Abstract
Background
Electrodermal activity (EDA) and other peripheral autonomic electrical parameters have been used as indicators of emotional states, including depressive states and suicidal state. We aimed to review EDA research systematically, focusing on EDA’s usefulness as a biomarker for depression and suicidal behaviour.
Methods
We searched MEDLINE, Scopus, Cochrane Library, and Web of Science databases, following PRISMA guidelines. The initial screening of articles was based on titles and abstracts; then the full text was reviewed. A preliminary synthesis of findings was developed using tables, thematic analysis and quality ratings.
Results
1287 articles were screened and 77 relevant studies were identified and included in the systematic review. The studies were fairly consistent in maintaining that hypoactive electrodermal response is an established feature of patients affected by depression. There is also preliminary evidence that monitoring EDA may help to differentiate the phases of mood disorders. A few studies provided evidence that EDA can be used to differentiate acutely suicidal subjects from depressed patients who are not severely suicidal. Although EDA has been shown to be a valid, sensitive marker of suicidal ideation, suicide attempts and violent suicidal behaviour, it also seems to be influenced to some extent by antidepressant treatment.
Conclusions
Most of the studies summarised in this review are quite outdated and employed a variety of designs and methods to evaluate EDA. This limits the generalisability of the results and makes it difficult to draw clear conclusions about the role of EDA in real-world settings. Electrodermal hypoactivity seems to be a reliable feature of depression and a valid marker of suicidal risk. Nevertheless, the potential utility of EDA in diagnosis, prevention, and treatment planning for depression and suicidal behaviour, should be thoroughly studied.
Similar content being viewed by others
Background
Rationale
Biological abnormalities may be risk factors for depression, suicidal behaviour or completed suicide; a recent review has identified the following major categories of potential biological predictors of suicide attempt behaviour: (1) results of structural and functional brain imaging and (2) biochemical and genomic findings relating to the major neurotransmitters (serotonin, catecholamines, GABA and glutamate), the hypothalamic pituitary adrenal (HPA) axis, the inflammasome, lipids and neuroplasticity [1]. Nevertheless, there is currently a lack of biomarkers for the psychiatric field, in particular for suicidal behaviour. The prediction of suicidal risk and identification of suicidal patients will only be possible when we have an accurate picture of the interplay between biological and psychosocial factors. The use of the autonomic responses as markers of emotion, attention, decision-making, motor preparation, anticipation of reward or punishment and unconscious detection has grown considerably since the 1980s [2]. The most basic indicators of the state of the autonomic nervous system are heart rate and electrodermal activity (EDA); the former is influenced by the sympathetic and parasympathetic systems, whereas the latter is under sympathetic control only. Since a useful biomarker should be measurable in a non-invasive manner [3], the features of EDA suggest that its potential utility as a biomarker warrants careful consideration.
EDA is now the preferred term for changes in the electrical conductance of the skin, which depend on the quantity of sweat secreted by eccrine sweat glands in the hypodermis of the palmar and plantar regions [4]. Sympathetic nervous activity and variations in the sweating of the skin are regulated by environmental temperature (thermoregulatory sweating) and by central nervous activity related to affective and cognitive states (palmar, mental or emotional sweating) [5, 6]. EDA has been used as an index of emotional stimulation in several experimental studies [7].
EDA has a tonic and a phasic component. The tonic component is related to the slower components and background characteristics of the signal (skin conductance level; SCL). The phasic components are the faster-changing elements of the signal that can be associated with a stimulus (skin conductance response; SCR) or “spontaneous” or “nonspecific” (nonspecific skin conductance response; NS.SCR) [8, 9].
It has been hypothesised that the central component of EDA originates in the left hemisphere, but this lateralisation remains controversial [10]. EDA may be modulated by two different pathways: ipsilateral modulation within the limbic system, via the hypothalamus and thermoregulatory pathways and, to a lesser degree, contralateral modulation by the premotor cortex and basal ganglia [8, 11, 12].
In subjects exposed to emotional stimuli the amplitude of electrodermal response increases linearly with perceived arousal [13,14,15,16], whilst repeated presentation of identical, non-significant stimuli elicits progressively smaller reactions, a phenomenon known as habituation [8]. Individual trait differences in EDA have been observed and labelled as EDA lability. Some individuals show a high rate of nonspecific EDA and slow habituation to specific stimuli (labile individuals), whereas others show less non-specific EDA and more rapid specific EDA habituation (stable individuals) [16, 17]. EDA lability is influenced by both genetic and environmental factors [18] and is often considered dependent on trait anxiety at the individual level. It has also been proposed as an endophenotype of individual disposition towards emotional expression, self-control and inhibition of contrasting impulses [19, 20]. EDA-labiles people may be described as calm, deliberative, restrained, more good-natured, cooperative, and responsible, whereas EDA-stable people tend to be active, emotionally expressive, animated, assertive, more irritable, more antagonistic, more impulsive and more irresponsible [21, 22]. Both depression and suicide have been extensively investigated from a physiological point of view, and the extant research has consistently demonstrated that depressed and suicidal patients show electrodermal hypoactivity and can therefore be described as EDA-stable [23,24,25,26].
To the best of our knowledge the first review of EDA in depression was published by Straub et al. in the early 1990s [25]. It acknowledged that electrodermal hypoactivity had been repeatedly associated with affective disorders, but raised doubts about whether EDA could be considered a marker of depression, pointing to conflicting results and differences in laboratory conditions and suggesting that the impact of person-situation-environment dynamics on EDA had been underestimated. A more recent review of galvanic skin response (GSR) confirmed that patients with mood disorders show low or flat EDA profiles, but the authors pointed out several limitations in the evidence, including that many of the EDA studies are quite old and were conducted using outdated methods and technology [26]. The Vahey & Becerra review [26] anyway focused specifically on mood disorders and yielded a smaller number of eligible studies (41 vs. 77).
A summary of the current knowledge about the relationships between EDA and depression and between EDA and suicidal behaviour is timely, given the lack of recent comprehensive reviews [24] and the recent publication of the study protocol for a multi-centre, naturalistic, clinical study of electrodermal orienting reactivity in a large sample of adult depressed patients [27].
Objectives
Our objective was to carry out a systematic review of studies investigating:
-
1.
The association between EDA and depression (research comparing depressed patients and controls, depression subgroups, depressed patients and other psychiatric patients, elicitation methods and EDA, EDA and hormones, EDA and antidepressants and other studies of EDA characteristics).
-
2.
The association between EDA and suicidal behaviour (including research on suicidal risk, suicidal ideation only, completed/attempted suicide, violent/non-violent suicidal behaviour, impulsive/non-impulsive suicidal behaviour and suicide prevention) in depressed patients.
Methods
Selection of studies
The inclusion criteria were the following:
-
EDA was measured.
-
Design: randomised controlled trial (RCTs), quasi-experimental (e.g. non-randomised controlled studies and before-and-after studies), observational or meta-analytical.
-
Participants of any age or gender in a community or clinical setting.
-
The following types of study were eligible for inclusion:
-
studies involving participants with a primary diagnosis of mood disorder (unipolar depression, bipolar disorder, dysthymia, cyclothymia) who were in a depressive phase;
-
studies investigating the effects of an antidepressant compound on EDA;
-
studies involving participants with suicidal ideation or suicidal behaviour (suicide attempts, completed suicide).
-
Exclusion criteria were:
-
Primary focus on psychiatric disorders other than mood disorders.
-
Investigation of the effects of EDA on medication other than antidepressants.
-
Case report, letter to the editor, conference paper, dissertation, personal opinion or commentary.
-
Papers not written in English.
Data sources and search strategy
We carried out an electronic literature search to identify relevant studies. PubMed, Scopus, Cochrane Library and Web of Science were searched from the inception of the databases up to 12th April 2017. The search strings used in each search engine are reported in the Appendix 1. Articles were selected in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram [28, 29]. The selection was done by three reviewers (M.S., M.I., V.C.) who independently selected titles, abstracts and full-text publications according to the inclusion and exclusion criteria described above. In the first stage of the selection process titles were screened to exclude those that were clearly not relevant to the review and then each reviewer read the abstract and full text of the remain articles and selected the relevant ones. Disagreements between reviewers were resolved through group discussion.
The following information was extracted from all publications: country, design, characteristics of study participants (number of subjects, diagnosis, mean age, % women), EDA variables and summary of main study findings. Regrettably, availability of information on several issues - sample and diagnosis; study design and protocol; type of EDA assessment; type of data presented in the results etc. - was very uneven and this meant that neither meta-analysis nor quantitative synthesis was possible, nor was it possible to adhere completely to the “Guidance on the conduct of narrative synthesis in systematic reviews”; nonetheless the guidance on data analysis and presentation was followed as closely as possible [30]. Study quality was appraised, where applicable, using the Newcastle-Ottawa Scale (NOS) [31].
Results
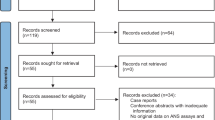
The electronic searches identified 1278 studies; once duplicates removed 823 records remained of which 606 were excluded because the title clearly had no relevance to the review; 109 records were excluded based on the Abstract; a further 68 studies were excluded because they were not written in English and 41 studies because the full text was not available. We assessed the full text of 108 articles and excluded 31 studies at this stage. The main reasons for exclusion were: study population did not match inclusion criteria (no primary clinical diagnosis of depression), focus on psychophysiological variables other than EDA (e.g. heart rate), basic, non-clinical research on EDA and wrong type of publication (conference session; letter to the editor; case report). Seventy-seven studies met all the inclusion criteria and were included in this review. The selection process is represented in Fig. 1.
Summaries of all the studies are presented in Tables 1 and 2 (EDA and depression) and 2 (EDA and suicidal behaviour).
Association between EDA and depression
Although low EDA in depressed patients was first described in 1890 [32], interest in this physiological variable as a marker of depressive disorders occurred mainly between the late 1970s and the 1990s.
The studies included in this section are very varied, both regarding sample and EDA variables. For clarity they have been grouped under the following headings: Depressed patients vs. controls; Depression subgroups; Depressed patients vs. other psychiatric patients; Elicitation methods and EDA; Other studies of EDA characteristics; EDA and hormones; EDA and antidepressants.
Depressed patients vs. controls
Nine studies, with a NOS score ranging from 2 (2 studies) to 5 (3 studies), mean NOS score 3.8, reported lower SCL, increased SCR latency and lower SCR amplitude in depressed subjects compared with healthy controls [33,34,35,36,37,38,39,40,41]. Thorell et al. reported a lower level of EDA in depressed patients than in healthy subjects, reflected in lower central values for SCL, SCR amplitude, SCR rate and an index of non-response during neutral tone stimulation [42].
The overall efficiency of SCR as a means of discriminating between depressed and healthy subjects has been estimated to be about 80% [43]. It has also been reported that depressed patients show marginally faster habituation of the skin resistance orienting response than healthy controls [44, 45], although one study [35] with a lower NOS score than the others (2 vs. 3 and 5) did not find this.
Some researchers looked at age- and gender-related EDA variability. In adolescents [46] the results were consistent with the notion that EDA is lower in major depressive disorder (MDD) patients than in control subjects. In contrast SCL was similar in patients with dementia, depressed patients (mean age 75.9 ± 7 years) and healthy controls, probably due to a decline in the number of active sweat glands and sweat production in the elderly. As far as gender is concerned, SCLs were lower in women than in men in both depressed patients and healthy controls [47].
A study of GSRfound that patients were hyporesponsive because of depressive inhibition in an experimental condition, but failed to find any difference in GSR variables between patients and controls in two rest periods and in a no-response experimental condition [48].
Unlike most studies in this field Lapierre and Butter reported a higher SCL and higher basal skin resistance in depressed patients than in controls. The amplitude of SCR and number of non-specific SCRs were similar in depressed patients and controls [49]. Other studies reported higher SCLs in depressed subjects than in healthy controls [50, 51] and psychiatric controls [50]. A couple of studies [52, 53] failed to find any difference between depressed patients and healthy controls with respect to SCL [52] and SCR [53] variables.
It should be noted that the Lapierre and Butter study scored 0 on the NOS because of the lack of information about the study design, whereas the other studies [50–52] described above had higher NOS scores (5,4, and 4, respectively).
Briefly, lower EDA, especially lower SCL and SCR, in depressed patients than in healthy controls was the most consistently reported result.
Depression subgroups
It has been suggested that in depressed patients level of EDA may be a function of the type of depression. A recent, cross-sectional study offered preliminary evidence that EDA levels may differentiate the phases (depressive vs. mixed vs. euthymic) of bipolar disorder [54].
Lower EDA levels were found in patients with psychomotor retardation or symptoms of inhibition than in agitated depressed patients ([55, 56, 58], mean NOS score 4.3; [57, 59] cross-sectional studies), except for the study by Lapierre and Butter, which was the least robust in terms of NOS score [49; NOS score 0]. Patients classified as suffering from “psychotic” (rather than “neurotic”) [60] and “endogenous” (rather than “non-endogenous”) [61, 62] depression had lower EDA levels, although this finding was not consistent across all studies. Moreover, the published research on this issue [40, 58, 59, 63] is highly heterogeneous with respect to NOS score (2, 5, not applicable, and 1, respectively).
The absence of a SCR was observed in depressed patients with predominance of anxiety symptoms [40].
In brief, patients classified as suffering from “psychotic” and “endogenous” depression had lower EDA levels compared to “neurotic” and “non-endogenous” depression. However, in view of the significant differences in the methodology and design of the relevant studies and their inconsistent results, the utility of EDA as a means of discrimination between subgroups of depressive patients remains to be demonstrated.
Depressed patients vs. other psychiatric patients
Comparisons of EDA in depressed patients and patients with other psychiatric disorders have been performed to ascertain whether EDA can be used as a marker of depressive states.
EDA abnormalities have been described in schizophrenic patients [64]. Compared with normal controls, both schizophrenic and depressed patients showed high levels of non-response in the habituation series, but schizophrenics - unlike the depressed patients - showed a decrease in SCR non-response to the target tone [55, 65]. In contrast Levinson [51] found no substantial differences between schizophrenic and depressed patients and normal controls with respect to SCR.
Pruneti et al. [66] found that patients with generalised anxiety disorder or panic disorder had higher SCRs than patients with major depression or obsessive-compulsive disorder.
Have et al. [67] reported that depressed patients, patients with degenerative dementia of the Alzheimer type and healthy controls had similar SCLs.
In summary, there have been only six studies comparing depressed patients to other psychiatric patients, and they deal with different disorders, making it hard to draw clear conclusions.
Elicitation methods and EDA
The published research using emotional elicitation protocols and other tasks to investigate EDA is highly heterogeneous with respect to sample, EDA variables, task and NOS score (range: 3 - 6), making it very hard to compare studies and to draw unequivocal conclusions.
Lower EDA, lower SCRs and higher SCL/SCRs were found in depressed subjects compared with controls in response to various emotional elicitation protocols (including exposure to pleasant and unpleasant pictures, exposure to sad and amusing film clips, and suppression of emotional reaction to pictures) [68,69,70,71]. Other studies found contrasting or mixed results depending on the type of task [72,73,73,74,75, 50]. For instance, in a standardised mood induction experiment, MDD patients had higher SCRs than controls in the cartoon condition, but not when mood was induced through happy and neutral pictures [73]. Schneider et al. [75] found that depressed subjects showed increased reactivity and autonomic arousal (elevated GSRs) in response to affective stimuli, compared to healthy controls [75]. Rohde and coworkers [76] studied depressed patients and healthy controls performing a Mindful Breathing Exercise task and found no difference in SCR between the two groups.
A couple of studies including elicitation protocols compared depressed patients with a seasonal pattern (seasonal affective disorder) and healthy controls. When exposed to overcast stimuli [77] or winter scenes [78] patients with seasonal affective disorder displayed more frequent SCRs and SCRs of greater magnitude, whereas the opposite pattern was found for sunny stimuli [77] and there was no disease by stimulus interaction for SCLs [77].
In short, the heterogeneity of study designs does not allow to draw clear conclusions in this field.
EDA and hormones
Only two studies dealt with this topic and they concerned different hormones, so it is not possible to generalise from the results. Based on the dexamethasone suppression test EDA in depressive patients does not appear to be related to dysfunctions of the hypothalamic-pituitary-adrenal axis. On the other hand, suicide attempters exhibited opposite correlations between EDA and cortisol in plasma and in urine, suggesting that there may be a complex relationship between EDA and cortisol production [79].
The positive correlations found between basal levels of thyroid hormones and SCL in healthy subjects were absent or reversed in depressed patients [80].
EDA and antidepressants
A cross-sectional study by Weckowicz [81] found GSR was a near-significant predictor of psychotherapy and drug therapy in depressed patients.
Several studies [37, 43, 55, 59, 82] failed to find any difference in EDA levels in depressed patients in response to antidepressant treatment or other medication, but it is hard to draw clear conclusions from these studies, many of which assessed non-specified antidepressants and/or antipsychotic medication.
Other studies investigating the effects of antidepressant compounds on EDA have found different results, but they cover a range of drugs and the results are mixed. Of the studies selected for this review, 6 assessed the effects of tricyclic antidepressants (imipramine [83, 84], amitriptyline [85,86,87,88]); 1 a tetracyclic antidepressant (maprotiline; [83]); 1 a serotonin antagonist and reuptake inhibitor (nefazodone; [89]); 3 selective serotonin reuptake inhibitors (paroxetine; [84], sertraline; [90], fluoxetine; [91]); 1 a noradrenaline reuptake inhibitor (reboxetine; [92]); 1 a serotonin-noradrenaline reuptake inhibitor (venlafaxine; [93]); 1 a reversible inhibitor of monoamine oxidase A (moclobemide; [94]); and 1 an unspecified antidepressant [95].
Imipramine-treated patients showed lower EDA than controls [84]. Regarding amitriptyline, findings were not consistent across studies, spanning from no correlation between the drug plasma level and EDA measures [86], to lower activation and decreased NS.SCRs and SCR in patients treated with amitriptyline [78, 87, 88]. Reboxetine reduced SCR after multiple dosing [92], while sertraline, moclobemide and nefazodone-treated patients showed no change in SCR [90, 94, 89]. On the other hand, sertraline-treated patients had lower SCL compared to controls [90]. Venlafaxine caused a reduction of EDA measures [93]. See Tables 1 and 2 for further details.
Very briefly, studies of EDA and antidepressants, either found no correlation between drugs and EDA measures, or a reduction of EDA measures (NS.SCRs, SCR, SCL) in subjects taking medications.
Other studies of EDA characteristics
A couple of follow-up studies investigated the temporal stability of EDA. In the studies of Iacono and coworkers [42, 45] the EDA variables were moderately stable at the one-year follow-up, whereas in the study of Thorell and d’Elia [43, 61] patients’ EDA was elevated in the remission phase and similar to that of the matched healthy subjects. It should be noted, however, that the mean follow-up period in this study was 2 years, which suggests that the recovery of tonic EDA in patients with affective disorders is probably a very slow process. Thorell and d’Elia’s study had a more robust design than the Iacono studies (NOS 5 vs. NOS 3).
A one-year cross-sectional follow-up study of depressive and depressive-anxious patients found mixed results with respect to the temporal stability of EDA: changes were smallest in stable patients, and greatest in all four labile-activated patients [85].
The laterality of EDA has also been explored and it has been hypothesised that there is right-hemisphere hyperexcitability in depressive conditions. After the original 1978 study by Myslobodsky and Horesh [96] others reported that EDA levels were lower on the right hand than the left under various experimental conditions, including rest [97]. Nevertheless, contrasting results were obtained both at rest and during stimulation [65, 98], and during different phases of illness, including remission [37, 99]. Notably, the first two studies cited in this paragraph had lower NOS scores (3 and 2) than the last four (respectively 4, 6, 5, 5); the existence of lateral differences in EDA remains to be confirmed.
EDA and suicidal behaviour
It has been suggested that differences in EDA may be specific to suicidality rather than depression [100]. Although there has been less research on EDA in individuals exhibiting suicidal behaviour than in people with depression, there is consistent evidence of electrodermal hypoactivity in depressed suicide attempters compared with non-suicidal depressed patients and healthy controls [61, 58, 101, 102].
Correlations between EDA and the type and level of suicide risk have been suggested. For instance, one study compared patients recently admitted to the hospital because of suicide threats or preoccupations, but with no history of attempts, controls with no history of suicide threats or attempts and no reported suicidal thoughts at the time of data collection and suicide attempters with a history of one or more suicide attempts. The first group showed the smallest GSR to the word ‘suicide’, suggesting generally lower reactivity [103].
Other studies have assessed SCR habituation in individuals with different patterns of suicidal behaviour. Violent suicide attempters and suicide completers were both found to be fast habituators [101, 104, 105]. The findings on non-violent suicide attempters, patients with suicidal ideation and non-suicidal depressed patients are less clear: one study found that non-violent suicide attempters showed either fast or slow habituation [101] but another found no differences among these groups (violent and non-violent suicide attempters, patients with suicidal ideation, non suicidal patients) [104]. Jandl et al. found no difference in the habituation of violent and non-violent suicide attempters, but corroborated the general finding of hyporeactivity in suicide attempters compared with non-attempters [106]. A study comparing parasuicidal adolescent girls with healthy controls found no differences in EDA variables [107].
Thorell et al. [24] carried out a meta-analysis of earlier research covering a total of 297 depressed patients and 59 healthy subjects. Electrodermal hyporeactivity was strongly associated with high suicide risk. Extremely low electrodermal reactivity had a sensitivity of 96.6% and a specificity of 92.9% for suicide and a sensitivity of 83.3% and specificity of 92.7% for suicide and/or violent suicide attempt.
A further analysis of data from 783 depressive patients by Thorell et al. [108] confirmed that electrodermal hyporeactivity is a marker of suicidal tendency in both unipolar and bipolar depression, independently of severity of depression, trait anxiety, gender and age.
Recently the protocol for a study involving 1573 patients with a primary diagnosis of depression recruited from 15 centres in nine European countries that will test the predictive value of electrodermal hyporeactivity (measured with the electrodermal orienting reactivity EDOR test) for suicide and suicide attempt has been published. The extant literature suggests that suicide attempt with intent to die and completed suicide will be associated with electrodermal hyporeactivity [27].
Discussion
The aim of this review is to offer a comprehensive overview of the EDA literature with a view to assessing its potential utility as a biomarker of depressive states and risk of suicidal behaviour, and its potential role in advanced, integrated physiological evaluation systems.
Overall, our review of the literature supports the hypothesis that electrodermal hypoactivity is a feature of depression. Nevertheless, considering the number and robustness of studies, EDA seems to be more useful in discriminating depressive patients from healthy controls than from other psychiatric patients. Moreover, specific EDA features (e.g., SCL, SCR, NS.SCR, habituation rate, etc.) seem to be differently affected, and the extremely contrasting nature of studies and of the variables used cannot be overlooked. Briefly, specific patterns of electrodermal hypoactivity may be a reliable marker of a depressive state at population level, but they should be carefully combined with other physiological and non-physiological indicators when used for preventive and diagnostic purposes.
Another area that deserves further investigation is the potential use of EDA to distinguish between subtypes of depression. At present the evidence suggests that patients with psychomotor retardation, endogenous and psychotic depression show lower EDA values than patients with agitation, non-endogenous and neurotic depression, respectively.
It has been hypothesised that electrodermal hypoactivity is a rather stable trait of patients affected by depression, although increases in EDA may indicate euthymia or remission [61, 54]. It should be noted that extremely hyporesponsive depressive patients, including suicide attempters and patients with recurrent major depression, may fail to reach the EDA levels of healthy subjects even when in remission [54].
There is even more debate about the effects of antidepressants on EDA and research has yielded mixed results. In the context of experimental anxiety conditioning tasks some antidepressants blunt EDA in healthy subjects, but the data from depressed patients are not consistent and there is no clear correlation between EDA and clinical improvement. As Thorell hypothesised, electrodermal hypoactivity may be a rather stable trait of depressed patients; EDA appears to be only marginally affected by treatment and clinical improvement, and normalisation may not occur until several months - or even years - after clinical recovery [61]. Moreover, there is only limited evidence in relation to each drug and for most drugs it comes from just one study.
As far as suicidal behaviour is concerned, extreme hyporeactivity has been consistently reported in both suicide attempters and at baseline in subjects who eventually committed suicide during a follow-up period; moreover, hyporeactivity seems to be related to the choice of a violent method for attempted or completed suicide [101, 104, 105]. On this basis it has been hypothesised that extreme electrodermal hypoactivity is a marker of suicidal tendencies in depressed patients, and it appears to be independent of severity of depression [61, 102]. Recent studies [24, 108] showed that EDA discriminates well between patients who will subsequently commit suicide, make a non-violent suicide attempt or make a violent suicide attempt, but it is less clear that EDA can be used to distinguish individuals with current suicidal ideation from depressed patients who are not currently suicidal. Nevertheless, it has been suggested that the evidence is sufficient to warrant strict monitoring of both euthymic and depressed patients who are show extreme electrodermal hyporeactivity, even in the absence of suicidal ideation. Obviously close monitoring and adequate antidepressant therapy are even more necessary in hyporeactive patients with suicidal ideation [24].
Assessment of the robustness of the synthesis and limitations
We adhered to the PRISMA statement [28, 29], which requires the use of defined inclusion/exclusion criteria, a rigorous search strategy and assessment of the quality assessment of included studies. Nevertheless, several limitations of this review should be acknowledged. First, as shown by the NOS scores, the quality of several of the included studies is questionable and most are quite old. The criteria for ‘depression’ vary somewhat between studies. Most of studies used the Diagnostic and Statistical Manual of Mental Disorders (DSM-III and DSM-IV) criteria [109, 110], but some used the International Classification of Diseases (ICD-9 and ICD-10) [111, 112], the Research Diagnostic Criteria [113], the Feighner Research Criteria or multiple classification systems [114]. Definitional inconsistency is also a problem in comparisons of subtypes of depression as there are no standard criteria for distinguishing between, for example, retarded and agitated depression, endogenous and non-endogenous depression or psychotic and neurotic depression. Furthermore, most studies included patients with various mood disorder diagnoses (e.g., unipolar depression, bipolar depression, dysthymia), without specifying the illness phase or the treatment patients were receiving. Finally, comorbidity was not always accounted for in comparisons between depression and other pathological conditions.
Besides diagnostic issues, over time the EDA assessment methods have changed, leading to inconsistencies. Not all the studies have assessed the same EDA parameters, and despite improvements in measuring equipment since the discovery of electrodermal phenomena more than 100 years ago [32], much of the research reviewed in the present article is limited to observational measurements performed over short periods of time, in laboratory settings or artificial clinical environments.
Conclusions
This review offers a more comprehensive assessment of the extant EDA literature than previous ones [25, 26] and it corroborates their findings, namely that there are associations between electrodermal hypoactivity and depression and suicidal behaviour. EDA appears to be a reliable marker, with high sensitivity and specificity, of depressive states, suicidal tendencies and suicidal behaviour [24, 39, 43, 108]. Nevertheless, further studies are required to validate EDA as an indicator of other clinical features, such as depression subtypes, response to treatment and acute suicide risk.
Abbreviations
- EDA:
-
Electrodermal activity
- GSR:
-
Galvanic skin response
- MDD:
-
Major depressive disorder
- NS.SCR:
-
Non-specific skin conductance response
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- SCL:
-
Skin conductance level
- SCR:
-
Skin conductance response
- SRR:
-
Skin resistance response
References
Sudol K, Mann JJ. Biomarkers of suicide attempt behavior: towards a biological model of risk. Curr Psychiatry Rep. 2017;19(6):31. https://doi.org/10.1007/s11920-017-0781-y. Review. PubMed PMID: 28470485
Öhman A, Hamm A, Hugdahl K. Cognition and the autonomic nervous system: orienting, anticipation, and conditioning. In Cacioppo JT, Tassinary LG & Berntson GG (Eds.), Handbook of psychophysiology (2nd ed.). New York, NY: US: Cambridge University Press; 2000. pp. 533–575.
Pandey GN, Dwivedy Y. The neurobiological basis of suicide. Boca Raton (FL): CRC Press, Taylor & Francis, Chapter 20 Peripheral biomarkers for suicide, 2012.
Groscurth P. Anatomy of sweat glands. Curr Probl Dermatol. 2002;30:1–9.
Gunnar Wallin B, Fagius J. The sympathetic nervous system in man — aspects derived from microelectrode recordings. Trends Neurosci. 1986;9:63–7. https://doi.org/10.1016/0166-2236(86)90024-X.
Asahina M, Suzuki A, Mori M, Kanesaka T, Hattori T. Emotional sweating response in a patient with bilateral amygdala damage. Int J Psychophysiol. 2003;47(1):87–93.
Bradley MM, Lang PJ. Measuring emotion: behavior, feeling and physiology. In Cognitive Neuroscience of Emotion. Eds. R.D. Lane and L. Nadel. New York: Oxford University Press. 2000.
Boucsein, W. Electrodermal activity (2nd ed.). New York: Springer Science & Business Media; 2012.
Braithwaite JJ, Watson DG, Jones R, Rowe M. A guide for analysing electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments. Psychophysiology. 2013;49:1017–34.
Yoon JH, Ko CM, Ahn YS, Park KS, Choe KH, Yoo KJ, et al. Mechanism of decrease in heart rate by peripheral dopaminergic D2-receptors. Yonsei Med J. 1994;35(4):411–9.
Lee GP, Arena JG, Meador KJ, Smith JR, Loring DW, Flanigin HF. Changes in autonomic responsiveness following bilateral amygdalectomy in humans. Neuropsychiatry, Neuropsychol Behav Neurol. 1988;1(2):119–29.
Mangina CA, Beuzeron-Mangina JH. Direct electrical stimulation of specific human brain structures and bilateral electrodermal activity. Int J Psychophysiol. 1996;22(1-2):1–8. https://doi.org/10.1016/0167-8760(96)00022-0.
Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. https://doi.org/10.1111/j.1469-8986.1993.tb03352.x.
Manning SK, Melchiori MP. Words that upset Urban College students: measured with GSRs and rating scales. J Soc Psychol. 1974;94(2):305–6. https://doi.org/10.1080/00224545.1974.9923225.
Winton WM, Putnam LE, Krauss RM. Facial and autonomic manifestations of the dimensional structure of emotion. J Exp Soc Psychol. 1984;20(3):195–216. https://doi.org/10.1016/0022-1031(84)90047-7.
Lacey JI, Lacey BC. The relationship of resting autonomic activity to motor impulsivity. Res Publ Assoc Res Nerv Ment Dis. 1958;36:144–209.
Mundy-Castle AC, McKiever BL. The psychophysiological significance of the galvanic skin response. J Exp Psychol. 1953;46(1):15–24. https://doi.org/10.1037/h0060100.
Crider A, Kremen WS, Xian H, Jacobson KC, Waterman B, Eisen SA, et al. Stability, consistency, and heritability of electrodermal response lability in middle-aged male twins. Psychophysiology. 2004;41(4):501–9. https://doi.org/10.1111/j.1469-8986.2004.00189.x.
Gottesman II, Hanson DR. Human development: biological and genetic processes. Annu Rev Psychol. 2005;56(1):263–86. https://doi.org/10.1146/annurev.psych.56.091103.070208.
Iacono WG. Identifying psychophysiological risk for psychopathology: examples from substance abuse and schizophrenia research. Psychophysiology. 1998;35(06):621–37.
Crider A. Electrodermal response Lability-stability: individual difference correlates. In J.-C. Roy, W. Boucsein, D. C. Fowles, & J. H. Gruzelier (Eds.), Progress in Electrodermal Research Springer US; 1993. New York: Plenum. 1993:173–186.
Crider A. Personality and Electrodermal response Lability: an interpretation. Applied Psychophysiology and Biofeedback. 2008;33(3):141–8. https://doi.org/10.1007/s10484-008-9057-y.
Straub R, Jandl M, Wolfersdorf M. Depressive state and Electrodermal activity of depressed inpatients during an acute suicidal state. Psychiatr Prax. 2003;30(Suppl 2):183–6. https://doi.org/10.1055/s-2003-39762.
Thorell LH. Valid electrodermal hyporeactivity for depressive suicidal propensity offers links to cognitive theory. Acta Psychiatr Scand. 2009;119(5):338–49. https://doi.org/10.1111/j.1600-0447.2009.01364.x.
Straub R, Hole G, Wolfersdorf M. Electrodermal hypoactivity in depression: psychobiological marker or differential psychophysiologic disposition? Schweiz Arch Neurol Und Psychiatrie (1985). 1992;143(1):41–59.
Vahey, R., Becerra, R. (2015). Galvanic Skin Response in Mood Disorders: A Critical Review. 2015. http://www.redalyc.org/articulo.oa?id=56041176008. Accessed 15 Dec 2016.
Sarchiapone M, Iosue M, Carli V, Amore M, Baca Garcia E, Batra A, et al. EUDORA multicentre research program: a naturalistic, European multicentre clinical study of EDOR test in adult patients with primary depression. BMC Psychiatry. 2017;17(1):108. https://doi.org/10.1186/s128880171246x.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. The BMJ. 2015;349:g7647. https://doi.org/10.1136/bmj.g7647.
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Programme. Version, 1. 2006 http://www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications/NS_Synthesis_Guidance_v1.pdf. Accessed 15 Dec 2016.
Wells GA, Shea B, O’connell D, Peterson JEA, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.medicine.mcgill.ca/rtamblyn/Readings/The%20Newcastle%20-%20Scale%20for%20assessing%20the%20quality%20of%20nonrandomised%20studies%20in%20meta-analyses.pdf. Accessed 15 Dec 2016.
Vigouroux A. Etude sur la résistance électrique chez les mélancoliques. Paris: Méd; 1890.
Carney RM, Hong BA, Kulkarni S, Kapila A. A comparison of EMG and SCL in normal and depressed subjects. Pavlov J Biol Scie. 1981;16(4):212–6.
Dawson ME, Schell AM, Catania JJ. Autonomic correlates of depression and clinical improvement following electroconvulsive shock therapy. Psychophysiology. 1977;14(6):569–78. https://doi.org/10.1111/j.1469-8986.1977.tb01201.x.
Miquel M, Fuentes I, Garcia-Merita M, Rojo L. Habituation and sensitization processes in depressive disorders. Psychopathology. 1999;32(1):35–42. https://doi.org/10.1159/000029064.
Storrie MC, Doerr HO, Johnson MH. Skin conductance characteristics of depressed subjects before and after therapeutic intervention. J Nerv. 1981;169(3):176–9.
Bonnet A, Naveteur J. Electrodermal activity in low back pain patients with and without comorbid depression. Int J Psychophysiol. 2004;53(1):37–44. https://doi.org/10.1016/j.ijpsycho.2004.01.004.
Donat DC, McCullough JP. Psychophysiological discriminants of depression at rest and in response to stress. J Clin Psychol. 1983;39(3):315–20. https://doi.org/10.1002/1097-4679(198305)39:3<%20315:AID-JCLP2270390303>%203.0.CO%202-H.
Ward NG, Doerr HO. Skin conductance: a potentially sensitive and specific marker for depression. J Nerv Ment Dis. 1986;174(9):553–9. https://doi.org/10.1097/00005053-198609000-00008.
Kamenskaya VM, Mikhailova FS. Ratios of electroencephalographic and autonomic indexes in a stress situation in patients with different types of depression. Neurosci Behav Physiol. 1985;15(6):483–7. https://doi.org/10.1007/BF01184258.
Iacono WG, Lykken DT, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB. Electrodermal activity in euthymic unipolar and bipolar affective disorders. A possible marker for depression. Arch Gen Psychiatry. 1983;40(5):557–65.
Thorell LH, Kjellman BF, d’Elia G. Electrodermal activity in antidepressant medicated and unmedicated depressive patients and in matched healthy subjects. Acta Psychiatr Scand. 1987;76(6):684–92. https://doi.org/10.1111/j.1600-0447.1987.tb02940.x.
Dawson ME, Schell AM, Braaten JR, Catania JJ. Diagnostic utility of autonomic measures for major depressive disorders. Psychiatry Res. 1985;15(4):261–70. https://doi.org/10.1016/0165-1781(85)90063-0.
Iacono WG, Peloquin LJ, Lykken DT, Haroian KP, Valentine RH, Tuason VB. Electrodermal activity in euthymic patients with affective disorders: one-year retest stability and the effects of stimulus intensity and significance. J Abnorm Psychol. 1984;93(3):304–11. https://doi.org/10.1037/0021-843X.93.3.304.
Giedke H, Heimann H. Psychophysiological aspects of depressive syndromes. Pharmacopsychiatry. 1987;20(5):177–80. https://doi.org/10.1055/s-2007-1017098.
Mestanikova A, Ondrejka I, Mestanik M, Hrtanek I, Snircova E, Tonhajzerova I. Electrodermal activity in adolescent depression. Adv Exp Med Biol. 2016;935(26):83–8. https://doi.org/10.1007/5584_2016_40.
Ward NG, Doerr HO, Storrie MC. Skin conductance: a potentially sensitive test for depression. Psychiatry Res. 1983;10:295–302.
Giedke H, Bolz J, Heimann H. Evoked potentials, expectancy wave, and skin resistance in depressed patients and healthy controls. Pharmakopsychiatr Neuropsychopharmakol. 1980;13(3):91–101.
Lapierre YD, Butter HJ. Agitated and retarded depression. Neuropsychobiology. 1980;6(4):217–23. https://doi.org/10.1159/000117755.
Lewinsohn PM, Lobitz WC, Wilson S. “Sensitivity” of depressed individuals to aversive stimuli. J Abnorm Psychol. 1973;81(3):259–63.
Levinson DF. Skin conductance orienting response in unmedicated RDC schizophrenic, schizoaffective, depressed, and control subjects. Biol Psychiatry. 1991;30(7):663–83. https://doi.org/10.1016/0006-3223(91)90012-B.
Pazderka-Robinson H, Morrison JW, Flor-Henry P. Electrodermal dissociation of chronic fatigue and depression: evidence for distinct physiological mechanisms. Int J Psychophysiol. 2004;53(3):171–82. https://doi.org/10.1016/j.ijpsycho.2004.03.004.
O'Kearney R, Parry L. Comparative physiological reactivity during script-driven recall in depression and posttraumatic stress disorder. J Abnorm Psychol. 2014;123(3):523–32. https://doi.org/10.1037/a0037326.
Greco A, Valenza G, Lanata A, Rota G, Scilingo EP. Electrodermal activity in bipolar patients during affective elicitation. IEEE J Biomed Health Inform. 2014;18(6):1865–73. https://doi.org/10.1109/JBHI.2014.2300940.
Bernstein AS, Schnur DB, Bernstein P, Yeager A, Wrable J, Smith S. Differing patterns of electrodermal and finger pulse responsivity in schizophrenia and depression. Psychol Med. 1995;25(01):51–62. https://doi.org/10.1017/S0033291700028087.
Lader MH, Wing L. Physiological measures in agitated and retarded depressed patients. J Psychiatr Res. 1969;7(2):89–100. https://doi.org/10.1016/0022-3956(69)90014-4.
Noble P, Lader M. The symptomatic correlates of the skin conductance changes in depression. J Psychiatr Res. 1971;9(1):61–9. https://doi.org/10.1016/0022-3956(71)90008-2.
Thorell LH, Kjellman BF, d’Elia G. Electrodermal activity in relation to diagnostic subgroups and symptoms of depressive patients. Acta Psychiatr Scand. 1987;76(6):693–701. https://doi.org/10.1111/j.1600-0447.1987.tb02941.x.
Williams KM, Iacono WG, Remick RA. Electrodermal activity among subtypes of depression. Biol Psychiatry. 1985;20(2):158–62. https://doi.org/10.1016/0006-3223(85)90075-7.
Byrne DG. A psychophysiological distinction between types of depressive states. Aust N Z J Psychiatry. 1975;9(3):181–5.
Thorell LH, d’Elia G. Electrodermal activity in depressive patients in remission and in matched healthy subjects. Acta Psychiatr Scand. 1988;78(2):247–53. https://doi.org/10.1111/j.1600-0447.1988.tb06332.x.
Mirkin AM, Coppen A. Electrodermal activity in depression: clinical and biochemical correlates. Br J Psychiatry. 1980;137(1):93–7. https://doi.org/10.1192/bjp.137.1.93.
Perez-Reyes M, Cochrane C. Differences in sodium thiopental susceptibility of depressed patients as evidenced by the galvanic skin reflex inhibition threshold. J Psychiatr Res. 1967;5(4):335–47.
Dawson ME, Schell AM. What does electrodermal activity tell us about prognosis in the schizophrenia spectrum? Schizophr Res. 2002;54(1):87–93.
Bernstein AS, Riedel JA, Graae F, Seidman D, Steele H, Connolly J, et al. Schizophrenia is associated with altered orienting activity: depression with electrodermal (cholinergic?) deficit and normal orienting response. J Abnorm Psychol. 1988;97(1):3–12. https://doi.org/10.1037/0021-843X.97.1.3.
Pruneti CA, Lento RM, Fante C, Carrozzo E, Fontana F. Autonomic arousal and differential diagnosis in clinical psychology and psychopathology. J Psychopathology. 2010;16:43–52.
Have G, Kolbeinsson H, Pétursson H. Dementia and depression in old age: psychophysiological aspects. Acta Psychiatr Scand. 1991;83(5):329–33. https://doi.org/10.1111/j.1600-0447.1991.tb05550.x.
Mardaga S, Hansenne M. Autonomic aspect of emotional response in depressed patients: relationships with personality. Neurophysiol Clin. 2009;39(4-5):209–16. https://doi.org/10.1016/j.neucli.2009.06.002.
Lemaire M, El-Hage W, Frangou S. Increased affective reactivity to neutral stimuli and decreased maintenance of affective responses in bipolar disorder. European Psychiatry. 2015;30(7):852–60. https://doi.org/10.1016/j.eurpsy.2015.07.008.
Tsai JL, Pole N, Levenson RW, Muñoz RF. The effects of depression on the emotional responses of Spanish-speaking Latinas. Cult Divers Ethn Minor Psychol. 2003;9(1):49–63. https://doi.org/10.1037/1099-9809.9.1.49.
Rottenberg J, Gross JJ, Wilhelm FH, Najmi S, Gotlib IH. Crying threshold and intensity in major depressive disorder. J Abnorm Psychol. 2002;111(2):302–12.
Branković SB. System identification of skin conductance response in depression–an attempt to probe the neurochemistry of limbic system. Psychiatr Danub. 2008;20(3):310–22.
Falkenberg I, Kohn N, Schoepker R, Habel U. (2012). Mood induction in depressive patients: a comparative multidimensional approach. PLoS ONE. 2012; 7(1) e30016; doi:https://doi.org/10.1371/journal.pone.0030016.
Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. Learning as a model for neural plasticity in major depression. Biol Psychiatry. 2010;68(6):544–52. https://doi.org/10.1016/j.biopsych.2010.05.026.
Schneider D, Regenbogen C, Kellermann T, Finkelmeyer A, Kohn N, Derntl B, et al. Empathic behavioral and physiological responses to dynamic stimuli in depression. Psychiatry Res. 2012;200(23):294–305. https://doi.org/10.1016/j.psychres.2012.03.054.
Rohde K, Adolph D, Dietrich DE, Michalak J. Mindful attention regulation and non-judgmental orientation in depression: a multi-method approach. Biol Psychol. 2014;101:36–43.
Lindsey KT, Rohan KJ, Roecklein KA, Mahon JN. Surface facial electromyography, skin conductance, and self-reported emotional responses to light- and season-relevant stimuli in seasonal affective disorder. J Affect Disord. 2011;133(1-2):311–9. https://doi.org/10.1016/j.jad.2011.04.016.
Sigmon ST, Whitcomb-Smith S, Boulard NE, Pells JJ, Hermann BA, Edenfield TM, et al. Seasonal reactivity: Attentional bias and Psychophysiological arousal in seasonal and nonseasonal depression. Cogn Ther Res. 2007;31:619–38.
Thorell LH, Kjellman BF, d’Elia G, Kagedal B. Electrodermal activity in relation to cortisol dysregulation in depressive patients. Acta Psychiatr Scand. 1988;78:743–53.
Thorell LH, Kjellman BF, d’Elia G. Electrodermal activity in relation to basal and post-dexamethasone of thyroid stimulating hormone and basal levels of thyroid hormones in major depressive patients and healthy subjects. Psychiatry Res. 1993;47(1):23–36. https://doi.org/10.1016/01651781(93)90052I.
Weckowicz TE, Yonge KA, Cropley AJ, Muir W. Objective therapy predictors in depression: a multivariate approach. J Clin Psychol. 1971;27(1):4–29.
Bob P, Jasova D, Raboch J. Subclinical Epileptiform process in patients with Unipolar depression and its indirect Psychophysiological manifestations. PLoS One. 2011;6(11):e28041. https://doi.org/10.1371/journal.pone.0028041.
Lapierre YD, Butter HI. Imipramine and maprotiline in agitated and retarded depression: a controlled psychiatric and psychiphysical assessment. Prog Neuro-Psychopharmacology. 1978;2:207–16.
Barg T, Wolfersdorf M, Ruppe A. The influence of various antidepressants on heart rate and Electrodermal activity during Psychophysiological examinations. Pharmacopsychiar. 1996;29:216–9.
Heimann H. Changes of psychophysiological reactivity in affective disorders. Arch Psychiatr Nervenkr. 1978;225(3):223–31. https://doi.org/10.1007/BF00344009.
Breyer-Pfaff U, Gaertner HJ, Giedke H. Plasma levels, psychophysiological variables, and clinical response to amitriptyline. Psychiatry Res. 1982;6(2):223–34. https://doi.org/10.1016/0165-1781(82)90010-5.
Ikeda Y, Nomura S, Sawa Y, Nakazawa T. The effects of antidepressants on the autonomic nervous system-a current investigation. J Neural Transm. 1982;54(1-2):65–73. https://doi.org/10.1007/BF01249279.
Siepmann M, Kirch W, Krause S, Joraschky P, Mueck-Weymann M. The effects of St. John’s wort extract and amitriptyline on autonomic responses of blood vessels and sweat glands in healthy volunteers. J Clin Psychopharmacol. 2004(a);24(1):79–82. https://doi.org/10.1097/01.jcp.0000104911.75206.f0.
Silva M, Hetem LAB, Guimaraes FS, Graeff FG. Opposite effects of nefazodone in two human models of anxiety. Psychopharmacology. 2001;156:454–60.
Siepmann M, Grossmann J, Mück-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology. 2003;168(3):293–8. https://doi.org/10.1007/s00213-003-1448-4.
Fraguas R, Marci C, Fava M, Iosifescu DV, Bankier B, Loh R, et al. Autonomic reactivity to induced emotion as potential predictor of response to antidepressant treatment. Psychiatry Res. 2007;151(1-2):169–72. https://doi.org/10.1016/j.psychres.2006.08.008.
Siepmann M, Mück-Weymann M, Joraschky P, Kirch W. The effects of reboxetine on autonomic and cognitive functions in healthy volunteers. Psychopharmacology. 2001;157(2):202–7.
Zullino D, Chatton A, Fresard E, Stankovic M, Bondolfi G, Borgeat F, et al. Venlafaxine versus applied relaxation for generalized anxiety disorder: a randomized controlled study on clinical and electrophysiological outcomes. Psychiatr Q. 2015;86(1):69–82. https://doi.org/10.1007/s11126-014-9334-2.
Siepmann M, Handel J, Mueck-Weymann M, Kirch W. The effects of moclobemide on autonomic and cognitive functions in healthy volunteers. Pharmacopsychiatry. 2004(b);37(2):81–7. https://doi.org/10.1055/s-2004-815530.
Hattangadi S, Lidsky A, Lee H, Ban TA. Orienting-reflex behavior and clinical psychopathology. Cond reflex. 1968;3(1):29–33. https://doi.org/10.1007/BF03001134.
Myslobodsky MS, Horesh N. Bilateral electrodermal activity depressive patients. Biol Psychol. 1978;6(2):111–20. https://doi.org/10.1016/0301-0511(78)90050-9.
Schneider SJ. (1983). Multiple measures of hemispheric dysfunction in schizophrenia and depression. Psychol Med. 1983;13(02):287–97. https://doi.org/10.1017/S003329170005090X.
Toone BK, Cooke E, Lader MH. Electrodermal activity in the affective disorders and schizophrenia. Psychol Med. 1981;11(03):497–508. https://doi.org/10.1017/S0033291700052818.
Iacono WG, Tuason VB. Bilateral electrodermal asymmetry in euthymic patients with unipolar and bipolar affective disorders. Biol Psychiatry. 1983;18(3):303–15.
Wolfersdorf M, Straub R, Barg T. Electrodermal activity (EDA) and suicidal behavior. Crisis. 1996;17(2):69–77.
Edman G, Åsberg M, Levander S, Schalling D. Skin conductance habituation and cerebrospinal fluid 5-hydroxyindoleacetic acid in suicidal patients. Arch Gen Psychiatry. 1986;43(6):586–92. https://doi.org/10.1001/archpsyc.1986.01800060080010.
Thorell LH. Electrodermal activity in suicidal and nonsuicidal depressive patients and in matched healthy subjects. Acta Psychiatr Scand. 1987;76(4):420–30.
Spiegel D. Autonomic reactivity in relation to the affective meaning of suicide. J Clin Psychol. 1969;25(4):359–62.
Keller F, Wolfersdorf M, Straub R, Hole G. Suicidal behaviour and electrodermal activity in depressive inpatients. Acta Psychiatr Scand. 1991;83(5):324–8. https://doi.org/10.1111/j.1600-0447.1991.tb05549.x.
Wolfersdorf M, Straub R, Barg T, Keller F, Kaschka WP. Depressed inpatients, electrodermal reactivity, and suicide - a study about psychophysiology of suicidal behavior. Arch Suicide Res. 1999;5(1):1–10. https://doi.org/10.1080/13811119908258311.
Jandl M, Steyer J, Kaschka WP. Suicide risk markers in major depressive disorder: a study of Electrodermal activity and event-related potentials. J Affect Disord. 2010;123(1-3):138–49. https://doi.org/10.1016/j.jad.2009.09.011.
Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Dev Psychopathol. 2005;17:1105–27.
Thorell LH, Wolfersdorf M, Straub R, Steyer J, Hodgkinson S, Kaschka WP, et al. Electrodermal hyporeactivity as a trait marker for suicidal propensity in uni- and bipolar depression. J Psychiatr Res. 2013;47(12):1925–31. https://doi.org/10.1016/j.jpsychires.2013.08.017.
American Psychiatric Association, APA. Diagnostic and statistical manual of mental disorders, fourth edition: DSM-IV-TR®: American Psychiatric Association; 2000.
American Psychiatric Association, APA. Diagnostic and statistical manual of mental disorders. 3rd ed. DSM-III: American Psychiatric Association; 1980.
ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification. Salt Lake City, Utah: Medicode, 1996. Print.
The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. Print.
Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35(6):773–82. https://doi.org/10.1001/archpsyc.1978.01770300115013.
Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26(1):57–63.
Acknowledgements
Not applicable.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and material
The dataset supporting the conclusions of this article is included within the article and its additional files.
Author information
Authors and Affiliations
Contributions
VC, AS, MS and PZ contributed to planning, supervision, writing, and analysis of the study; MS, MI, VC independently selected titles, abstract and full text; CG, MI, LM and DM each contributed to data collection, writing the manuscript and review of the literature. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sarchiapone, M., Gramaglia, C., Iosue, M. et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: A systematic review and narrative synthesis. BMC Psychiatry 18, 22 (2018). https://doi.org/10.1186/s12888-017-1551-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-017-1551-4