Abstract
Background
Mangosteen, Garciniam angostana L., is a juicy fruit commonly found in Thailand. The rinds of Garciniam angostana L.have been used as a traditional medicine for the treatment of trauma, diarrhea and skin infection. It is also used in dermatological product such as in cosmetics. The mangosteen pericarp can be used to extract valuable bioactive xanthone compounds such as α-mangostin and gartanin. This study is aimed to predict the metabolism of α-mangostin and gartanin using in silico and in vitro skin permeation strategies.
Methods
Based on their 2D molecular structures, metabolites of those compounds were predicted in silico using ADMET Predictor™. The Km and Vmax, for 5 important recombinant CYP isozymes 1A2, 2C9, 2C19, 2D6 and 3A4 were predicted. Moreover, the in vitro investigation of metabolites produced during skin permeation using human epidermal keratinocyte cells, neonatal (HEKn cells) was performed by LC-MS/MS.
Results
It was found that the results derived from in silico were in excellent alignment with those obtained from in vitro studies for both compounds. The prediction referred that gartanin and α-mangostin were the substrate of CYP1A2, 2C9, 2C19 and 3A. In the investigation of α-mangostin metabolites by LC-MS/MS system, the MW of the parent compound was increased from 411.200 to 459.185 Da. Therefore, α-mangostin might be metabolized via tri-oxidation process. The increased molecular weight of parent compound (397.200 to 477.157 Da) illustrated that gartanin might be conjugated to sulfated derivatives.
Conclusions
In all the studies, α-mangostin and gartanin were predicted to be.
metabolized via phase I and phase II metabolism (sulfation), respectively.
Similar content being viewed by others
Background
Mangosteen (Garcinia mangostana L.) is a popular tropical fruit that grows mainly in Southeast Asia and other tropical areas. In Thailand, it has been used as a traditional medicine for the treatment of trauma, diarrhea and skin infections [1]. It is used in dermatological products such as in cosmetics. Mangosteen contains two major bioactive xanthone compounds, α-and γ-mangostins, that are major bioactive compounds. The biological activities of xanthone compounds are antimicrobial activity against methicillin-resistant Staphylococcus aureus [2], inhibition of oxidative damage by human low-density lipoprotein (LDL) [3] and weak antioxidant activity [4]. Skin is a major interface between the environment and the body. Previous studies revealed that P-gp has a physiologic function during the migration of dendritic cells from skin via lymphatic vessels [5] and that keratinocytes show a high expression of CYP enzymes, such as CYP2B19 [6]. Uptake of xenobiotics into normal human epidermal keratinocytes (HEKn) and subsequent excretion of these substances has long been thought to be a process of passive diffusion. Other studies by Abels et al. [7] showed that the cellular uptake of indocyanine green in HaCaT keratinocytes is inhibited by bromosulfophthalein indicating the possible involvement of an organic anion transporting polypeptide (OATP) in the active uptake of organic cations like indocyanine green. Also, normal human epidermal keratinocytes have been shown to express a cell-type-specific pattern of extrahepatic cytochrome P450 enzymes and efflux transport proteins showing that these cells metabolize and excrete a variety of xenobiotics [8]. Cultured skin alternatives have appeared during the last decade, and there is an increasing demand for good cell culture models that can be used in pharmaceutical and toxicological studies. Cultured skin models can remain viable for long period in vitro experiments, as long as culture conditions are maintained. This allows more opportunities to perform more elaborate experiments [9]. In silico research, in which mathematical models of a physiologic or pharmacologic system are developed and tested on a computer, are a hybrid of in vivo and in vitro techniques. This techniques, thus, offer the clinician-scientist the opportunity to answer questions that, for a variety of reasons, could not otherwise be easily addressed [10]. Many conferences have included sessions that focus on the in silico prediction of ADME properties and toxicity, highlighting the interest in moving these approaches to a point at which they can be used routinely in the drug-discovery process [11,12,13]. The application of in silico modelling and simulation within drug development is rapidly increasing in the R&D sector of the pharmaceutical industry [14]. The in silico approach can be used from ADMET property predication to clinical trial simulation. It was used to predict the drug-like properties of Chinese herbal compounds include the virtual screening method, pharmacophore model method and machine learning method [15]. For a metabolism prediction, in silico tools are most commonly used for predicting substrates and inhibitors of metabolic enzymes, sites of metabolism, and the structures of probable metabolites. ADMET predictor™ was used to predict of CYP isoforms potentially involved in phytocannabinoid metabolism. The software correctly predicted several of the many metabolites reported in the literature for the main phytocannabinoids [16].
Accordingly, the purpose of this study was to predict the metabolite of α-mangostin and gartanin for skin permeation and characterize the metabolism of α-mangostin and gartanin in human epidermal keratinocyte cells, neonatal (HEKn cells). The concentrations of the xanthone compounds were determined by LC-MS/MS and the metabolite of two xanthones compounds were predicted by in silico method using ADMET Predictor™ program.
Method
Plant material
The G. mangostana is a common tropical fruit consumed by household in Thailand. The hull of G. mangostana was collected in June 2011 at Chumporn Province, Southern part of Thailand. Botanical identification was achieved through comparison with a voucher specimen of Assoc. Prof. Dr. Pichaet Wiriyachitra, which was deposited in the herbarium of Department of Biology, Prince of Songkla University.
Extraction and Isolations.
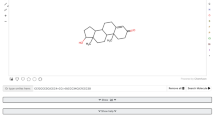
The crude CH2Cl2 extract (260 mg) of the hulls of G. mangostana was subjected to quick column chromatography (QCC) on silica gel (Merck 60 F254) using hexane as a first eluent and then increasing the polarity with Acetone to give 21 fractions (F1-F21). Fraction F3 was further separated by column chromatography (CC) eluting with a gradient of EtOAc-hexane to give 39 subfractions (F31-F339). Subfraction F35 was purified by LH20 eluting with 25% H2O-MeOH to afford 9 subfractions (F35A-F35I) and gartanin (5.2 mg). Fraction F8 was further separated by CC eluting with a gradient of EtOAc-hexane to give 16 subfractions (F81-F816) and α-mangostin (336.3 mg) (Fig. 1) [17, 18].
Compound (1): Gartanin. Yellow powder, m.p. 168–170 °C; 1H NMR 300 MHz (CDCl3) δ (ppm): 12.29 (1H, s, 1-OH), 11.20 (1H, s, 8-OH), 7.17 (1H, d, J = 9.0 Hz, H-6), 6.60 (1H, d, J = 9.0 Hz, H-7), 6.54 (1H, s, 3-OH), 5.18 (1H, m, H-2′), 3.40 (2H, d, J = 6.9 Hz, H-1′), 1.72 (1H, s, H-4′), 1.51 (1H, s, H-5′), 5.20 (1H, m, H-2′′), 3.46 (2H, d, J = 6.9 Hz, H-1′′), 1.79 (1H, s, H-4′′), 1.69 (1H, s, H-5′′).
Compound (2): α-mangostin. Deep-yellow powder, m.p. 180–182 °C; UV-Vis (CHCl3) λmax (log ε) 243 (3.90), 289 (4.10), 298 (4.13), 320 (3.68), 351 (3.47), 391 (3.33) nm; FT-IR (neat) νmax 3397, 1649, 1613 cm− 1. 1H NMR 300 MHz (CDCl3) δ (ppm): 13.77 (1H, s, 1-OH), 6.29 (1H, s, H-4), 6.82 (1H, s, H-5), 3.45 (2H, d, J = 7.2 Hz, H-1′), 5.29 (1H, m, H-2′), 1.77 (1H, s, H-4′), 1.84 (1H, s, H-5′), 4.09 (2H, d, J = 6.0 Hz, H-1′′), 5.26 (1H, m, H-2′′), 1.69 (3H, s, H-4′′), 1.84 (3H, s, H-5′′), 3.81 (3H, s, 7-OCH3).
In vitro study
Primary cell culture
Human epidermal keratinocyte (neonatal) (HEKn) (InvitrogenTM, Cat# C-001-5C Lot#1654391, Oregon, USA) cells were grown in T-25 flask at 37 °C in an atmosphere of 5% CO2. The adherent keratinocytes were cultured in low calcium (0.09 mM), serum-free, Epilife® medium, and supplement with Human Keratinocyte Growth Supplement (HKGS) at 1% v/v concentration. When a 500 ml of Epilife® medium was supplemented with HKGS the final concentration of the components in the supplement medium were: bovine pituitary extract (BPE) 0.2% v/v, bovine insulin 5 μg/ml, hydrocortisone 0.18 μg/ml, bovine transferrin 5 μg/ml and human epidermal growth factor 0.2 ng/ml. The medium was replaced regularly three times a week until the flask reaches 90% confluence (Fig. 2). The cells were moved from the flasks by incubating the monolayers with 0.5% trypsin for 2–3 min at 37 °C. The cells were collected into centrifuge tubes, and then centrifuged at 1000 rpm for 4 min and the pellets were resuspended in Epilife® medium. Cells used for this study were in third and fourth passage in late sub confluency.
Culturing of HEKn cells on permeable supports
HEKns cells were seeded at a density of 1 × 105 cells/cm2 on permeable supports (Transwell® Costar, 0.4 μm pore size) as shown in Fig. 3. The complete media were hanged every other day. Before the experiments, the cells were washed with D-PBS. Then, the xanthone compounds dissolved in DMSO were added into the cell cultures. DMSO (0.1%) treatment was used as control. The integrity of the HEKns cell monolayer in the transwell plates was determined by measuring Trans Epithelial Electrical Resistance (TEER). Acceptable TEER value is > 400 Ω •.cm2 by using Millicell ERS-2 Voltohmmeter (Sigma- Aldrich, Germany). Moreover, the mannitol was used as a marker compound for paracellular transport permeation. The cells were incubated at 37 °C at eight time points (i.e. on days 1,3, 5, 7, 10, 14, 20, and 21).
In silico study (in silico prediction)
ADMET Predictor™ version 8, formerly known as QMPRPlus™, a state-of-the-art computer program, was used to estimate certain ADMET properties of gartanin and α-mangostin from their molecular structures. The program required 2D or 3D molecular structure information, parses the structure and calculates the values of molecular descriptors. The program uses the molecular descriptor values as inputs to independent mathematical models (generally, nonlinear machine learning techniques) in order to generate estimates for each of the ADMET properties. It provided a human CYP450 enzyme kinetic parameters including Km and Vmax models for five important recombinant CYP isozymes which were 1A2, 2C9, 2C19, 2D6, and 3A4 and qualitative assessment of the molecules as human CYP450 substrate and inhibition was predicted.
Liquid chromatography-mass spectrometry
LC-MS/MS analysis
The LC-MS/MS system consisted of a Q-Trap 5500 triple quadrupole/ion trap mass spectrometer (ABSCIEX™, USA) equipped with a Turbo Spray ion source operated at 350 °C. The pMRM–IDA-EPI (Predicted Multiple reaction monitoring mode – Information dependent acquisition - Enhanced production scan) method used pMRM (predicted MRM) as a survey scan. The information-dependent acquisition (IDA) method was employed to trigger the enhanced product ion (EPI) scans by analyzing MRM signals. A total of 66 MRM transitions were used as the survey experiment in positive mode. The system included an Agilent 1200 HPLC pump, degasser, auto sampler and column heater (Agilent, USA). The in house developed and validated HPLC separation was performed using a 50 × 2.1 mm, Luna 5 μm NH2 100 Å column (Agilent, USA) operated at a flow rate of 1000 μl/min. A mobile phase system consisted of (A) water and (B) acetonitrile and was used with the following gradient: 30% B for 0.75 min, 30 to 90% B from 0.75 to 7 min, and finally 30% B isocratic from 7 to 12 min.
Results
Metabolism of gartanin and α-mangostin
Two xanthones with different substitutions were investigated on a LC-MS/MS system. Total 10 processes were run in positive scan mode. There were oxidation (+ 15.995 Da), di-oxidation (+ 31.990 Da), tri-oxidation (+ 47.985 Da), hydrogenation (+ 2.061 Da), sulfation (+ 79.957 Da), methylation (+ 14.016 Da), di-methylation (+ 28.032 Da), glucuronidation (+ 176.034 Da), de-methylation (− 14.016 Da) and de-hydrogenation (− 2.016 Da). Common features were displayed that gartanin and α-mangostin exhibited gain of small neutral molecules from the precursors. These were helpful to summarize the mechanism of two xanthone compounds. The increased molecular weight (MW) of parent compounds (397.200 to 477.157 Da) illustrated that gartanin might be conjugated to sulfated derivatives. The conjugated gartanin could permeate from apical to basolateral side and vice versa as showed in Fig. 4 and there were almost stable at the intensity approximately 8.00E+ 3 cps. This indicated that the conjugated gartanin could not have the ability to attach the efflux transporters as the parent compounds. Thus, the metabolite accumulated in both apical and basolateral side of the cells. For α-mangostin, the MW of parent compound was increased from 411.200 to 459.185 Da. Therefore, α-mangostin might be metabolized via tri-oxidation process. In addition, the intensity was increased within 30 min then constant in both absorptive and secretory directions and the intensity were equal in apical and basolateral side (Fig. 5). The transport of gartanin and α-mangostin in both parent compounds and metabolites were illustrated in Fig. 6-7.
In silico prediction by ADMET predictor™
The program obtained 2D molecular structure information and then parsed the structure and calculated the values of molecular descriptors.
Simulation of apparent permeability coefficient (Papp)
The simulations plus S + MDCK model predicted Papp measured using the MDCK (Madin-Darby Canine Kidney) cells-on-sheet (COS) technology. The calculation informed that Papp of gartanin and α-mangostin were 52.62 and 93.51 nm/sec, respectively. When Papp was compared to the Papp from the previous experiments [16] Papp, A-B of gartanin (9 μM) could not be detected and Papp, A-B of α-mangostin was lower than predicted 3-fold. These could be concluded that these two compounds might be permeated through the intestinal better than skin when using each compound alone.
In silico prediction of drug metabolism
ADMET Predictor™ provided a human CYP450 enzyme kinetic models including Km and Vmax models for five important recombinant CYP isozymes which were 1A2, 2C9, 2C19, 2D6, and 3A4. By using the 2D molecular structure of gartanin and α-mangostin, qualitative assessment of a molecule being the human CYP450 substrate and inhibition were shown in Table 1. The gartanin and α-mangostin were predicted to be a substrate of CYP1A2, 2C9, 2C19 and 3A4. The intrinsic clearance for each CYP isozymes of both molecules were also estimated by Vmax/Km and listed in Table 1. As a substrate for the CYP isozymes, the CLint per nmoL of P450 for CYP2C9 is highest for both α-mangostin and gartanin. Both α-mangostin and gartanin had the inhibitory properties against 1A2, 2C9, 2D6 and 3A4. The Km of α-mangostin were predicted to be 6.740 and 0.556 μM for CYP1A2 and CYP2C9, respectively. The possible atomic sites for metabolism were illustrated in Figs. 8. The scores range from 0 to 1 with higher scores indicating a greater propensity of being a metabolic site. The possible chemical structures of phase I oxidation for α-mangostin were proposed in Fig. 9. By the way, the metabolite of gartanin was not found to be metabolized in phase I metabolism in LC/MS/MS identification although the prediction from the program was identified.
Discussion
In the present study, the possible involvement of the active transport system of two compounds (gartanin and α-mangostin) was demonstrated. The bi-directional studies were conducted across the HEKn cells. Mannitol was used as the marker compound to compare and clarify the permeation of the test compounds.
The result of in silico method showed that the Papp from the experiments, Papp, A-B of gartanin (9 μM) could not be detected and Papp, A-B of α-mangostin was approximately lower than predicted 3-fold. The two compounds have more ability that can permeate through the intestinal better than skin when using each compound alone. It is possible pathway that α-mangostin may act as a co-effector to improve the bioavailability of gartanin [19]. Then, the ADMET Predictor™ provided a human CYP450 enzyme kinetic models including Km and Vmax models for five important recombinant CYP isozymes which were 1A2, 2C9, 2C19, 2D6, and 3A4. The gartanin and α-mangostin were predicted to be the substrate of CYP1A2, 2C9, 2C19 and 3A4 and had the inhibitory properties against 1A2, 2C9, 2D6 and 3A4. Km of α-mangostin on CYP1A2 was higher than a CYP2C9. Our results of the prediction were similar to previously studies by Foti et al. [20], which they reported that α-mangostin was metabolized primarily by CYP1A2 and was potent inhibited the CYP2C family of enzymes.
Moreover, based on the investigation of metabolites by LC-MS/MS system, mangostin might be metabolized via phase I metabolism (tri-oxidation reaction) which correlated to the results from the prediction. This might be due to the appropriate structure of mangostin that eases the oxidation. However, gartanin was found to be metabolized via phase II sulfation by LC-MS/MS identification, the results of which contradicted those from the in silico prediction. Although, based on its structure, gartanin could probably be metabolized via phase I oxidation, the level of metabolites might be lower than the detection limit of LC-MS/MS.
Conclusion
Due to the increase in using Garciniam angostana L. as a complimentary medicine and cosmetics in dermatological products. Skin is the barrier for the penetration of the xanthones active compounds including α-mangostin and gartanin in the extract. The comprehension of permeability and skin first pass metabolism can help determine the bioavailability of these compounds. The application of in silico modelling and simulation within drug development is rapidly increasing in the R&D sector of the pharmaceutical industry and it is increasing possibility of using them in natural product research. Thus, LC-MC/MS and ADMET predictor™ were used and compared for prediction of possibility of cutaneous metabolism of these two compounds. The investigation of the metabolites by a LC-MS/MS system found that α-mangostin might be metabolized via phase I metabolism (tri-oxidation reaction) which correlated to the result from the prediction that from the program. For gartanin, it was metabolized via phase II metabolism in sulfation process. Although, many computer modeling methods of metabolic pathways have already been developed, they are not perfect. Similar to our findings, the in silico prediction and the in vitro cell culture study may not demonstrate perfect matching. We need more investigational efforts which are continuously developed in our laboratory. More efficient and reliable in silico methods are essential to motivate more studies in the field in the near future and they can help reduce the cost for complimentary medicine development.
Abbreviations
- BPE:
-
Bovine pituitary extract
- cells/cm2 :
-
Cell per square centimeter
- COS:
-
Cells-on-sheet
- cps:
-
Count Per Second
- Da:
-
Dalton
- DMSO:
-
Dimethyl sulfoxide
- HEKn:
-
Human epidermal keratinocyte (neonatal)
- HKGS:
-
Human Keratinocyte Growth Supplement
- HPLC:
-
High Performance Liquid Chromatography
- Km :
-
Michaelis-Menten constant
- LC-MS/MS:
-
Liquid Chromatograph-Mass Spectrometer
- LDL:
-
Low-density lipoprotein
- MDCK:
-
Madin-Darby Canine Kidney
- ml:
-
Milliliter
- mM:
-
Millimolar
- MW:
-
Molecular weight
- ng/ml:
-
Nanogram per milliliter
- nm/sec:
-
Nanometer per second
- Papp :
-
Permeability coefficient
- Papp, A-B :
-
Permeability coefficient from apical side to Basolateral side
- Vmax :
-
Maximum metabolic capacity
- % v/v:
-
Percentage of volume by volume
- μg/ml:
-
Microgram per milliliter
- μm:
-
Micrometer
References
Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46(2):688–93.
Iinuma M, Tosa H, Tanaka T, Asai F, Kobayashi Y, Shimano R, et al. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol. 1996;48(8):861–5.
Iikubo K, Ishikawa Y, Ando N, Umezawa K, Nishiyama S. The first direct synthesis of α-mangostin, a potent inhibitor of the acidic sphingomyelinase. Tetrahedron Lett. 2002;43(2):291–3.
Suvarnakuta P, Chaweerungrat C, Devahastin S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011;125(1):240–7.
Randolph GJBS, Pope M, Sugawara I, Hoffman L, Steinman RM, et al. A physiologic function for p-glycoprotein (MDR-1) during migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad USA. 1998;95:6924–9.
Keeney DSSC, Travers JB, Capdevila JH, Nanney LB, King LE, et al. Differentiating keratinocytes express a novel cytochrome P450 enzyme, CYP2B19, having arachidonate monooxygenase activity. J Biol Chem. 1998a;273:32071–9.
Abels CFS, Weiderer P. BaumlerW, Hofstadter F, Landthaler M et al. Indocyanine green (ICG) and laser irradiation induce photooxidation. Arch Dermatol Res. 2000;29:404–11.
Barbotteau YGE, Barberet P, Cappadoro M, Wever BD, Habchi C, et al. Reconstructed human epidermis: a model to study the barrier function. Nuclear Instruments and Methods in Physics Research. 2005;231:286–91.
Pappinena STS, Seppanen SP, Murtomaki L, Suhonena M, Urtti A. Rat epidermal keratinocyte organotypic culture (ROC) compared to human cadaver skin: The effect of skin permeation enhancer. Eur J Pharm Sci. 2007;30:240–50.
Colquitt RBCD, Thiele RH. In silico modelling of physiologic systems. Best Pract Res Clin Anaesthesiol. 2011;25:499–510.
Clark DE GP. Progress in computational methods for the prediction of ADMET properties. Curr Opin Drug Discov Dev 2002;5:382–390.
Butina DSM, Frankcombe K. Predicting ADME properties in silico: methods and models. Drug Discov Today. 2002;7:S83–8.
Ekins SWC, Swaan PW, Cruciani G, Wrighton SA, Wikel JH. Progress in predicting human ADME parameters in silico. J Pharmacol Toxicol Methods. 2000;44(1):251–72.
Duque MD, Issa MG, Silva DA, Barbosa EJ, Löbenberg R, Ferraz HG. In silico simulation of dissolution profiles for development of extended-release doxazosin tablets. Dissolution Technologies. 2018;25(4):14–21.
Han K, Zhang L, Wang M, Zhang R, Wang C, Zhang C. Prediction methods of herbal compounds in Chinese medicinal herbs. Molecules. 2018;23(9):2303.
Amaral Silva D, Pate DW, Clark RD, Davies NM, El-Kadi AOS, Löbenberg R. Phytocannabinoid drug-drug interactions and their clinical implications. Pharmacol Ther. 2020;215:107621.
Chairungsrilerd N, Takeuchi K, Ohizumi Y, Nozoe S, Ohta T. Mangostanol, a prenyl xanthone from Garcinia mangostana. Phytochemistry. 1996;43(5):1099–102.
Mahabusarakam W, Wirichitra P, Phongpaichit S. Antimicrobial activities of chemical constituents from Garcinia mangostana. J Sci Soc Thail. 1986;12:239–42.
Rukthong P, Sereesongsang N, Kulsirirat T, Nimprayoon B, Sathirakul K. Effect of α-mangostin on enhanced transdermal bioavailability of Gartanin via efflux transporters. JBB. 2017;9(4):455–62.
Foti RS, Pearson JT, Rock DA, Wahlstrom JL, Wienkers LC. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab Dispos. 2009;37(9):1848–55.
Acknowledgements
Special thanks for Prof. Yuichi Sugiyama, Assoc. Prof. Maeda Kazuya and Assoc. Prof. Plemchitt Rojanapanthu and Assoc. Prof. Chonlaphat Sukasem for all of comment and good suggestion. We also benefited by outstanding works from Mr. Takano’s help with his skill in handling precisely delicate equipment and technique. We would like to special thanks for all of staff in Department of Molecular Pharmacokinetics, Faculty of Pharmaceutical Science at Tokyo University and all staff in Faculty of Pharmacy at Mahidol University for their helpful in providing facilities and materials for our experiment.
Funding
The authors gratefully acknowledge research grant from Commission on Higher Education (Staff Development Project), Thailand for financial support. N.B. thanks IPST for research funds for DPST graduate with first replacement (No.03/2557).
Author information
Authors and Affiliations
Contributions
RP, SN, KT and BN carried out the experiment. A.B. and D.E. RP and SK analysed the data wrote the manuscript in consultation with BN and SK. SK helped supervise the project and conceived the original idea. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
“The authors declare that they have no competing interests.”
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rukthong, P., Sereesongsang, N., Kulsirirat, T. et al. In vitro investigation of metabolic fate of α-mangostin and gartanin via skin permeation by LC-MS/MS and in silico evaluation of the metabolites by ADMET predictor™. BMC Complement Med Ther 20, 359 (2020). https://doi.org/10.1186/s12906-020-03144-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-020-03144-7








 , gartanin in absorptive direction;
, gartanin in absorptive direction;  , conjugated gartanin in absorptive direction;
, conjugated gartanin in absorptive direction;  , gartanin in secretory direction;
, gartanin in secretory direction;  , conjugated gartanin in secretory direction)
, conjugated gartanin in secretory direction)
 , α-mangostin in absorptive direction;
, α-mangostin in absorptive direction;  , oxidized α-mangostin in absorptive direction;
, oxidized α-mangostin in absorptive direction;  , α-mangostin in secretory direction;
, α-mangostin in secretory direction;  , oxidized α-mangostin in secretory direction)
, oxidized α-mangostin in secretory direction)


