Abstract
Background
The symptom burden in people with HIV (PWH) is considerable. Nonetheless, the identification of a central symptom, or bridge symptom, among the myriad symptoms experienced by PWH remains unclear. This study seeks to establish networks of symptom experiences within different clusters and investigate the relationships and interconnectedness between these symptoms in PWH.
Methods
A multicenter, cross-sectional descriptive design was carried out in China over two periods: November 2021 to January 2022 and April 2022 to May 2022. A total of 711 PWH completed online questionnaires, providing information on demographics and the 27-item Self-Report Symptom Scale. The symptom network was analyzed using Network/Graph theory, allowing for the exploration of connections between physical, cognitive, and psychological symptoms. This analysis was based on data from a subset of 493 individuals out of the total 711 PWH.
Results
A total of 493 PWH who exhibited symptoms out of a total of 711 PWH were analyzed. The average number of symptoms reported was 5.367. The most prevalent symptom was sleep disturbance (37.98%). In the node centrality analysis, a cognitive symptom, ‘becoming confusing’, emerged as the most central symptom with significant values for node centrality (strength = 1.437, betweenness = 140.000, closeness = 0.003). Fever was identified as the bridge symptom with the highest bridge strength (0.547), bridge closeness (0.053), lower bridge betweenness (23.000), and bridge expectedinfluence (0.285). Overall, our network displayed good accuracy and stability.
Conclusion
Early identification and assessment of the central or bridge symptoms should be emphasized in clinical practice. According to the findings from network analysis, healthcare providers should proactively explore intervention strategies or bundle care to alleviate the burden of symptoms and enable anticipatory care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Since the widespread use of antiretroviral therapy (ART), people with HIV (PWH) have experienced a significant improvement in life expectancy, nearing that of the general population [1]. However, PWH often encounter a wide range of complex and interconnected symptoms throughout their battle against HIV, stemming from the infection itself, the side effects of ART, and coexisting health conditions [2]. Research indicates that PWH typically experience five or more symptoms concurrently in various countries such as the US [3], Vietnam [4], and China [3]. These symptoms can manifest as physical issues like fatigue, pain, and gastrointestinal disturbances, as well as psychological challenges, including depression, anxiety, and cognitive impairments [4]. The cumulative burden of these complex symptoms negatively affects the clinical outcomes, quality of life, and treatment adherence of PWH [5].
Modern medicine embraces the biopsychosocial model, which views biological, psychological, and social factors as interconnected aspects of an individual’s health [6]. This model acknowledges that physical symptoms can be influenced by psychological factors like stress, emotions, and cognition, while physical conditions can also impact mental well-being. Various studies have highlighted the bidirectional relationship between physical and psychological symptoms, underscoring the significance of a comprehensive healthcare approach [7,8,9]. Traditional Chinese medicine and modern medicine have explored the concept of integrated treatment of the body and mind [10, 11]. Prior research has indicated that symptoms in persons with health conditions are often interconnected and interact with one another rather than existing in isolation [12]. For instance, fatigue may be linked to sleep disturbances, depression, and pain [13, 14], intensifying the overall symptom burden. Using a network analysis approach, this study provides valuable insights into managing symptoms in individuals with health conditions. The network approach conceptualizes the complex network structure formed by the interconnection of all symptoms, where symptoms act as nodes and causal interactions between symptoms serve as connections. This interpretation of the network approach by creating a network of symptoms and identifying those with high centrality, we can pinpoint the most impactful symptoms and determine how central symptoms affect others, suggesting that if an intervention alters the state of one symptom, it can affect the probability distribution of other symptoms [15]. In turn, bridge symptoms are broadly defined as symptoms linking various symptom clusters or subgroups within a cluster [16]. They are considered important treatment targets because deactivating these symptoms might prevent the development of comorbidity. Therefore, identifying central and bridge symptoms within this intricate network will enhance the efficiency and effectiveness of interventions.
Traditional approaches to studying symptoms often focus on individual symptoms in isolation, potentially overlooking their interconnected nature and the cascading effects they may have on overall health. Network analysis offers a new framework for examining symptom experiences by analyzing the relationships between symptoms and revealing how they influence and interact. Symptom networks have been applied to various clinical issues, including depression [17, 18], autism, schizophrenia [19, 20], anxiety disorders [21], eating disorders [22, 23], pediatric cough [24], substance use [25], and more. These interconnected symptom networks undoubtedly impact clinical outcomes,well-being, treatment adherence, and quality of life. Zhu Z and his team in China have pioneered this technique to explore the network of symptom severity among PHW. Their research has highlighted the importance of critical symptoms, often referred to as bridge symptoms [26, 27].
Symptoms not only involve issues such as frequency and severity but also encompass the situational significance of symptom distress, which is integrated into what is known as the symptom experience [28]. This concept reflects the multidimensional nature of symptoms and provides a more precise and comprehensive description than mere severity alone. Therefore, this study aimed to establish a symptom network for PWH based on the symptom experience. The second aim was to identify central or bridging symptoms within the network.
Materials and methods
Setting and study sample
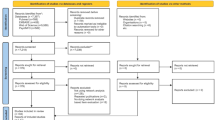
This is a large-scale, multi-center, cross-sectional survey conducted in China, and we have previously reported on some of the research data. The content of this manuscript is a secondary analysis of the overall research data [29,30,31]. This investigation was conducted in nine HIV-designated medical institutions in China, including three high-incidence areas involving Xinjiang Province, Guangxi, and Sichuan Province, three areas with slightly high incidence involving Beijing, Hunan, and Guangdong Province, two moderate-incidence areas involving Qinghai Province and Henan Province as well as Shenyang City located in northeast China’s Liaoning Province that is low incidence area. In the period of 4 months, from November 2021 to January 2022 and April 2022 to May 2022, 711 PWH who were diagnosed with HIV-infected and at least 18 years old were enrolled to participate in this study from the above medical institutions by convenient and quota sampling according to sex, age, and residential areas. Excluded participants were those diagnosed with severe conditions who were unable to complete the survey. On this basis, participants in the network analysis were also required to fulfill the criteria of having at least one and more symptoms. We recruited the target population by putting up posters, and field investigators were responsible for providing explanations. All participants signed written informed consent. The ethics review board approved this study, and the reference number is DTEC-KY2021-015-01.
Measurements
The 27-item Self-Report Symptom Scale Chinese version [32] used in this study was employed to quantify the symptom experience(including symptom frequency, symptom severity, and symptom distress, namely symptom multidimensionality) of PWH, which was developed based on the Self-report Symptom Checklist (SRSC) from the School of Nursing at Fudan University [4]. In our study, it was combined with the horizontal content of the Chinese version of the Memorial Symptom Assessment Scale (MSAS) based on suggestions from Vincenzi [33], with a content validity index of 0.918 and a Cronbach’s α coefficient of 0.916. This scale may be used to screen for physical, cognitive, and psychological symptoms and rate their frequency, severity, and distress in the past two weeks. The frequency and severity of these 27 symptoms were evaluated with a four-point Likert scale, and the distress of symptoms was rated by a five-point Likert scale with higher values indicating more burden symptoms. Each symptom’s experience score is the sum of the three dimension scores.
Demographic information for the PWH included age, sex, ethnicity, education, marital status, employment status, economic income, residential districts, and personal monthly income in the previous 12 months. Clinical information such as years since HIV diagnosis, years of ART, the latest CD4 + T cell count, disease staging, and comorbidities was obtained from the hospital’s electronic medical record system.
Data collection
All participants were investigated using questionnaires administered by field investigators through an online survey platform (https://www.wjx.cn/). Each question was set as a required answer. We assigned nine field investigators to oversee on-site data collection in the above HIV-designated medical institutions. They were responsible for explaining and guiding but not inducing activity. The self-report took approximately 10–15 min to complete. At the end of the survey, those who completed the study received 50 yuan RMB.
Statistical analysis
The study analyzed the sociodemographic characteristics of all participants through descriptive statistics, such as frequencies, percentages, means, and standard deviations (SDs). A sociodemographic and clinical characteristics were compared using the chi-squared test or t-test. Statistical significance was determined by P-values < 0.05. The sample size in the study was calculated following Constantin’s suggestion [34]: a minimum sample size of greater than 20 times the number of nodes or more than 250–350 cases, or using the formula n = N*(N-1)/2 (where N represents the number of nodes and n represents the sample size).
To explore the connected structure of symptom networks, we used the symptom network analysis (SNA) approach [15] to demonstrate the aims of our study by graphical summary. In SNA, symptoms are represented by independent nodes, and an edge between nodes reflects the conditional dependence relation between them; the thicker the edges are, the stronger the association between the two nodes [27]. A centrality analysis was conducted to examine each item’s critical role within the symptom networks. Three centrality measures were calculated in this study: strength (i.e., the sum of the edge weights connected to a node), betweenness (i.e., the number of times that node lies on the shortest path between two other nodes), and closeness (i.e., indicated by the average distance between one symptom and all other nodes. The shorter the route is, the greater the closeness value) [35]. A higher strength centrality indicates a greater likelihood of the symptom co-occurring with other symptoms [35]. On the other hand, closeness centrality highlights the central position of the symptom in the network [36]. Additionally, nodes with higher betweenness centrality have a more significant impact on the network [35]. Regarding centrality indices, strength centrality is considered the most vital and dependable [37], while closeness centrality and betweenness centrality are less practical. Age and sex, being confounders, were adjusted in network analysis.
Difference tests of edge weights tested network robustness and accuracy from the observed data network and those estimated under non-parametric bootstrapping [38]. The 95% bootstrap confidence intervals (CIs) derived from nonparametric bootstrapping with 1000 iterations were employed for the edge weights [39]. The centrality stability coefficient (considering strength and betweenness) was used to determine the maximum proportion of cases that can be dropped to retain the same centrality values. We calculated the stability coefficient concerning the expected impact of nodes using a case-dropping subset bootstrap approach [40]. This coefficient should not be below 0.25 [40].
Testing whether the centrality of different nodes or the edge weights of different edges are significant is still done using the bootstrap difference test. This calculates the difference in the nodes’ centrality or edge weights and constructs confidence intervals obtained from their repeated sampling. According to the null hypothesis test, if the zero lies within the confidence interval, the difference in node centrality or edge weights is insignificant [41].
Our study conducted data analysis in the software packages R (version 3.6.3, available at https://www.r-project.org), packages qgraph [42], botnet, and Free Statistics software versions 1.7 were also used to perform all statistical analyses. All data analysis was the responsibility of statisticians who were not directly involved in the research.
Results
Baseline characteristics of the study population
We recruited a total of 711 HIV-infected individuals to complete an online survey. Among them, 493 individuals (69.34%) reported at least one symptom in the past two weeks and were included in the symptom network analysis. Table 1 shows no statistically significant differences in demographic characteristics between symptomatic and asymptomatic groups (P > 0.05). The average age was 39.22 years (SD = 11.60), and the male-to-female ratio was 2.59:1. Among the participants, 623 individuals (87.62%) were regularly using ART, and 218 individuals (30.66%) were in the asymptomatic stage(see Table 1).
Symptom description
Of all participants, 69.34% reported having at least one symptom in the last two weeks, 8.86% reported having only one symptom, 13.08% had 2–3 symptoms, 11.39% had 4–5 symptoms, and others reported more than six. The mean number of symptoms was 5.367. The most common symptoms were sleep disturbance (37.98%), fatigue (35.16%), memory loss (33.90%), hair loss (26.58%), and slow reactions (25.04%) (Table 2). The results showed that ranking the symptoms’ multidimensionality scores (frequency, severity, distress) remained largely consistent with the ordering of the prevalence.
Symptom network
Figure 1 displays the unadjusted and adjusted by age and sex network plot for twenty-seven symptoms. The unadjusted plot highlights the strong relationship between muscle/joint ache and hand/foot pain among eighteen physical symptoms, as well as difficulty reasoning and confusion among five cognitive symptoms. The four psychological symptoms showed a positive influence on each other. Additionally, there was a significant association between confusion and uncontrollable worrying in different symptom clusters. After adjusting the network analysis by age and sex, similar partial relationships were found. However, we can see that there was still some variation in the degree of correlation among the partial symptoms in this visualization graph.
Symptom network plot. A Unadjusted estimated network plot for 27 symptoms in 493 PWH; B Adjusted (by age and sex) estimated network plot for 27 symptoms in 493 PWH.
An edge represents a connection between two nodes and is interpreted as a connection between two nodes that applies to all other nodes. Each edge of the network represents a positively regulated matching link (blue edge) or a negatively regulated matching link (red edge). The thickness of an edge reflect its weight (the strength of the connection between two nodes)
PHYS1: Fatigue; PHYS2: Dizziness; PHYS3: Headache; PHYS4: Fever; PHYS5:Cough; PHYS6: Sleep disturbance; PHYS7:Vision blur; PHYS8: Rash; PHYS9: Mouth ulcer; PHYS10: Muscle/joint ache; PHYS11: Hand/foot pain; PHYS12: Appetite loss; PHYS13: Bloating/abdominal pain /diarrhea; PHYS14: Nausea/vomit; PHYS15: Lipodystrophy; PHYS16: Weight loss; PHYS17: Low sex drive; PHYS18: Hair loss; COGS1: Having difficulty in concentrating; COGS2: Slow react; COGS3: Memory loss; COGS4: Having difficulty in reasoning; COGS5: Becoming confusing; PSYS1: Uncontrollable worrying; PSYS2: Feeling nervous; PSYS3: Little interest in doing things; PSYS4: Feeling down
The gray area in Fig. 2 represents the 95% CI area of the edge weight obtained using the bootstrap method. The 95% CIs (gray intervals) were small, indicating good network accuracy. For the subset bootstrap in Fig. 3, the stability of closeness and betweenness dropped steeply while the stability of node strength was better, and the correlation stability coefficient was solid and trustworthy, which suggested that the network remained stable. The value of correlation stability (CS) coefficients is as follows: betweenness = 0.361, closeness = 0.438, strength = 0.671, expected Influence = 0.671. According to the null hypothesis test, if zero falls within the confidence interval, it indicates that the difference in node centrality is not statistically significant. In Fig. 4, the black boxes signified the presence of a difference, while the gray area suggested no statistical distinction. The values on the diagonal represented the expectedinfluence or strengh values obtained through multiple samplings of a node. Our finding showed that the symptom “becoming confusing” emerged as both the strongest and most influential within the network and falls under the category of cognitive symptoms.
The accuracy of the symptom network: is demonstrated through bootstrapped edge weight estimates with 95% confidence intervals plotted for the adjusted network. In the plot, the red line indicates the sample values, the black line represents bootstrap means, and the gray area signifies the bootstrapped confidence intervals. To prevent visual clutter, the names of the node pairs (Y axis labels and ticks) have been removed
Stability coefficient for centrality indices of 27 symptoms network among PWH.
The correlation between the centrality index of a network measured in terms of headcount and the network constructed from the entire input dataset was calculated across multiple time points. The mean values are represented by lines, while the range from the 2.5th quantile to the 97.5th quantile is shown by shaded areas
Bootstrapped different test of expectedInfluence and strength for nodes. The larger the coefficient, the greater the magnitude or intensity of its influence, and its position is located in the upper right corner of the entire graph.The bootstrap difference test is utilized to determine if the centrality of various nodes varies significantly. This method assesses the disparity in centrality of nodes and generates a confidence interval based on repeated sampling. In the figure, black boxes denote the presence of a significant difference, while the gray area indicates no statistical variance. The values on the diagonal reflect the expectedinfluence or strength values acquired through multiple repeated samplings of a node
PHYS1: Fatigue; PHYS2: Dizziness; PHYS3: Headache; PHYS4: Fever; PHYS5:Cough; PHYS6: Sleep disturbance; PHYS7:Vision blur; PHYS8: Rash; PHYS9: Mouth ulcer; PHYS10: Muscle/joint ache; PHYS11: Hand/foot pain; PHYS12: Appetite loss; PHYS13: Bloating/abdominal pain /diarrhea; PHYS14: Nausea/vomit; PHYS15: Lipodystrophy; PHYS16: Weight loss; PHYS17: Low sex drive; PHYS18: Hair loss; COGS1: Having difficulty in concentrating; COGS2: Slow react; COGS3: Memory loss; COGS4: Having difficulty in reasoning; COGS5: Becoming confusing; PSYS1: Uncontrollable worrying; PSYS2: Feeling nervous; PSYS3: Little interest in doing things; PSYS4: Feeling down
Figure 5(A) shows the centrality of bridge symptoms. PHYS4 (Fever) had the highest bridge strength (0.547), bridge closeness (0.053), lower bridge betweenness (23.000), and bridge expected influence (0.285). Figure 5(B) presents three centrality indices of all nodes. Among physical symptoms, PHYS10 (Muscle/joint ache) had the most significant values for node centrality (strength = 1.112, betweenness = 12.000, closeness = 0.002). Among cognitive symptoms, COGS5 (Becoming confusing) had the most significant values for node centrality (strength = 1.437, betweenness = 140.000, closeness = 0.003) and identified as the central symptom in the entire network. Among psychological symptoms, PSYS4 (Feeling down) had the most significant values for node centrality (strength = 1.077, betweenness = 16.000, closeness = 0.002). The node predictability values ranged from 11.8 to 68.8%. Our results showed that the psychological symptom cluster had the highest predictability, showing that their neighbors can explain 58.6%~68.8% of their variance.
Centrality measures of all symptoms across study groups: strength, betweenness, closeness, and expextedInfluence of 27 symptoms. A Bridge node centrality; B Node centrality
PHYS1: Fatigue; PHYS2: Dizziness; PHYS3: Headache; PHYS4: Fever; PHYS5:Cough; PHYS6: Sleep disturbance; PHYS7:Vision blur; PHYS8: Rash; PHYS9: Mouth ulcer; PHYS10: Muscle/joint ache; PHYS11: Hand/foot pain; PHYS12: Appetite loss; PHYS13: Bloating/abdominal pain /diarrhea; PHYS14: Nausea/vomit; PHYS15: Lipodystrophy; PHYS16: Weight loss; PHYS17: Low sex drive; PHYS18: Hair loss; COGS1: Having difficulty in concentrating; COGS2: Slow react; COGS3: Memory loss; COGS4: Having difficulty in reasoning; COGS5: Becoming confusing; PSYS1: Uncontrollable worrying; PSYS2: Feeling nervous; PSYS3: Little interest in doing things; PSYS4: Feeling down
Discussion
This multi-center study conducted across China ensured a representative population sample and investigated the relationships and interconnectedness of various symptoms experienced by PWH using network analysis. By analyzing data from 493 PWH, the research identifies ‘becoming confusing’ as the most central symptom and ‘fever’ as the bridge symptom that connects other symptoms. The pivotal roles of these symptoms within the network underscore their significance in symptom management, suggesting that targeted interventions could alleviate the overall symptom burden and enhance the quality of life for patients. This approach emphasizes a shift towards a more comprehensive and precise care model focusing on central and bridging symptoms rather than managing isolated symptoms. Additionally, our study revealed that the average number of symptoms reported by Chinese PWH was 5.367, in contrast to the 9 symptoms documented in a previous study involving Chinese PWH [4]. Conversely, a study conducted by the National AIDS Treatment Center of Vietnam in 2021 reported an average of 7.660 symptoms [43]. The discrepancies in reported symptom numbers across different regions and countries may arise from variations in population selection, the application of diverse measurement tools, or ongoing advancements in AIDS treatment programs.
Analysis conducted on two groups of PWH, one group with symptoms and the other without, showed no statistically significant differences in sociodemographic characteristics such as sex, education, marital status, employment, family income, and residential area. However, there were significant differences in clinical features such as duration of HIV infection, disease stages, and ART use. The symptomatic group had a higher proportion of individuals who had been living with HIV for more than 6 years, consistent with previous research by Araya [44]. Additionally, a larger proportion of individuals in the symptomatic group were in the AIDS stage compared to the asymptomatic group. These findings are in line with the known disease characteristics and progression observed in clinical practice.
Our findings identified the top five common symptoms in PWH as sleep disturbance, fatigue, memory loss, hair loss, and slowed reaction time. The prevalence of physical and cognitive symptoms was particularly notable, aligning with existing research and underscoring the significant impact of fatigue and sleep disturbances on individuals with HIV. Previous studies [45,46,47,48] on fatigue in PWH have shed light on the burden and underlying mechanisms of fatigue, including its association with sleep disorders, mental health issues, and other symptoms. A separate survey conducted among PWH in various regions of China [4] yielded similar results to our study, offering valuable insights for clinical interventions and care strategies.
The distribution of symptom incidence in our study did not align with the central or bridge symptoms identified in the complex network. Understanding how symptoms are interconnected provides a more thorough understanding of the complex dynamics of symptomatology within this population. Malgaroli and colleagues discovered that central symptoms could trigger other symptoms, potentially influencing the onset and persistence of additional symptoms [12]. This suggests that targeting specific symptoms within interconnected networks could have a broader impact on overall symptom burden. Such insights can guide targeted interventions and enhance the quality of care for PWH, promoting a more holistic approach to symptom management. In contrast to similar studies that primarily focus on symptom severity, our study was rooted in a comprehensive and holistic understanding of the symptom experience. It incorporated the patient’s perspective and considered the real-world implications of symptoms.
Our analysis of the symptom network revealed the central symptoms and clusters. Within the physical symptoms, we found that muscle/joint ache and hand/foot pain, rash and mouth ulcer, appetite loss and nausea/vomit exhibited stronger correlations. This suggested that these symptoms can be classified into three sub-categories: pain-related, cutaneous/mucosal, and digestive system symptoms. From the visualized network, it can be observed that four nodes belonging to the psychological symptom cluster are closely interconnected. The close connections among symptoms within each cluster validate the categorization accuracy in this symptom scale [13]. However, muscle/joint ache and feeling down were separately ranked as the most prominent physical or psychological symptoms from the node centrality analysis. The mechanisms underlying pain are complex and involve various physiological and neuropathological processes. Pain is associated with inflammatory responses, nerve dysfunction, tissue damage, viremia, and exposure to many different ART [49, 50]. Being a significant presenting symptom or cause of patients seeking medical care, pain profoundly affects their physical and mental well-being and social behavior [14]. Previous studies revealed that PWH with pain had increased depression symptom severity [51] and incidence of Dementia [52]. Therefore, health providers should pay more attention to the assessment and management of pain and explore scientific palliative care models that can effectively reduce the overall burden of physical symptoms.
In the network graph, becoming confused was a central symptom, and fever was identified as the most important bridge symptom in the entire network system. These two symptoms exerted a cascading effect on physical and psychological symptoms by influencing other symptoms among PWH. This means that cognitive symptoms, becoming confused, are most likely accompanied by the appearance of any symptoms. Fever as a bridge symptom often suggests that other symptom clusters will be triggered or that the patient is comorbid with other diseases. These above viewpoints bring an important value for our clinical precision intervention in the future. Considering its significant impact on PWH’s quality of life and medication adherence, the researchers believe that managing cognitive symptoms should be a core concern in healthcare practice. Meanwhile, previous studies have shown that cognitive symptoms related to psychological status, especially depression and anxiety, also intertwine with physical symptoms such as pain, palpitations, dizziness, and nausea. There is a clear correlation between cognitive-psychological vulnerability and the experience of HIV symptoms [53], suggesting that cognitive symptoms may serve as essential factors in predicting overall HIV symptom severity. An important report from The Lancet highlighted that cognitive symptoms or impairment often arise from the interplay between long-term HIV infection, persistent inflammatory response, potential treatment toxicity, aging, and neurodegenerative changes caused by HIV infection [54]. Cognitive impairment has become a major concern in AIDS treatment [55]. The findings of this study also support this viewpoint, emphasizing the need to strengthen the assessment, intervention, and care for cognitive impairment in PWH in the future. Fever, as the primary physical symptom, serves as a bridging symptom connecting two different symptom clusters. Fever in HIV patients often indicates the presence of opportunistic infections, tuberculosis, or central nervous system (CNS) diseases [56]. CNS diseases, including progressive multifocal leukoencephalopathy (PML) and HIV-associated dementia (HAD) [57], are also linked to fever and can involve complex neurological symptoms and cognitive decline. The presence of fever in PWH necessitates thorough diagnostic evaluation to identify and treat these underlying conditions effectively.
Strength and limitation
This study demonstrates a significant strength in utilizing network analysis to uncover intricate relationships among physical, cognitive, and psychological symptoms. Additionally, the incorporation of multiple dimensions of symptoms, including frequency, severity, and distress, enhances the depth of data analysis. However, there are several limitations. The cross-sectional design restricts the ability to observe changes in symptom networks over time. Moreover, causal relationships between symptoms and other variables could not be established. The use of a convenience sample introduces bias, particularly as the majority of participants were PWH from healthcare settings in China, potentially limiting the generalizability of the findings. Lastly, reliance on self-reported data collection methods may introduce further bias. Future research should aim to validate findings in more diverse samples across different settings and investigate the dynamic nature of symptom networks.
Conclusion
PWH often experience a combination of physical, cognitive, and psychological symptoms that have a significant impact on their clinical outcomes and well-being. Utilizing network analysis can help identify key symptoms that are interconnected and play a central role or bridge in symptom management for these individuals. Our study highlights the importance of addressing ‘fever’ as a bridge symptom, suggesting healthcare providers should focus on intervention strategies to alleviate symptom burden and prevent the development of other associated symptoms or comorbidities. These insights can inform targeted interventions and support strategies to enhance the quality of life for PWH, while also enabling anticipatory care.
Data availability
The data can be disclosed with the consent of all researchers after publication.
References
Smiley CL, Rebeiro PF, Cesar C, Belaunzaran-Zamudio PF, Crabtree-Ramirez B, Padgett D, Gotuzzo E, Cortes CP, Pape J, Veloso VG, et al. Estimated life expectancy gains with antiretroviral therapy among adults with HIV in Latin America and the Caribbean: a multisite retrospective cohort study. Lancet HIV. 2021;8(5):e266–73.
ANAC. ANAC’s Core Curriculum for HIV AIDS. JONES AND BARTLETT; 2010.
Zhu Z, Hu Y, Guo M, Williams AB. Urban and rural differences: unmet needs for Symptom Management in people living with HIV in China. J Assoc Nurses AIDS Care. 2019;30(2):206–17.
Zhu Z, Hu Y, Xing W, Guo M, Zhao R, Han S, Wu B. Identifying Symptom clusters among people living with HIV on antiretroviral therapy in China: A Network Analysis. J Pain Symptom Manage. 2019;57(3):617–26.
Olson B, Vincent W, Meyer JP, Kershaw T, Sikkema KJ, Heckman TG, Hansen NB. Depressive symptoms, physical symptoms, and health-related quality of life among older adults with HIV. Qual Life Res. 2019;28(12):3313–22.
Papadimitriou G. The Biopsychosocial Model: 40 years of application in Psychiatry. Psychiatriki. 2017;28(2):107–10.
Gevers-Montoro C, Liew BXW, Deldar Z, Conesa-Buendia FM, Ortega-De Mues A, Falla D, Khatibi A. A network analysis on biopsychosocial factors and pain-related outcomes assessed during a COVID-19 lockdown. Sci Rep. 2023;13(1):4399.
Chew NWS, Lee GKH, Tan BYQ, Jing M, Goh Y, Ngiam NJH, Yeo LLL, Ahmad A, Ahmed Khan F, Napolean Shanmugam G, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–65.
Nipp RD, El-Jawahri A, Moran SM, D’Arpino SM, Johnson PC, Lage DE, Wong RL, Pirl WF, Traeger L, Lennes IT, et al. The relationship between physical and psychological symptoms and health care utilization in hospitalized patients with advanced cancer. Cancer. 2017;123(23):4720–7.
Wang P, Jiang C, Liu J. Interaction between Traditional Chinese Medicine and modern medicine from the perspective of mind-body unity. China Mod Traditional Chin Med. 2021;23(4):685–91.
Wu Y, Dong X, Wang L. Analysis of the interaction between traditional Chinese Medicine and modern medicine. Henan Traditional Chin Med. 2022;42(1):55–7.
Malgaroli M, Calderon A, Bonanno GA. Networks of major depressive disorder: a systematic review. Clin Psychol Rev. 2021;85:102000.
Zhao R, Han S, Zhu Z, Hu Y, Xing W, Guo M, Wu B. Identifying Symptom clusters among people living with HIV on antiretroviral therapy in China: A Network Analysis. J Pain Symptom Manag. 2019;57(3):617–26.
Li Y, Jia S, Cao B, Chen L, Shi Z, Zhang H. Network analysis of somatic symptoms in Chinese patients with depressive disorder. Front Public Health. 2023;11:1079873.
Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13.
Castro D, Ferreira F, de Castro I, Rodrigues AR, Correia M, Ribeiro J, Ferreira TB. The Differential Role of Central and Bridge symptoms in deactivating Psychopathological Networks. Front Psychol. 2019;10:2448.
Corponi F, Anmella G, Verdolini N, Pacchiarotti I, Samalin L, Popovic D, Azorin JM, Angst J, Bowden CL, Mosolov S, et al. Symptom networks in acute depression across bipolar and major depressive disorders: a network analysis on a large, international, observational study. Eur Neuropsychopharmacol. 2020;35:49–60.
Groen RN, Snippe E, Bringmann LF, Simons CJP, Hartmann JA, Bos EH, Wichers M. Capturing the risk of persisting depressive symptoms: a dynamic network investigation of patients’ daily symptom experiences. Psychiatry Res. 2019;271:640–8.
Han GT, Trevisan DA, Foss-Feig J, Srihari V, McPartland JC. Distinct Symptom Network Structure and Shared Central Social Communication Symptomatology in Autism and Schizophrenia: a bayesian network analysis. J Autism Dev Disord 2022.
Ye H, Zalesky A, Lv J, Loi SM, Cetin-Karayumak S, Rathi Y, Tian Y, Pantelis C, Di Biase MA. Network Analysis of Symptom Comorbidity in Schizophrenia: relationship to Illness Course and Brain White Matter Microstructure. Schizophr Bull. 2021;47(4):1156–67.
Ellwardt E, Muthuraman M, Gonzalez-Escamilla G, Chirumamilla VC, Luessi F, Bittner S, Zipp F, Groppa S, Fleischer V. Network alterations underlying anxiety symptoms in early multiple sclerosis. J Neuroinflammation. 2022;19(1):119.
Giles S, Hughes EK, Fuller-Tyszkiewicz M, Treasure J, Fernandez-Aranda F, Karwautz AFK, Wagner G, Anderluh M, Collier DA, Krug I. Bridging of childhood obsessive-compulsive personality disorder traits and adult eating disorder symptoms: a network analysis approach. Eur Eat Disord Rev. 2022;30(2):110–23.
Mares SHW, Burger J, Lemmens L, van Elburg AA, Vroling MS. Evaluation of the cognitive behavioural theory of eating disorders: a network analysis investigation. Eat Behav. 2022;44:101590.
Huang M, Wang J, Zhang R, Ni Z, Liu X, Liu W, Kong W, Chen Y, Huang T, Li G, et al. Symptom network topological features predict the effectiveness of herbal treatment for pediatric cough. Front Med. 2020;14(3):357–67.
Rutten RJT, Broekman TG, Schippers GM, Schellekens AFA. Symptom networks in patients with substance use disorders. Drug Alcohol Depend. 2021;229(Pt B):109080.
Zhu Z, Wen H, Yang Z, Han S, Fu Y, Zhang L, Hu Y, Wu B. Evolving symptom networks in relation to HIV-positive duration among people living with HIV: a network analysis. Int J Infect Dis. 2021;108:503–9.
Liu X, Wang H, Zhu Z, Zhang L, Cao J, Zhang L, Yang H, Wen H, Hu Y, Chen C, et al. Exploring bridge symptoms in HIV-positive people with comorbid depressive and anxiety disorders. BMC Psychiatry. 2022;22(1):448.
Armstrong TS. Symptoms experience: a concept analysis. Oncol Nurs Forum. 2003;30(4):601–6.
Xie M, Wang A, Wang K, Yu Y, Lin Z. Effects of perceived social support on health-related quality of life in asymptomatic and symptomatic people living with HIV in China: a cross-sectional study. Curr HIV Res 2023.
Xie M, Wang A, Wang K, Yu Y, Lin Z. Association between HIV duration and symptom distress among middle-aged and elderly people with HIV-infected in China: a cross-sectional study. BMC Geriatr. 2022;22(1):728.
Xie M, Wang A, Lin Z. Prevalence of Gynecological Related Symptoms and Quality of Life in Women Living with HIV/AIDS: a secondary analysis from an Online, cross-sectional survey in China. Int J Womens Health. 2022;14:1425–33.
Xie ML. Development of the perceived symptom manageability scale and its impact on quality of life among people living with HIV from a multidimensional perspective of symptom experience. Doctor. 2023.
Vincenzi CB, Moody K, Spirig R. Development and pretesting of a set of symptom assessment tools in HIV: the HIV Symptom Experience Assessment Scale and the HIV Symptom Manageability Scale. Appl Nurs Res. 2009;22(3):204–10.
Constantin M. Sample size recommendations for estimating cross-sectional network models. 2018.
Wen H, Zhu Z, Hu T, Li C, Jiang T, Li L, Zhang L, Fu Y, Han S, Wu B, et al. Unraveling the central and bridge psychological symptoms of people living with HIV: a network analysis. Front Public Health. 2022;10:1024436.
Zhu Z, Sun Y, Kuang Y, Yuan X, Gu H, Zhu J, Xing W. Contemporaneous symptom networks of multidimensional symptom experiences in cancer survivors: a network analysis. Cancer Med. 2023;12(1):663–73.
Kuang Y, Jing F, Sun Y, Zhu Z, Xing W. Symptom networks in older adults with cancer: a network analysis. J Geriatr Oncol. 2024;15(3):101718.
Costenbader E, Valente TW. The stability of centrality measures when networks are sampled. Social Networks. 2003;25(4):283–307.
Efron B. The bootstrap and modern statistics. J Am Stat Assoc. 2000;95(452):1293–6.
Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212.
Junwen Y, Zheng Z, Tiantian H, Yilin J, Jiamin H, Zhongfang Y. Specificity indices of Symptom Network. J Nurses Train. 2023;38(24):2229–34.
Sacha Epskamp, Ang´elique OJ, Cramer, Lourens J, Waldorp AR, Borsboom D. qgraph: Network Visualizations of Relationships in Psychometric Data. Journal of Statistical Software 2012, 48(4):1–8.
Le Dai D, Than TNH, Pham TVA, Bui TTH, Pham VT, Luong NK, Harding R, Krakauer EL. Symptom prevalence, burden and correlates among people living with HIV in Vietnam: a two-centre self-report study. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV 2021.
Araya AX, Norr KF, Pérez CM, Levy JA, Park CG, Kim MJ. HIV-related symptoms and patient clusters among chileans living with HIV. AIDS Care. 2013;25(4):488–95.
Gebreyesus T, Belay A, Berhe G, Haile G. Burden of fatigue among adults living with HIV/AIDS attending antiretroviral therapy in Ethiopia. BMC Infect Dis. 2020;20(1):280.
Lee KA, Jong S, Gay CL. Fatigue management for adults living with HIV: a randomized controlled pilot study. Res Nurs Health. 2020;43(1):56–67.
Coetzee BJ, Loades ME, Du Toit S, Kagee A. Correlates of fatigue among South African adolescents living with HIV and receiving antiretroviral therapy. AIDS Behav. 2019;23(3):602–8.
’ … But i’m still tired’: the experience of fatigue among South African adolescents receiving antiretroviral therapy. AIDS Care 2018, 30(6):746–750.
Hu Y, Liu J, Zhuang R, Zhang C, Lin F, Wang J, Peng S, Zhang W. Progress in pathological and therapeutic research of HIV-Related Neuropathic Pain. Cell Mol Neurobiol. 2023;43(7):3343–73.
Slawek DE. People living with HIV and the emerging field of chronic pain-what is known about epidemiology, etiology, and management. Curr HIV/AIDS Rep. 2021;18(5):436–42.
Parker R, Jelsma J, Stein DJ. Pain in amaXhosa women living with HIV/AIDS: a cross-sectional study of ambulant outpatients. BMC Womens Health. 2017;17(1):31.
Xavier Hall CD, Okantey B, Meng Z, Sabuncu C, Lane B, Millender E, Queiroz A, Kim JH, Okada L, Gillespie A, et al. Examining biopsychosocial predictors of risk for cognitive impairment among a racially diverse sample of men who have sex with men living with HIV. Ther Adv Infect Dis. 2024;11:20499361241249657.
Leyro TM, Vujanovic AA, Bonn-Miller MO. Examining associations between cognitive-affective vulnerability and HIV symptom severity, perceived barriers to treatment adherence, and viral load among HIV-positive adults. Int J Behav Med. 2015;22(1):139–48.
Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86.
Deng L, Zhang X, Gao Y, Turner D, Qian F, Lu H, Vermund SH, Zhang Y, Qian HZ. Association of HIV infection and cognitive impairment in older adults: a meta-analysis. Ageing Res Rev. 2021;68:101310.
Holtzclaw BJ. Managing fever and febrile symptoms in HIV: evidence-based approaches. J Assoc Nurses AIDS Care. 2013;24(1 Suppl):S86–102.
Luma HN, Tchaleu BC, Temfack E, Doualla MS, Ndenga DP, Mapoure YN, Njamnshi AK, Djientcheu VD. HIV-Associated Central Nervous System Disease in patients admitted at the Douala General Hospital between 2004 and 2009: a retrospective study. AIDS Res Treat. 2013;2013:709810.
Acknowledgements
We would like to express our special gratitude to all study participants for their time during data collection. We are grateful to our colleagues who gave strong support to the study, especially Junli Cao from the First affiliated Hospital of China Medical University, Shenyang, Liaoning Province; Dandan Ma from Henan Infectious Diseases Hospital; Weixia Bai from Center for Disease Control and Prevention in Shuimogou District, Urumqi, Xinjiang; Liumei Xu from the third People’s Hospital from Shenzhen, Guangdong Province; Zhihui Guo from Public Health Clinical Center of Chengdu, Sichuan Province; Yongjuan Yang from Chengzhong Center for Disease Control and Prevention, Xining, Qinghai Province. Kerong Wang and Yanping Yu from Beijing Home of Red Ribbon, Beijing Ditan Hospital Capital Medical University, Beijing, China.
Funding
This research received a specific grant from Capital’s Funds for Health Improvement and Research (CFH): 2024-2G-2174.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the study design and data interpretation. Meilian Xie and Aiping Wang recruited a sample population and performed this online survey. Meilian Xie, Xiaoyu Liu and Yiwei Hao completed the data analysis. Meilian Xie wrote the manuscript mainly, and other authors read and revised the manuscript. All authors are responsible for the content and have approved this final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Beijing Ditan Hospital Capital Medical University, where the study was conducted, and the reference number is DTEC-KY2021-015-01. All participants signed written informed consent.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, M., Liu, X., Wang, A. et al. Symptom network connectivity and interaction among people with HIV in China: secondary analysis based on a cross-sectional survey. BMC Public Health 24, 2331 (2024). https://doi.org/10.1186/s12889-024-19728-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19728-8