(3):281–91.
Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, et al. Effects of fixed or self-titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend. 2016;161:298–306.
Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PLoS One. 2018;13(1):e0190768.
López-Sendón Moreno JL, García Caldentey J, Trigo Cubillo P, Ruiz Romero C, García Ribas G, Alonso Arias MAA, et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J Neurol. 2016;263(7):1390–400.
Kavia RBC, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult Scler. 2010;16(11):1349–59.
Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, Asherson P. Cannabinoids in attention-deficit/hyperactivity disorder: a randomised-controlled trial. Eur Neuropsychopharmacol. 2017;27(8):795–808.
Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord. 2007;45(8):551–62.
Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, et al. Nabiximols for the treatment of cannabis dependence: a randomized clinical trial. JAMA Intern Med. 2019;179(9):1242–53.
Rintala DH, Fiess RN, Tan G, Holmes SA, Bruel BM. Effect of dronabinol on central neuropathic pain after spinal cord injury: a pilot study. Am J Phys Med Rehabil. 2010;89(10):840–8.
Killestein J, Hoogervorst ELJ, Reif M, Kalkers NF, Van Loenen AC, Staats PGM, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology. 2002;58(9):1404–7.
Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Adv Alcohol Subst Abuse. 1987;7(1):39–50.
Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D, et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12(9):857–65.
Zadikoff C, Wadia PM, Miyasaki J, Chen R, Lang AE, So J, et al. Cannabinoid, CB1 agonists in cervical dystonia: failure in a phase IIa randomized controlled trial. Basal Ganglia. 2011;1(2):91–5.
Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatric Psychiatr. 1997;12:913–9 (US: John Wiley & Sons).
Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81(10):1135–40.
Brisbois TD, de Kock IH, Watanabe SM, Mirhosseini M, Lamoureux DC, Chasen M, et al. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol Off J Eur Soc Med Oncol. 2011;22(9):2086–93.
Gilbert CJ, Ohly KV, Rosner G, Peters WP. Randomized, double-blind comparison of a prochlorperazine-based versus a metoclopramide-based antiemetic regimen in patients undergoing autologous bone marrow transplantation. Cancer. 1995;76(11):2330–7.
Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol. 2002;20(2):567–73.
Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang H-M, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533–43.
Neidhart JA, Gagen MM, Wilson HE, Young DC. Comparative trial of the antiemetic effects of THC and haloperidol. J Clin Pharmacol. 1981;21(S1):38S-42S.
Orr LE, McKernan JF. Antiemetic effect of delta 9-tetrahydrocannabinol in chemotherapy-associated nausea and emesis as compared to placebo and compazine. J Clin Pharmacol. 1981;21(S1):76S-80S.
Sallan SE, Zinberg NE, Frei E 3rd. Antiemetic effect of delta-9-tetrahydrocannabinol in patients receiving cancer chemotherapy. N Engl J Med. 1975;293(16):795–7.
Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer: a randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. N Engl J Med. 1980;302(3):135–8.
de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2017;15(7):1079-1086.e4.
Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, et al. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannab. J Clin Oncol. 2006;24(21):3394–400.
Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy: a comparison of oral delta-9-THC and prochlorperazine. Cancer. 1982;50(4):636–45.
Lane M, Vogel CL, Ferguson J, Krasnow S, Saiers JL, Hamm J, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. J Pain Symptom Manage. 1991;6(6):352–9.
Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89–97.
Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181(1):170–8.
Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, et al. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45(5):545–54.
Kleine-Brueggeney M, Greif R, Brenneisen R, Urwyler N, Stueber F, Theiler LG. Intravenous Delta-9-tetrahydrocannabinol to prevent postoperative nausea and vomiting: a randomized controlled trial. Anesth Analg. 2015;121(5):1157–64.
Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Vol. 86, Drug and Alcohol Dependence. Budney, Alan J.: 4301 W. Markham St., Slot 843, Little Rock, AR, US, 72206, ajbudney@uams.edu: Elsevier Science; 2007. p. 22–9.
Lundahl LH, Greenwald MK. Effect of oral THC pretreatment on marijuana cue-induced responses in cannabis dependent volunteers. Drug Alcohol Depend. 2015;149:187–93.
Tomida I, Azuara-Blanco A, House H, Flint M, Pertwee RG, Robson PJ. Effect of sublingual application of cannabinoids on intraocular pressure: a pilot study. J Glaucoma. 2006;15(5):349–53.
de Vries M, Van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, Van Goor H. Single dose delta-9-tetrahydrocannabinol in chronic pancreatitis patients: analgesic efficacy, pharmacokinetics and tolerability. Br J Clin Pharmacol. 2016;81(3):525–37.
Klooker TK, Leliefeld KEM, Van Den Wijngaard RM, Boeckxstaens GEE. The cannabinoid receptor agonist delta-9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil. 2011;23(1):30–5, e2.
Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(6):636–41.
Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int J Eat Disord. 2014;47(1):18–23.
Andries A, Gram B, Støving RK. Effect of dronabinol therapy on physical activity in anorexia nervosa: a randomised, controlled trial. Eat Weight Disord. 2015;20(1):13–21.
Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol. 1983;3(3):165–71.
Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, et al. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015;154:38–45.
Müller-Vahl KR, Koblenz A, Jöbges M, Kolbe H, Emrich HM, Schneider U. Influence of treatment of Tourette syndrome with delta9-tetrahydrocannabinol (delta9-THC) on neuropsychological performance. Pharmacopsychiatry. 2001;34(1):19–24.
Müller-Vahl KR, Schneider U, Koblenz A, Jöbges M, Kolbe H, Daldrup T, et al. Treatment of Tourette’s syndrome with Delta 9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry. 2002;35(2):57–61.
Rabinak CA, Blanchette A, Zabik NL, Peters C, Marusak HA, Iadipaolo A, et al. Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology. 2020;237(6):1813–26.
van den Elsen GAH, Ahmed AIA, Verkes R-J, Feuth T, van der Marck MA, Olde Rikkert MGM. Tetrahydrocannabinol in behavioral disturbances in dementia: a crossover randomized controlled trial. Am J Geriatr Psychiatry. 2015;23(12):1214–24.
Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, et al. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology. 2011;141(5):1638–47.
van den Elsen GAH, Ahmed AIA, Verkes R-J, Kramers C, Feuth T, Rosenberg PB, et al. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: a randomized controlled trial. Neurology. 2015;84(23):2338–46.
D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608.
Carley DW, Prasad B, Reid KJ, Malkani R, Attarian H, Abbott SM, et al. Pharmacotherapy of apnea by cannabimimetic enhancement, the PACE clinical trial: Effects of dronabinol in obstructive sleep apnea. Sleep. 2018;41(1):zsx184.
Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–50.
Lofwall MR, Babalonis S, Nuzzo PA, Elayi SC, Walsh SL. Opioid withdrawal suppression efficacy of oral dronabinol in opioid dependent humans. Drug Alcohol Depend. 2016;164:143–50.
Müller-Vahl KR, Schneider U, Prevedel H, Theloe K, Kolbe H, Daldrup T, et al. Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry. 2003;64(4):459–65.
Muller-Vahl KR, Prevedel H, Theloe K, Kolbe H, Emrich HM, Schneider U. Treatment of Tourette syndrome with delta-9-tetrahydrocannabinol (delta 9-THC): no influence on neuropsychological performance. Neuropsychopharmacology. 2003;28(2):384–8.
Herrmann N, Ruthirakuhan M, Gallagher D, Verhoeff NPLG, Kiss A, Black SE, et al. Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr psychiatry Off J Am Assoc Geriatr Psychiatry. 2019;27(11):1161–73.
Cote M, Trudel M, Wang C, Fortin A. Improving quality of life with nabilone during radiotherapy treatments for head and neck cancers: a randomized double-blind placebo-controlled trial. Ann Otol Rhinol Laryngol. 2016;125(4 CC-ENT):317–24.
Turcott J, Guillen-Núñez MDR, Flores D, Oñate L, Zatarain-Barrón Z, Barrón F, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. J Thorac Oncol. 2018;13(10):S360–1.
Ball S, Vickery J, Hobart J, Wright D, Green C, Shearer J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess. 2015;19(12):vii–viii (xxv--xxxi, 1--187).
Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153(10):2073–82.
Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J pain. 2008;9(2):164–73.
Ware MA, Fitzcharles M-A, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):604–10.
Pini LA, Guerzoni S, Cainazzo MM, Ferrari A, Sarchielli P, Tiraferri I, et al. Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. J Headache Pain. 2012;13(8):677–84.
Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med. 2015;16(1):149–59.
Frank B, Serpell MG, Hughes J, Matthews JNS, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336(7637):199–201.
Pinsger M, Schimetta W, Volc D, Hiermann E, Riederer F, Pölz W. Benefits of an add-on treatment with the synthetic cannabinomimetic nabilone on patients with chronic pain–a randomized controlled trial. Wien Klin Wochenschr. 2006;118(11–12):327–35.
Peball M, Krismer F, Knaus H-G, Djamshidian A, Werkmann M, Carbone F, et al. Non-motor symptoms in Parkinson’s disease are reduced by nabilone. Ann Neurol. 2020;88(4):712–22.
Wissel J, Haydn T, Müller J, Brenneis C, Berger T, Poewe W, et al. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain : a double-blind placebo-controlled cross-over trial. J Neurol. 2006;253(10):1337–41.
Pooyania S, Ethans K, Szturm T, Casey A, Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch Phys Med Rehabil. 2010;91(5):703–7.
Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. 2017;78(5–6):320–9.
Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. Br J Cancer. 1983;48(5 CC-SR-CANCER CC-Lung Cancer CC-Pain, Palliative and Supportive Care CC-Gynaecological, Neuro-oncology and Orphan Cancer):657–63.
Chan HS, Correia JA, MacLeod SM. Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: a double-blind, crossover trial. Pediatrics. 1987;79(6):946–52.
Crawford SM, Buckman R. Nabilone and metoclopramide in the treatment of nausea and vomiting due to cisplatinum: a double blind study. Med Oncol Tumor Pharmacother. 1986;3(1):39–42.
Dalzell AM, Bartlett H, Lilleyman JS. Nabilone: an alternative antiemetic for cancer chemotherapy. Arch Dis Child. 1986;61(5):502–5.
Einhorn LH, Nagy C, Furnas B, Williams SD. Nabilone: an effective antiemetic in patients receiving cancer chemotherapy. J Clin Pharmacol. 1981;21(S1):64S-69S.
Johansson R, Kilkku P, Groenroos M. A double-blind, controlled trial of nabilone vs. prochlorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treat Rev. 1982;9(Suppl B):25–33.
Niederle N, Schütte J, Schmidt CG. Crossover comparison of the antiemetic efficacy of nabilone and alizapride in patients with nonseminomatous testicular cancer receiving cisplatin therapy. Klin Wochenschr. 1986;64(8):362–5.
Niiranen A, Mattson K. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. Am J Clin Oncol. 1985;8(4):336–40.
Pomeroy M, Fennelly JJ, Towers M. Prospective randomized double-blind trial of nabilone versus domperidone in the treatment of cytotoxic-induced emesis. Cancer Chemother Pharmacol. 1986;17(3):285–8.
Priestman SG, Priestman TJ, Canney PA. A double-blind randomised cross-over comparison of nabilone and metoclopramide in the control of radiation-induced nausea. Clin Radiol. 1987;38(5):543–4.
Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329(7460):253.
Wada JK, Bogdon DL, Gunnell JC, Hum GJ, Gota CH, Rieth TE. Double-blind, randomized, crossover trial of nabilone vs placebo in cancer chemotherapy. Cancer Treat Rev. 1982;9(Suppl B):39–44.
Lewis IH, Campbell DN, Barrowcliffe MP. Effect of nabilone on nausea and vomiting after total abdominal hysterectomy. Br J Anaesth. 1994;73(2):244–6.
Levin DN, Dulberg Z, Chan A-W, Hare GMT, Mazer CD, Hong A. A randomized-controlled trial of nabilone for the prevention of acute postoperative nausea and vomiting in elective surgery. Can J Anesth. 2017;64(4):385–95.
Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology. 2016;233(13):2469–78.
Curtis A, Mitchell I, Patel S, Ives N, Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington’s disease. Mov Disord. 2009;24(15):2254–9.
Fox SH, Kellett M, Moore AP, Crossman AR, Brotchie JM. Randomised, double-blind, placebo-controlled trial to assess the potential of cannabinoid receptor stimulation in the treatment of dystonia. Mov Disord. 2002;17(1):145–9.
Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology. 2001;57(11):2108–11.
Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. 1981;21(8–9 Suppl CC-Common Mental Disorders):377–82.
Glass RM, Uhlenhuth EH, Hartel FW, Schuster CR, Fischman MW. Single-dose study of nabilone in anxious volunteers. J Clin Pharmacol. 1981;21(S1):383S-396S.
Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse. Addict Biol. 2019;24(4):765–76.
van Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al. Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clin Ther. 2018;40(9):1467–82.
Hill KP, Palastro MD, Gruber SA, Fitzmaurice GM, Greenfield SF, Lukas SE, et al. Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am J Addict. 2017;26(8):795–801.
Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8.
Vela J, Dreyer L, Petersen K, Lars A, Duch K, Kristensen S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: a randomized, double-blind placebo-controlled trial. Pain. 2022;163(6):1206–14.
Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777–86.
Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40(3):701–8.
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85.
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med. 2017;376(21):2011–20.
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the lennox–gastaut syndrome. N Engl J Med. 2018;378(20):1888–97.
Miller I, Scheffer IE, Gunning B, Sanchez-Carpintero R, Gil-Nagel A, Perry MS, et al. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77(5):613–21.
Thiele EA, Marsh ED, French JA, Mazurkiewicz MB, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–96.
Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. 2017;78(5–6):320–9.
Turcott JG, Del RocíoGuillenNúñez M, Flores-Estrada D, Oñate-Ocaña LF, Zatarain-Barrón ZL, Barrón F, et al. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: a randomized, double-blind clinical trial. Support care cancer Off J Multinatl Assoc Support Care Cancer. 2018;26(9):3029–38.
Freeman TP, Craft S, Wilson J, Stylianou S, ElSohly M, Di Forti M, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction . 2020.
McGuire P, Robson P, Cubała W, Vasile D, Morrison P, Barron R, et al. A randomized controlled trial of cannabidiol in schizophrenia. Schizophr Bull. 2018;44:S27.
Poyatos L, Pérez-Acevedo AP, Papaseit E, Pérez-Mañá C, Martin S, Hladun O, et al. Oral administration of cannabis and Δ-9-tetrahydrocannabinol (THC) preparations: a systematic review. Medicina (Kaunas). 2020;56(6):309.
Furyk JS, Meek RA, Egerton‐Warburton D. Drugs for the treatment of nausea and vomiting in adults in the emergency department setting. Cochrane Database Syst Rev. 2015;(9):CD010106.
Spanagel R, Bilbao A. Approved cannabinoids for medical purposes - comparative systematic review and meta-analysis for sleep and appetite. Neuropharmacology. 2021;196:108680.
Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159(10):1932–54.
Higgins JPT Page MJ, Elbers RG, Sterne JAC. SJ. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) Cochrane Handbook for Systematic Reviews of Interventions version 62 (updated February 2021) Cochrane, 2021
GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University, (developed by Evidence Prime, Inc.). 2012.
Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9.
Schünemann H Guyatt G, Oxman A, editors. BJ. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42(S1):82S-89S.
Brezing CA, Choi CJ, Pavlicova M, Brooks D, Mahony AL, Mariani JJ, et al. Abstinence and reduced frequency of use are associated with improvements in quality of life among treatment-seekers with cannabis use disorder. Am J Addict. 2018;27(2):101–7.
Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106(1–2):169–72.
Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, et al. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer. 1981;47(7):1746–51.
Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, et al. Delata-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Ann Intern Med. 1979;91(6):819–24.
Freeman D, Dunn G, Murray RM, Evans N, Lister R, Antley A, et al. How cannabis causes paranoia: using the intravenous administration of DELTA9-Tetrahydrocannabinol (THC) to identify key cognitive mechanisms leading to paranoia. Schizophr Bull. 2015;41(2 // (NIHR) *National Institute for Health Research*):391–9.
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157–68.
Issa MA, Narang S, Jamison RN, Michna E, Edwards RR, Penetar DM, et al. The subjective psychoactive effects of oral dronabinol studied in a randomized, controlled crossover clinical trial for pain. Clin J Pain. 2014;30(6):472–8.
Jansma JM, van Hell HH, Vanderschuren LJMJ, Bossong MG, Jager G, Kahn RS, et al. THC reduces the anticipatory nucleus accumbens response to reward in subjects with a nicotine addiction. Transl Psychiatry. 2013;3(2): e234.
Jatoi A, Yamashita J, Sloan JA, Novotny PJ, Windschitl HE, Loprinzi CL. Does megestrol acetate down-regulate interleukin-6 in patients with cancer-associated anorexia and weight loss? A North Central Cancer Treatment Group investigation. Support Care Cancer. 2002;10(1):71–5.
Jicha CJ, Lofwall MR, Nuzzo PA, Babalonis S, Elayi SC, Walsh SL. Safety of oral dronabinol during opioid withdrawal in humans. Drug Alcohol Depend. 2015;157:179–83.
Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem. 2011;57(1):66–75.
Lane M, Smith FE, Sullivan RA, Plasse TF. Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. Am J Clin Oncol. 1990;13(6):480–4.
Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, et al. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;159:53–60.
McCabe M, Smith FP, Macdonald JS, Woolley PV, Goldberg D, Schein PS. Efficacy of tetrahydrocannabinol in patients refractory to standard antiemetic therapy. Invest New Drugs. 1988;6(3):243–6.
Merritt JC, Perry DD, Russell DN, Jones BF. Topical delta 9-tetrahydrocannabinol and aqueous dynamics in glaucoma. J Clin Pharmacol. 1981;21(S1):467S-471S.
Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. Vol. 4, Frontiers in Psychiatry. Carley, David W.: Department of Biobehavioral Health Science, University of Illinois at Chicago, 215W CON MC 802, 845 S Damen Ave, Chicago, IL, US, 60612, dwcarley@uic.edu: Frontiers Media S.A.; 2013.
Reichenbach ZW, Sloan J, Rizvi-Toner A, Bayman L, Valestin J, Schey R. A 4-week pilot study with the cannabinoid receptor agonist dronabinol and its effect on metabolic parameters in a randomized trial. Clin Ther. 2015;37(10):2267–74.
Schlienz NJ, Cone EJ, Herrmann ES, Lembeck NA, Mitchell JM, Bigelow GE, et al. Pharmacokinetic characterization of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in urine following acute oral cannabis ingestion in healthy adults. J Anal Toxicol. 2018;42(4):232–47.
Ungerleider JT, Sarna G, Fairbanks LA, Goodnight J, Andrysiak T, Jamison K. THC or Compazine for the cancer chemotherapy patient–the UCLA study. Part II: Patient drug preference. Am J Clin Oncol. 1985;8(2):142–7.
Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139(4):258–66.
van den Elsen GA, Tobben L, Ahmed AI, Verkes RJ, Kramers C, Marijnissen RM, et al. Effects of tetrahydrocannabinol on balance and gait in patients with dementia: a randomised controlled crossover trial. J Psychopharmacol. 2017;31(2):184–91.
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17(1):21–9.
Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J pain. 2015;16(7):616–27.
Wong BS, Camilleri M, Eckert D, Carlson P, Ryks M, Burton D, et al. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24(4):358-e169.
Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry. 2012;83(11):1125–32.
Lile JA, Kelly TH, Hays LR. Separate and combined effects of the cannabinoid agonists nabilone and $Δ$9-THC in humans discriminating $Δ$9-THC. Drug Alcohol Depend. 2011;116(1–3):86–92.
Williams CJ, Bolton A, de Pemberton R, Whitehouse JM. Antiemetics for patients treated with antitumor chemotherapy. Cancer Clin Trials. 1980;3(4):363–7.
Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. 2013;18(5):872–81.
Cunningham D, Bradley CJ, Forrest GJ, Hutcheon AW, Adams L, Sneddon M, et al. A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues. Eur J Cancer Clin Oncol. 1988;24(4):685–9.
Cunningham D, Forrest GJ, Soukop M, Gilchrist NL, Calder IT, McArdle CS. Nabilone and prochlorperazine: a useful combination for emesis induced by cytotoxic drugs. Br Med J (Clin Res Ed). 1985;291(6499):864–5.
Almog S, Aharon-Peretz J, Vulfsons S, Ogintz M, Abalia H, Lupo T, et al. The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: a randomized, double-blinded, placebo-controlled trial. Eur J Pain. 2020;24(8):1505–16.
Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). Vol. 15, CNS Neuroscience & Therapeutics. Fraser, George A.: Operational Trauma and Stress Support Centre, Canadian Forces Health Services Centre, 1745 Alta Vista Drive, Ottawa, ON, Canada, K1A 0K6, fraser.ga2@forces.gc.ca: Wiley-Blackwell Publishing Ltd.; 2009. p. 84–8.
Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional Phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treat Rev. 1982;9(Suppl B):45–8.
Kalliomaki J, Philipp A, Baxendale J, Annas P, Karlsten R, Segerdahl M. Lack of effect of central nervous system-active doses of nabilone on capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol. 2012;39(4):336–42.
Kayser RR, Raskin M, Snorrason I, Hezel DM, Haney M, Simpson HB. Cannabinoid augmentation of exposure-based psychotherapy for obsessive-compulsive disorder. J Clin Psychopharmacol. 2020;40(2):207–10.
Nakano S, Gillespie HK, Hollister LE. A model for evaluation of antianxiety drugs with the use of experimentally induced stress: comparison of nabilone and diazepam. Clin Pharmacol Ther. 1978;23(1):54–62.
Niiranen A, Mattson K. Antiemetic efficacy of nabilone and dexamethasone: a randomized study of patients with lung cancer receiving chemotherapy. Am J Clin Oncol. 1987;10(4):325–9.
Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 “N of 1” studies. Anaesthesia. 2004;59(5):440–52.
Peball M, Werkmann M, Ellmerer P, Stolz R, Valent D, Knaus H-G, et al. Nabilone for non-motor symptoms of Parkinson’s disease: a randomized placebo-controlled, double-blind, parallel-group, enriched enrolment randomized withdrawal study (The NMS-Nab Study). J Neural Transm. 2019;126(8):1061–72.
Steele N, Gralla RJ, Braun DWJ, Young CW. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treat Rep. 1980;64(2–3):219–24.
Appiah-Kusi E, Petros N, Wilson R, Colizzi M, Bossong MG, Valmaggia L, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology. 2020;237(4):1121–30.
Andries A, Frystyk J, Flyvbjerg A, Støving RK. Changes in IGF-I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: results from a randomised, controlled trial. Growth Horm IGF Res. 2015;25(5):247–52.
Ben-Menachem E, Gunning B, Arenas Cabrera CM, VanLandingham K, Crockett J, Critchley D, et al. A phase II randomized trial to explore the potential for pharmacokinetic drug–drug interactions with stiripentol or valproate when combined with cannabidiol in patients with epilepsy. CNS Drugs. 2020;34(6):661–72.
Bhattacharyya S, Wilson R, Appiah-Kusi E, O’Neill A, Brammer M, Perez J, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiat. 2018;75(11):1107–17.
Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60(8):1586–92.
Bristot G, Hizo GH, Pinto JV, da Ponte FDR, Valiati FE, De Moura Silveira É, et al. P.369 Potential anti-inflammatory and antioxidant effects of cannabidiol in bipolar depression. Eur Neuropsychopharmacol. 2020;40:S212–3.
Davies C, Wilson R, Appiah-Kusi E, Blest-Hopley G, Brammer M, Perez J, et al. A single dose of cannabidiol modulates medial temporal and striatal function during fear processing in people at clinical high risk for psychosis. Transl Psychiatry. 2020;10(1):311.
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–11.
Efron D, Freeman JL, Cranswick N, Payne JM, Mulraney M, Prakash C, et al. A pilot randomised placebo-controlled trial of cannabidiol to reduce severe behavioural problems in children and adolescents with intellectual disability. Br J Clin Pharmacol. 2021;87(2):436–46.
Good P, Haywood A, Gogna G, Martin J, Yates P, Greer R, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat Care. 2019;18(1):110.
Hardy J, Haywood A, Gogna G, Martin J, Yates P, Greer R, et al. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Trials. 2020;21(1):611.
Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJA, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. 2015;25(3):325–34.
Attal N, Brasseur L, Guirimand D, Clermond-Gnamien S, Atlami S, Bouhassira D. Are oral cannabinoids safe and effective in refractory neuropathic pain? Vol. 8, European Journal of Pain. Bouhassira, D.: INSERM E-332, Centre d’Evaluation et de Traitement de la Douleur, Hopital Ambroise Pare, AP-HP, Boulogne-Billancourt, France, 92100, didier.bouhassira@apr.ap-hop-paris.fr: Elsevier Science; 2004. p. 173–7.
Hundal H, Lister R, Evans N, Antley A, Englund A, Murray RM, et al. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. J Psychopharmacol. 2018;32(3):276–82.
Hussain SA, Dlugos DJ, Cilio MR, Parikh N, Oh A, Sankar R. Synthetic pharmaceutical grade cannabidiol for treatment of refractory infantile spasms: a multicenter phase-2 study. Epilepsy Behav. 2020;102:106826.
Irving PM, Iqbal T, Nwokolo C, Subramanian S, Bloom S, Prasad N, et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis. 2018;24(4):714–24.
Klein P, Tolbert D, Gidal BE. Drug–drug interactions and pharmacodynamics of concomitant clobazam and cannabidiol or stiripentol in refractory seizures. Epilepsy Behav. 2019;99:106459.
Leweke FM, Rohleder C, Müller JK, Hirjak D, Meyer-Lindenberg A. Enhancing recovery in early schizophrenia by randomized controlled add-on of cannabidiol to an individualized antipsychotic treatment. Nervenheilkunde. 2018;37(5):319–23.
Müller-Vahl KR. Cannabinoids reduce symptoms of Tourette’s syndrome. Expert Opin Pharmacother. 2003;4(10):1717–25.
Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci. 2017;62(6):1615–20.
Nitecka-Buchta A, Nowak-Wachol A, Wachol K, Walczyńska-Dragon K, Olczyk P, Batoryna O, et al. Myorelaxant effect of transdermal cannabidiol application in patients with TMD: a randomized, double-blind trial. J Clin Med. 2019;8(11):1886.
Santos de Alencar S, Crippa JAS, Brito MCM, Pimentel ÂV, CecilioHallak JE, Tumas V. A single oral dose of cannabidiol did not reduce upper limb tremor in patients with essential tremor. Park Relat Disord. 2021;83:37–40.
Szaflarski JP, Hernando K, Bebin EM, Gaston TE, Grayson LE, Ampah SB, et al. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy Behav. 2019;95:131–6.
Baker NL, Gray KM, Sherman BJ, Morella K, Sahlem GL, Wagner AM, et al. Biological correlates of self-reported new and continued abstinence in cannabis cessation treatment clinical trials. Vol. 187, Drug and Alcohol Dependence. Baker, Nathaniel L.: Department of Public Health Sciences, Medical University of South Carolina, 135 Cannon Street, Suite 303, Charleston, SC, US, 29425, bakern@musc.edu: Elsevier Science; 2018. p. 270–7.
Szaflarski M, Hansen B, Bebin EM, Szaflarski JP. Social correlates of health status, quality of life, and mood states in patients treated with cannabidiol for epilepsy. Epilepsy Behav. 2017;70(Part B):364–9.
Thiele EA, Bebin EM, Filloux F, Kwan P, Loftus R, Sahebkar F, et al. Long-term safety and efficacy of add-on Cannabidiol (CBD) for treatment of seizures associated with tuberous sclerosis complex (TSC) in an open-label extension (OLE) trial (GWPCARE6). Dev Med Child Neurol. 2021;63(SUPPL 1):69.
van Amsterdam J, Vervloet J, de Weert G, Buwalda VJA, Goudriaan AE, van den Brink W. Acceptance of pharmaceutical cannabis substitution by cannabis using patients with schizophrenia. Harm Reduct J. 2018;15(1):47.
Wall MB, Pope R, Freeman TP, Kowalczyk OS, Demetriou L, Mokrysz C, et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol. 2019;33(7):822–30.
Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, et al. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 2019;33(6):593–604.
Wilson R, Bossong MG, Appiah-Kusi E, Petros N, Brammer M, Perez J, et al. Cannabidiol attenuates insular dysfunction during motivational salience processing in subjects at clinical high risk for psychosis. Transl Psychiatry. 2019;9(1):203.
Winton-Brown TT, Allen P, Bhattacharrya S, Borgwardt SJ, Fusar-Poli P, Crippa JA, et al. Modulation of auditory and visual processing by delta-9- tetrahydrocannabinol and cannabidiol: An fMRI study. Neuropsychopharmacology. 2011;36(7):1340–8.
Wright S, Duncombe P, Altman DG. Assessment of blinding to treatment allocation in studies of a cannabis-based medicine (Sativex®) in people with multiple sclerosis: a new approach. Trials. 2012;13:189.
Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 2020;21(5):390–402.
Centonze D, Mori F, Koch G, Buttari F, Codecà C, Rossi S, et al. Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Vol. 30, Neurological Sciences. Centonze, Diego: Clinica Neurologica, Dipartimento di Neuroscienze, Universita Tor Vergata, Via Montpellier 1, Rome, Italy, 00133, centonze@uniroma2.it: Springer; 2009. p. 531–4.
Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, et al. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Vol. 212, Psychopharmacology. Haney, Margaret: Division on Substance Abuse, New York State Psychiatric Institute, 1051 Riverside Drive, Unit 120, New York, NY, US, 10032, mh235@columbia.edu: Springer; 2010. p. 675–86.
Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice—results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Vol. 71, European Neurology. Flachenecker, Peter: Neurologisches Reha-Zentrum Quellenhof Bad Wildbad, Kuranlagenallee 2, Bad Wildbad, Germany, DE 75323, peter.flachenecker@sana.de: Karger; 2014. p. 271–9.
Haupts M, Vila C, Jonas A, Witte K, Álvarez-Ossorio L. Influence of previous failed antispasticity therapy on the efficacy and tolerability of THC:CBD oromucosal spray for multiple sclerosis spasticity. Eur Neurol. 2016;75(5–6):236–43.
Hindocha C, Freeman TP, Schafer G, Gardner C, Bloomfield MAP, Bramon E, et al. Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict Biol. 2020;25(3):e12762.
Libzon S, Schleider LB-L, Saban N, Levit L, Tamari Y, Linder I, et al. Medical cannabis for pediatric moderate to severe complex motor disorders. J Child Neurol. 2018;33(9):565–71.
Lintzeris N, Mills L, Dunlop A, Copeland J, Mcgregor I, Bruno R, et al. Cannabis use in patients 3 months after ceasing nabiximols for the treatment of cannabis dependence: results from a placebo-controlled randomised trial. Drug Alcohol Depend. 2020;215:108220.
Lus G, Cantello R, Danni MC, Rini A, Sarchielli P, Tassinari T, et al. Palatability and oral cavity tolerability of THC:CBD oromucosal spray and possible improvement measures in multiple sclerosis patients with resistant spasticity: a pilot study. Neurodegener Dis Manag. 2018;8(2):105–13.
Marinelli L, Balestrino M, Mori L, Puce L, Rosa GM, Giorello L, et al. A randomised controlled cross-over double-blind pilot study protocol on THC:CBD oromucosal spray efficacy as an add-on therapy for post-stroke spasticity. BMJ Open. 2017;7(9):e016843.
Meuth SG, Henze T, Essner U, Trompke C, Vila Silván C. Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: Consistency of response across subgroups from the SAVANT randomized clinical trial. Vol. 130, International Journal of Neuroscience. Meuth, Sven G.: Department of Neurology, Institute of Translational Neurology, University Hospital Munster, Albert-Schweitzer-Campus 1, Gebaude A1, Munster, Germany, D-48149, sven.meuth@ukmuenster.de: Taylor & Francis; 2020. p. 1199–205.
Naftali T, Bar-Lev Schleider L, Almog S, Meiri D, Konikoff FM. Oral CBD-rich cannabis induces clinical but not endoscopic response in patients with Crohn’s disease, a randomized controlled trial. J Crohns Colitis. 2021;15(11):1799–806.
Schoedel KA, Chen N, Hilliard A, White L, Stott C, Russo E, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the subjective abuse potential and cognitive effects of nabiximols oromucosal spray in subjects with a history of recreational cannabis use. Hum Psychopharmacol. 2011;26(3):224–36.
Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS One. 2021;16(3 March):e0246990.
Trigo JM, Soliman A, Staios G, Quilty L, Fischer B, George TP, et al. Sativex associated with behavioral-relapse prevention strategy as treatment for cannabis dependence: a case series. Vol. 10, Journal of Addiction Medicine. Le Foll, Bernard: Centre for Addiction and Mental Health, ON, Canada, bernard.lefoll@camh.ca: Lippincott Williams & Wilkins; 2016. p. 274–9.
Van De Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, Van Velzen M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain. 2019;160(4):860–9.
Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006;12(5):639–45.
Heinz A, Kiefer F, Smolka MN, Endrass T, Beste C, Beck A, et al. Addiction Research Consortium: losing and regaining control over drug intake (ReCoDe)-From trajectories to mechanisms and interventions. Addict Biol. 2020;25(2):e12866.
Acknowledgements
We are thankful to Hans Bomhard who was involved in all steps of assessment of search results and full-text articles. Rick Bernardi did a final English editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. Financial support for this work was provided by the Bundesministerium für Bildung und Forschung (BMBF) funded SysMedSUDs consortium (FKZ: 01ZX1909A), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 402170461 – TRR 265 [264] and SFB1158 (B04). The funding bodies had no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study concept and design, acquisition, analysis or interpretation of data and drafting of and intellectual input the manuscript. All author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Methodological details.
Additional file 2.
Abbreviations and characteristics of excluded and included studies.
Additional file 3.
Risk of bias assessments of included studies.
Additional file 4.
Forest-plot for primary outcomes.
Additional file 5.
Meta-regression analysis.
Additional file 6.
Forest-plot for secondary outcomes: retention and adverse events.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bilbao, A., Spanagel, R. Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med 20, 259 (2022). https://doi.org/10.1186/s12916-022-02459-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02459-1