Abstract
Background
Sedation and/or analgesia can relieve the patient-ventilator asynchrony. However, whether sedation and/or analgesia can benefit the clinical outcome of the patients with interface intolerance is still unclear.
Methods
A retrospective study was performed on patients with interface intolerance who received noninvasive positive pressure ventilation (NIPPV) after extubation in seven intensive care units (ICU) of West China Hospital, Sichuan University. The primary outcome was rate of NIPPV failure (defined as need for reintubation and mechanical ventilation); Secondary outcomes were hospital mortality rate and length of ICU stay after extubation.
Results
A total of 80 patients with oral-nasal mask (90%) and facial mask (10%) were included in the analysis. 41 out of 80 patients received sedation and/or analgesia treatment (17 used analgesia, 11 used sedation and 13 used both) at some time during NIPPV. They showed a decrease of NIPPV failure rate, (15% vs. 38%, P = 0.015; adjusted odd ratio [OR] 0.29, 95% confidence interval [CI] 0.10–0.86, P = 0.025), mortality rate (7% vs. 33%, P = 0.004; adjusted OR 0.14, 95% CI 0.03–0.60, P = 0.008), and the length of ICU stay after extubation.
Conclusion
This clinical study suggests that sedation and/or analgesia treatment can decrease the rate of NIPPV failure, hospital mortality rate and ICU LOS in patients with interface intolerance after extubution during NIPPV.
Similar content being viewed by others
Background
The early use of noninvasive positive pressure ventilation (NIPPV) can reduce the reintubation rates of patients after extubation for its role in decreasing the work of breathing and improving gas exchange [1,2,3,4,5,6,7]. However, the interface intolerance could result in patient-ventilator asynchrony [8, 9], which could also cause the discontinuation of NIPPV and thus lead to unplanned requirement for endotracheal intubation with a rate up to 9–22% [10].
Sedation and/or analgesia can release the discomfort of patients about NIPPV interface [11, 12]. Whether sedation and/or analgesia during NIPPV is safe and feasible has been assessed in several clinical trials but the results still remains unclear. In the review of Hilbert, the author held the view that although lack of evidence, the mask intolerance could be the ideal indication of sedation and/or analgesia and is clearly to avoid intubation [13]. On the contrary, Conti thought patients could not benefit from sedation and/or analgesia [14].
Therefore, the role of sedation and/or analgesia in reducing the rate of NIPPV failure in post extubation patients with interface intolerance is unclear.
Thus, we hypothesized that the use of sedation and/or analgesia could decrease the failure of NIPPV and a retrospective study was conducted to examine the effect of sedation and/or analgesia on post-extubation patients with interface intolerance.
Methods
Study design
Patients hospitalized between December 2014 to August 2016 at 7 intensive care units (ICUs) in West China Hospital, Sichuan University, China were studied in the clinical investigation. The study was approved by the Institutional Ethical Committee for Clinical and Biomedical Research of West China Hospital (Sichuan, China). Written informed consent was waived given that the investigation was a retrospective observational study and involved no therapeutic intervention.
All adult patients received NIPPV after extubation were screened (the weaning protocol could be seen in Additional file 1 and the inclusion criteria for patients used NIPPV directly after extubation could be seen in Additional file 2). Patients were eligible if they were recorded as interface intolerance (claimed by patients themselves) in the medical records and/or nursing records and received more than 2 h of NIPPV after extubation, or fulfiled at least one of the following criteria for using NIPPV directly after extubation: 1) Age older than 65 years; 2) Heart failure as the primary indication for mechanical ventilation; 3) Moderate to severe chronic obstructive pulmonary disease; 4) An Acute Physiology and Chronic Health Evaluation II(APACHE II) score higher than 12 on extubation day; 5) Body mass index of more than 30 (calculated as weight in kilograms divided by height in meters squared); 6) Airway patency problems, including high risk of developing laryngeal edema; 7) Inability to deal with respiratory secretions (inadequate cough reflex or suctioning > 2 times within 8 h before extubation); 8) Difficult or prolonged weaning, in brief, a patient failing the first attempt at disconnection from mechanical ventilation; 9) 2 or more comorbidities; 10) Mechanical ventilation for more than 7 days.
Patients with short duration of NIPPV were excluded to guard against reverse causality because these groups of patients were already discontinued from the NIPPV when sedation and/or analgesia plays a role.
Data extraction
We collected information on each patient’s age, gender, illness severity obtained at ICU admission and extubation manifested as the score of Acute Physiology And Chronic Health Evaluation (APACHEII), arterial blood gases prior to NIPPV started (half an hour before extubation), interface used to apply the NIPPV. After NIPPV treatment started, we recorded the following variables: interface for NIPPV, the history of NIPPV usage before mechanical ventilation, the maximum inspiratory positive airway pressure and expiratory positive airway pressure, respiratory rate, inspired fraction of oxygen, body temperature, heart rate, arterial blood gases and level of consciousness–sedation by Richmond Agitation Sedation Scale (RASS). At each change of ventilator settings, we registered the same variables and the administration of sedatives (dexmedetomidine, propofol) or analgesia (fentanyl, sufentanyl). In addition, the arterial blood gas was recorded at the beginning of the sedation and/or analgesia treatment and 4 h after the treatment began. During the study, all patients would be monitored until they were discharged from the hospital or dead. We obtained data of the rate of NIPPV failure, hospital mortality, length of ICU stay (ICU LOS) and rate of delirium after extubation. The failure of NIPPV was defined as the requirement for intubation and invasive mechanical ventilation (the standard of reintubation could be seen in Additional file 1).
Outcomes
The outcomes included the failure of NIPPV, the hospital mortality and ICU LOS after extubation.
Statistical analysis
Consecutive variables were reported as mean ± standard deviation or median (Inter-Quartile Range [IQR]), while categorical variables were reported as frequency and proportion. The student’s t, Mann-Whitney U-test and Kruskal-Wallis tests were used for comparisons between continuous variables and the Chi-squared test or Fisher’s exact test were for comparisons between categorical variables.
To assess the relationship between the use of sedation and/or analgesia with the rate of NIPPV failure and the hospital mortality, we structured a logistic regression model and the OR was adjusted by variables independently associated with failure of NIPPV or hospital mortality that had P value less than 0.10. Kaplan-Meier curves were analysed to assess the time from extubation to failure of NIPPV or death. In addition, the time was compared by means of the log-rank test.
All the analyses were performed with SPSS 19.0 (SPSS Inc) and 2-sided P-value less than 0.05 was considered statistically significant.
Results
Patients
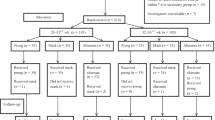
From December 2014 through August 2016, a total of 4913 patients were admitted to the 7 ICUs. Among them, 309 patients received NIPPV directly after extubation and 80 patients were recorded as interface intolerance in the medical records and/or nursing records (Fig. 1). Forty-one out of 80 patients (51%) received intravenous sedation and/or analgesia at any time during NIPPV: 17 used analgesia, 11 used sedation and 13 used both. The sedation drugs included propofol and dexmedetomidine, while the analgesia drugs included fentanyl and sufentanil. And 90% of the patients used oral-nasal mask while the other 10% used facial mask.
Characteristics at inclusion
The characterisitics of the patients at enrollment were shown in the Table 1 . The mean age and proportion of male patients in sedation-analgesia and non-sedoanalgesia groups were 68 vs.78 (P = 0.077) and 73% vs. 64% (P = 0.471), respectively. There was no significant difference in the score of APACHE II at ICU admission (17.95 ± 6.87 vs. 19.28 ± 6.49, P = 0.376) nor at the extubation (10.61 ± 3.89 vs. 10.97 ± 5.29, P = 0.725) as well as PaO2/FiO2 before extubation (230.43 vs. 260.59, oP = 0.059) between the two groups. We did not find significant difference in body temperature, respiratory rate, heart rate, blood pressure as well as the pH and PaCO2 before extubation.
Outcomes
Failure of NIPPV and NIPPV duration
Overall, 21 of 80 patients (26%) failed NIPPV. (Table 2) The characteristics of patients with failed and successed NIPPV were presented in Table 2. No difference was found between two groups. In the unadjusted analysis, sedation and/or analgesia was significant associated with failure of NIPPV (29% vs. 59%, P = 0.015). After adjusting for sex before extubation (Table 3), we found that sedation and/or analgesia can reduce the rate of NIPPV failure (OR 0.29, 95% CI 0.10–0.86, P = 0.025). In the log-rank test, significant differences also existed in median time from extubation to failure of NIPPV (P = 0.021) (Fig. 2). Moreover, NIPPV duration was also shorter in the group of sedoanalgesia (46.5 vs. 70 h, P = 0.041)(Table 4).
Hospital mortality
Table 5 presented the characteristics of the patients survived and dead. No difference was found about the basic characteristics between the two groups. Hospital mortality was lower in patients who received sedation and/or analgesia compared with those who did not (19% vs. 59%, P = 0.004). After adjustment for PaCO2 before extubation and half an hour after NIPPV initiate, diagnosis and SBP, the difference remained significantly (Table 3): OR 0.14, 95% CI 0.03–0.60 (P = 0.008). In the log-rank test, significant differences also existed in median time from extubation to death (P = 0.013) (Fig. 3).
ICU LOS after extubation
ICU LOS was shorter in patients who received sedation and/or analgesia vs. those who did not receive drugs (5 vs. 8 days, P = 0.030).
Moreover, the rate of delirium of sedoanalgesia group was not different from the one of non-sedoanalgesia group (29% vs. 18%, P = 0.234).
Discussion
In this retrospective study, patients with interface intolerance after extubation could benefit from sedation and/or analgesia during NIPPV treatment, resulting in the decrease of NIPPV failure rate, hospital mortality, and length of ICU stay after extubation. Our study showed that sedation and/or analgesia could reduce the rate of NIPPV failure and NIPPV duration. The mechanisms that explain the decrease of NIPPV failure rate and NIPPV duration have been reported as the followings. Firstly, the rate of delirium and NIPPV duration can be decreased when sedation and/or analgesia treatment applied because the treatment can relieve anxiety and stimulate sleep [15,16,17]. In addition, physiology responses to stress such as hypertension and tachycardia can also be modulated, which might facilitate NIPPV [18] and improve the respiratory status of patients. Secondly, sedation and/or analgesia could abate the uncomfortable feelings about NIPPV especially in terms of the interface intolerance, resulting in improved synchronizing between patients and ventilator, which could promote gas exchange [11, 12]. A study about 36 patients showed that patients who complained of discomfort and asked for interruption of NIPPV session had lower rate of failure when received remifentanil-based sedation protocol [19]. Moreover, although the potential effects of sedation and/or analgesia on depressing respiration and hypoxic drive were worried by many clinicians, physiological studies on the impacts of sedation and/or analgesia on ventilator response demonstrated that a continuous infusion of sedation and/or analgesia will not have significant influence on respiratory drive, respiratory pattern, minute volume, and blood gases [20,21,22]. In our study, we found that sedation and/or analgesia would not influence the oxygenation and that the OI of patients in the sedation and/or analgesia remained unchanged. On the contrary, the extraction of CO2 in patients can be facilitated by conducting sedoanalgesia, showed by the lower PaCO2 after the administration of the drug (51.33 ± 13.49 vs. 48.53 ± 13.54 mmHg, P = 0.019). Also, the study of Clouzeau et al. found a significant increase of oxygenation index in 10 acute respiratory failure (ARF) patients (from 167 ± 68 to 195 ± 68; P < 0.05) to verify the safety of sedation and analgesia [23]. A result from another study about 13 patients also support this point of view (PaCO2 decrease from 57.8 ± 15.3 to 49 ± 9.8 mmHg, P < 0.05) [11].
However, some other studies showed opposite results. They found that the use of sedation and/or analgesia may be related to the failure of NIPPV [24]. The different result might be caused by the discrepancy between patients enrolled in our study and theirs. In our study, only the patients with interface intolerance according to the records were enrolled in, while in other studies, the premise is uncertain. Therefore, the exact clinical aim of the sedation and/or analgesia might not be comforting patients or facilitating the NIPPV in those studies, but patients may suffer from the adverse events of sedation and/or analgesia, such as hypotension, arrhythmias, respiratory acidosis, and infections from contaminated vials or tubing [25]. In our study, NIPPV was applied after extubation, when patients received respiratory support mainly due to low ability of airway protection and cough strength [26]. On the contrary, when NIPPV was used as first line therapy in the other studies, the initial disease,which often had been released in the patients after extubation, was the primary problem of ARF. Further more, in our study, the RASS score of the patients enrolled in our study was controlled between −2 to 2, while the RASS score of a big proportion of patients in other studies were lower than −2. As we all know, excess sedation will bring numerous complications such as delirium, and result in increasing mortality [27].
We also found the management of sedation and/or analgesia could reduce the hospital mortality and ICU LOS after extubation. This may mainly due to the lower rate of NIPPV failure in the patients received sedation and/or analgesia [28,29,30]. Studies showed that a lot of the adverse events caused by the reintubation were related to invasive mechanical ventilation that would multiple the rate of mortality such as ventilator associated pneumonia (VAP), ventilator-associated lung injury and barotrauma [31,32,33].
A recent study showed that VAP would affect approximately 10% of the ventilated patients [34]. It was a risk factor for ICU mortality (OR 2.20; 95%CI 1.91–2.54) [35] and prolonged the length of hospital stay compared with patients without VAP (22.7 ± 2.9 vs. 16.8 ± 2.9; P < 0.0006) [36]. Moreover, Studies showed that the rate of barotrauma induced by invasive mechanical ventilation can be up to 50% and the mortality of patients with barotrauma also increased (51.4 vs 39.2%; P = 0.04) as well as the ICU LOS (14 ± 13.6 vs. 10.9 ± 11.4; P = 0.04) [37].
Moreover, our study also showed that sedoanalgesia could better improve the prognosis of patients with hypercapnia than the ones only with hypoxemia. This might because that NIPPV plays different role in different occasions and different diseases [38]. For example, the advantages of NIPPV used in patients with AECOPD has been recognized already, but whether NIPPV can improve the clinical outcomes in patients with asthma remains debated [39, 40].
Our study has several limitations. The small sample size of this study did not allow us to divide patients into sedation, analgesia, sedation and/or analgesia, and non sedoanalgesia groups. Moreover, in the clinical practice, it is difficult to distinguish the potential role of the two kinds of drugs because both of the sedative and analgesia drugs had the underlying function of respiratory inhibition and can have significant impact on moderating the intolerance. Moreover, because of shortcomings of retrospective study, we could not unify the criterion of the drug dose and the duration of administration. Also, other potential factors contribute to the clinical outcomes such as the ability of airway protection and cough strength also were unable to measure.
Conclusion
The use of sedation and/or analgesia could improve the survival rate among patients with interface intolerance after extubation and decrease the rate of NIPPV failure and ICU LOS, especially in patients with hypercapnia. Therefore, under the 24 h monitoring in the ICU, sedation and/analgesia is safe and effective in patients with interface intolerance after extubation. However, more large randomized controlled trials are still needed to verify the result.
Abbreviations
- APACHE:
-
The Acute Physiologic and Chronic Health Evaluation
- CI:
-
Confidence interval
- FiO2 :
-
Fraction of inspired oxygen
- GOLD:
-
The Global Initiative for Chronic Obstructive Lung Disease
- ICU:
-
Intensive care unit
- LOS:
-
Length of stay
- MV:
-
Mechanical ventilation
- NIPPV:
-
Noninvasive positive pressure ventilation
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- PaO2 :
-
Arterial partial pressure of oxygen
- RASS:
-
Richmond agitation sedation scale
- SD:
-
Standard derivation
References
Antonelli M, Bello G. Noninvasive mechanical ventilation during the weaning process: facilitative, curative, or preventive? Crit Care. 2008;12(2):136.
Lin C, Yu H, Fan H, Li Z. The efficacy of noninvasive ventilation in managing postextubation respiratory failure: a meta-analysis. Heart Lung. 2014 Mar-Apr;43(2):99–104.
Hess DR. The role of noninvasive ventilation in the ventilator discontinuation process. Respir Care 2012 Oct;57(10):1619–1625. Review.
Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, Beltrame F, Navalesi P. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005 Nov;33(11):2465–70.
Su CL, Chiang LL, Yang SH, Lin HI, Cheng KC, Huang YC, Wu CP. Preventive use of noninvasive ventilation after extubation: a prospective, multicenter randomized controlled trial. Respir Care. 2012 Feb;57(2):204–10.
Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, Epstein SK, Hill NS, Nava S, Soares MA, D'Empaire G, Alía I, Anzueto A. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004 Jun 10;350(24):2452–60.
Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006 Jan 15;173(2):164–70.
Kacmarek RM. NIPPV: patient-ventilator synchrony, the difference between success and failure? Intensive Care Med. 1999 Jul;25(7):645–7.
Vignaux L, Vargas F, Roeseler J, et al. Patient-ventilator asynchrony during non-invasive ventilation foracute respiratory failure: a multicenter study. Intensive Care Med. 2009 May;35(5):840–6.
Hilbert G, Clouzeau B, Nam Bui H, et al. Sedation during non-invasive ventilation. Minerva Anestesiol. 2012 Jul;78(7):842–6.
Constantin JM, Schneider E, Cayot-Constantin S, et al. Remifentanil-based sedation to treat noninvasive ventilation failure: apreliminary study. Intensive Care Med. 2007 Jan;33(1):82–7.
Senoglu N, Oksuz H, Dogan Z, et al. Sedation during noninvasive mechanical ventilation with dexmedetomidine or midazolam: a randomized, double-blind, prospective study. Curr Ther Res Clin Exp. 2010 Jun;71(3):141–53.
Hilbert G, Navalesi P, Girault C. Is sedation safe and beneficial in patients receiving NIV? Yes Intensive Care Med. 2015 Sep;41(9):1688–91.
Conti G, Hill NS, Nava S. Is sedation safe and beneficial in patients receiving NIV? No Intensive Care Med. 2015 Sep;41(9):1692–5.
O'Mahony R, Murthy L, Akunne A, et al. Synopsis of the National Institute for health and clinical excellence guideline for prevention of delirium. Ann Intern Med. 2011 Jun 7;154(11):746–51.
Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013 Mar 5;158(5):375–80.
Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010 Feb;38(2):477–85.
Triltsch AE, Welte M, von Homeyer P, et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002 May;30(5):1007–14.
Rocco M, Conti G, Alessandri E, et al. Rescue treatment for noninvasive ventilation failure due to interface intolerance with remifentanil analgosedation: a pilot study. Intensive Care Med. 2010 Dec;36(12):2060–5.
Conti G, Arcangeli A, Antonelli M, et al. Sedation with sufentanil in patients receiving pressure support ventilation has no effects on respiration: a pilot study. Can J Anaesth. 2004 May;51(5):494–9.13.
Cavaliere F, Antonelli M, Arcangeli A, et al. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Can J Anaesth. 2002 Dec;49(10):1088–94.14.
Bilgin H, Başaðan Moðol E, Bekar A, et al. A comparison of effects of alfentanil, fentanyl, and remifentanil on hemodynamic and respiratory parameters during stereotactic brain biopsy. J Neurosurg Anesthesiol. 2006 Jul;18(3):179–184. 15.
Clouzeau B, Bui HN, Vargas F, et al. Target-controlled infusion of propofol for sedation in patients with non-invasive ventilation failure due to low tolerance: a preliminary study.Intensive Care Med. 2010 Oct;36(10):1675–1680.21.
Muriel A, Peñuelas O, Frutos-Vivar F, et al. Impact of sedation and/or analgesia during noninvasive positive pressure ventilation on outcome: a marginal structural model causal analysis. Intensive Care Med. 2015 Sep;41(9):1586–600.
Bennett SN, McNeil MM, Bland LA, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med 1995 Jul 20;333(3):147–154.
Salam A, Tilluckdharry L, Amoateng-Adjepong Y, et al. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004 Jul;30(7):1334–9.
Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013 May;39(5):910–8.32.
Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006 Jan 15;173(2):164–70.
Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011 Dec;39(12):2612–8.
de Lassence A, Alberti C, Azoulay E, et al. Impact of unplanned extubation and reintubation after weaning on nosocomial pneumonia risk in the intensive care unit: a prospective multicenter study. Anesthesiology. 2002;97(1):148–56.
Antonelli M, Conti G, Bufi M, et al. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000 Jan 12;283(2):235–41.
Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002 Jan 16;287(3):345–55.
Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005 Jun;50(6):714–21.
Metersky ML, Wang Y, Klompas M, et al. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA. 2016 Nov;11
Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013 Aug;13(8):665–71.
Leone M, Bourgoin A, Giuly E, et al. Influence on outcome of ventilator-associated pneumonia in multiple trauma patients with head trauma treated with selected digestive decontamination. Crit Care Med. 2002 Aug;30(8):1741–6.
Anzueto A, Frutos-Vivar F, Esteban A, et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004 Apr;30(4):612–9.
Organized jointly by the American Thoracic Society, the European Respiratory Society, the European Society of Intensive Care Medicine, and the Société de Réanimation de Langue Française, and approved by ATS Board of Directors, December 2000. International Consensus Conferences in Intensive Care Medicine: noninvasive positive pressure ventilation in acute Respiratory failure. Am J Respir Crit Care Med. 2001 Jan;163(1):283–91.
Pallin M, Naughton MT. Noninvasive ventilation in acute asthma. J Crit Care. 2014 Aug;29(4):586–93.
Ferrer M, Sellarés J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009 Sep 26;374(9695):1082–8.
Acknowledgements
We thank Professor Dongtao Lin (College of Foreign Languages, Sichuan University), who is specialized in biomedical writing and editing, for copyediting this manuscript.
Funding
This study was partly supported by Sichuan Science and Technology Agency Grant (2014SZ0010) and the National Science Foundation of China (81600057).
Availability of data and materials
The data supporting our findings can be found by contacted us (liangzatg@126.com).
Author information
Authors and Affiliations
Contributions
Y-NN and TW designed the study, did the data acquisition, analysis and drafted the manuscript; He Yu collected data and helped to do the critical manuscript revision; B-ML and Z-AL designed the study and did the critical manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our study was approved by the Institutional Ethical Committee for Clinical and Biomedical Research of West China Hospital (Sichuan, China). Because this was a retrospective observational cohort study and included no therapeutic intervention, written informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Weaning protocol and standard of reintubation. (DOC 19 kb)
Additional file 2:
Inclusion criteria for patients used NIPPV directly after extubation. (DOC 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ni, YN., Wang, T., Yu, H. et al. The effect of sedation and/or analgesia as rescue treatment during noninvasive positive pressure ventilation in the patients with Interface intolerance after Extubation. BMC Pulm Med 17, 125 (2017). https://doi.org/10.1186/s12890-017-0469-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-017-0469-4