Abstract
Background
Pancreatic cancer is anatomically divided into pancreatic head and body/tail cancers, and some studies have reported differences in prognosis. However, whether this discrepancy is induced from the difference of tumor biology is hotly debated. Therefore, we aimed to evaluate the differences in clinical outcomes and tumor biology depending on the tumor location.
Methods
In this retrospective cohort study, we identified 800 patients with pancreatic ductal adenocarcinoma who had undergone upfront curative-intent surgery. Cox regression analysis was performed to explore the prognostic impact of the tumor location. Among them, 153 patients with sufficient tumor tissue and blood samples who provided informed consent for next-generation sequencing were selected as the cohort for genomic analysis.
Results
Out of the 800 patients, 500 (62.5%) had pancreatic head cancer, and 300 (37.5%) had body/tail cancer. Tumor location in the body/tail of the pancreas was not identified as a significant predictor of survival outcomes compared to that in the head in multivariate analysis (hazard ratio, 0.94; 95% confidence interval, 0.77–1.14; P = 0.511). Additionally, in the genomic analyses of 153 patients, there were no significant differences in mutational landscapes, distribution of subtypes based on transcriptomic profiling, and estimated infiltration levels of various immune cells between pancreatic head and body/tail cancers.
Conclusions
We could not find differences in prognosis and tumor biology depending on tumor location in pancreatic ductal adenocarcinoma. Discrepancies in prognosis may represent a combination of lead time, selection bias, and clinical differences, including the surgical burden between tumor sites.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related deaths worldwide and has been characterized by dismal prognosis [1]. Regarding tumor location, PDAC was generally divided into pancreatic head and body/tail cancer. Therefore, the type of surgery that remains the backbone of treatment varies depending on the tumor location. Tumors located to the right of the superior mesenteric vein are considered pancreatic head cancers that are potentially suitable for pancreaticoduodenectomy, and tumors located to the left of the superior mesenteric vein are considered body/tail cancers that are potentially suitable for distal pancreatectomy [2].
In addition to differences in surgical methods, part of the pancreatic head, including the uncinate process, has a different embryonic origin. Because of this embryological difference, the head and body/tail of the pancreas have different innervations, blood supplies, and lymphatic and venous drainage. From a clinical perspective, pancreatic head cancer, which can induce biliary obstruction, is typically diagnosed at an early stage compared to body/tail cancer [3]. From these backgrounds, there has been a long debate regarding whether there is a real difference in prognosis between pancreatic head and body/tail cancers. Artinyan et al. (2008), using Surveillance, Epidemiology, and End Results registry, reported that pancreatic body/tail cancer had a lower resection rate and worse outcomes than those of head cancer [4]. Winer et al. (2019), using the National Cancer Database, reported that cancer localized to the pancreatic head had worse outcomes than those of body/tail cancers [5]. These contradictory findings call for further investigations because they raise the possibility that either lead time and selection bias played a role or that biological differences exist.
The recent large-scale sequencing studies have demonstrated the inter-tumoral and intra-tumoral genomic heterogeneity of PDAC [6]. With frequent alterations in main driver genes, including KRAS, TP53, CDKN2A, and SMAD4, Moffitt et al. (2015) and Bailey et al. (2016) established the molecular subtypes of PDAC, which showed the difference in survival according to tumor biology [7,8,9]. Some studies have noted genetic differences between pancreatic head and body/tail cancer, but most of them have flaws in that clinical information is either not taken sufficiently into account or data from various institutions are combined, making it challenging to completely rule out the impact of batch effects as well as different clinical stage and management [10,11,12]. Therefore, we aimed to evaluate the impact of tumor location on prognosis with consideration of other clinical and pathologic confounding factors. In addition, we aimed to explore the differences in tumor biology depending on tumor location using uniformly generated sequencing data with clinical details.
Materials and methods
Patient cohort
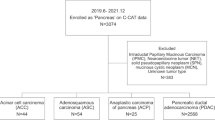
This retrospective cohort study was approved by the Institutional Review Board of Seoul National University Hospital (H-2309-159-1471). This study was conducted in accordance with the 1975 Declaration of Helsinki and its later versions. This work was registered at Research Registry (researchregistry9782). Data from patients who underwent pancreatectomy for pancreatic cancer between January 2005 and December 2020 were retrieved from prospectively maintained databases. Patients who underwent palliative surgery, received neoadjuvant treatment, had histology other than adenocarcinoma, underwent total pancreatectomy, lacked sufficient clinical information, had a previous history of pancreatectomy, or died within six months after surgery were excluded. Subsequently, 800 qualifying patients were selected as the cohort for analysis of clinical aspects (the entire cohort). Among them, 153 patients with sufficient tumor tissue and blood samples and who provided informed consent for next generation sequencing were selected as the cohort for the analysis of genomic aspects (genomic cohort). The genomic analysis cohort was designed to avoid discrepancies in clinical characteristics between patients with pancreatic head cancer and those with pancreatic body/tail cancer. A flow diagram of this study is presented in Fig. 1.
Clinical data collection
Detailed clinical characteristics, including demographics, pathology, and treatment information, were collected. Patient demographics included age, sex, American Society of Anesthesiologists physical status classification, presence of symptom, and carbohydrate antigen 19 − 9 at the time before surgery. Because the period included in this study was so extensive, we classified the years 2005 to 2012 as Phase I and the years 2013 to 2020 as Phase II, depending on the time of surgery, to adjust the chronological bias. A clear resection margin was defined as the presence of tumor cells 1 mm away from any margin or circumferential surface. The treatment information included details regarding the operation and adjuvant treatment. In particular, data on both the initiation and completion of adjuvant chemotherapy were collected to evaluate the actual impact of adjuvant chemotherapy and the surgical burden. Adjuvant chemotherapy for a period of 6 months was usually recommended to all patients with appropriate physical status. Completion of adjuvant chemotherapy was defined the completion of scheduled adjuvant chemotherapy regardless of the delay. Medical oncologists made the decision to reduce the chemotherapy dose based on adverse effects, and dose reduction was not one of the requirements for completion of adjuvant chemotherapy. Patients with a microscopically positive resection margin or metastatic lymph nodes who had a high chance of recurrence were the primary candidates for adjuvant radiotherapy. Usual prescription of adjuvant radiotherapy was 50.0 Gy to the tumor bed and 45.0 Gy to the regional lymphatics in 25 fractions using simultaneous integrated boost for the concurrent chemo-radiotherapy. Follow-up data were also retrieved to evaluate the prognosis. Overall survival (OS) was measured from the date of pancreatectomy until death or the last hospital visit.
Sample preparation for next generation sequencing
Tumor and blood samples were collected from patients who were admitted for surgery and provided informed consent for next-generation sequencing. Blood samples were collected one day before surgery, and tumor samples were collected immediately after surgical resection. Subsequently, the samples were placed in RNA later within 15 min to preserve RNA integrity and stored at -80 °C. DNA and RNA were extracted according to the Allspin (GeneAll) total DNA/RNA purification protocol.
Whole exome sequencing
Libraries were prepared using the SureSelect Human All Exon v5 probe set, based on the Agilent SureSelect Target Enrichment protocol (version B. June 3, 2015). The libraries were loaded onto an Illumina HiSeq 2500 platform for 101-bp paired-end sequencing. Sequencing depths were set to a minimum of 100X and 300X for the blood and tumor tissue samples, respectively. The sequencing reads were mapped to the human reference genome (GRCh38) through the Burrows–Wheeler Aligner [13]. The mapping result files were further pre-processed using the Genome Analysis Tool Kit [14]. Somatic variant calling with paired tumor tissues and blood samples per patient was performed using Mutect2 according to the Genome Analysis Tool Kit best practice [15]. The somatic variants were annotated using ANNOVAR, and annotated tables were converted to mutation annotation format [16]. The R package maftools was used to analyze and visualize the mutational information.
KRAS targeted sequencing
Due to the notably lower KRAS mutation frequency (73.1%) observed in whole exome sequencing in comparison to the previously reported rate (90% or higher), KRAS amplicons were sequenced at over 1,000,000X depth targeting the previously reported mutational hotspots, aiming to counteract the impact of low tumor purity [9, 17, 18]. All libraries were sequenced using the Illumina NovaSeq platform to generate paired-end 151 base pairs reads.
RNA sequencing
RNA exome capture sequencing of tumor tissue samples was performed instead of Total RNA sequencing to overcome RNA degradation caused by pancreatic enzymes. Total RNA was quantified using Quant-IT RiboGreen. Subsequently, the mRNA-encoding exome was extracted from > 100 ng of total RNA using an Illumina TruSeq RNA exome. The cDNA library was constructed by adaptor ligation and loaded onto an Illumina HiSeq 2500 platform for 101-bp paired-end sequencing. The sequencing reads were pre-processed with Trimmomatic to remove reads containing low-quality bases and adapters [19]. Trimmed reads were mapped to GRCh38 using STAR [20]. The mapped result files were processed to quantify the expression levels of genes according to GENCODE v27 GTF annotation using RSEM [21].
Transcriptomic profiling
To compare the distribution of previously known transcriptome-based subtypes with clinical significance between pancreatic head and body/tail cancers, the R package ConsensusClusterPlus was employed [22]. The classification of samples was based on molecular signatures defined in Bailey et al. (2016) and Moffitt et al. (2015) [8, 9].
Deconvolution analysis
To compare the characteristics of the tumor microenvironment between pancreatic head and body/tail cancer, deconvolution analysis was performed using the Tumor Immune Estimation Resource version 2.0, a web-based tool [23]. The analysis estimated immune cell infiltration levels, including B cells, CD4 + T cells, CD8 + T cells, neutrophils, macrophages, and myeloid dendritic cells, with transcripts per million values.
Statistical analysis
Categorical variables were expressed as numbers with percentages, while continuous variables were expressed as median values with interquartile ranges. To compare clinical characteristics according to tumor location, the chi-square and Fisher’s exact tests were used for categorical variables, and the independent t-test was used for continuous variables. Cox proportional hazards regression models, for calculating hazard ratios (HR) and 95% confidence intervals (CI), were employed to explore prognostic factors. Variables previously identified as prognostic factors were selected for multivariate analysis. Survival analysis was performed using Kaplan–Meier estimates and compared using the log–rank test. Statistical significance was set at P < 0.05. All statistical analyses were performed using the R software, version 4.2.3 (R Foundation for Statistical Computing).
Results
Clinical characteristics of entire cohort
The baseline characteristics of the entire cohort, stratified by tumor location, are summarized in Table 1. Out of the 800 patients, 500 (62.5%) had pancreatic head cancer and 300 (37.5%) had pancreatic body/tail cancer. Patients with pancreatic body/tail cancer were older than those with pancreatic head cancer (P < 0.001). There were no significant differences in sex and preoperative physical status between the two groups.
Regarding operation, there were several disparities between the pancreatic head and body/tail cancer groups. All patients with pancreatic head cancer underwent pancreaticoduodenectomy, whereas most patients with pancreatic body/tail cancer (298/300, 99.3%) underwent distal or subtotal pancreatectomy without reconstruction. Minimally invasive surgery was performed more frequently for pancreatic body/tail (22.3%) cancers than for pancreatic head (5.8%) cancers (P < 0.001). Compared to patients with pancreatic head cancer, those with pancreatic body/tail cancer experienced a much shorter operation time and lower intraoperative blood loss.
There were no discernible differences in the rates of initiating adjuvant chemotherapy between patients with pancreatic head (79.4%) and body/tail (80.7%) cancers (P = 0.733). However, the completion rate of adjuvant chemotherapy was noticeably higher in patients with pancreatic body/tail cancer (60.0%) than in those with pancreatic head cancer (51.2%; P = 0.019).
To evaluate the differences in baseline characteristics according to the treatment period, the baseline characteristics stratified by treatment period are summarized in Supplementary Table 1. There were no differences in the initiation and completion rates of adjuvant chemotherapy between phase I and phase II, however there were more patients with body/tail cancer and those who underwent minimally invasive surgery in phase II compared to phase I.
Prognostic power of tumor location
Survival and univariate and multivariate Cox regression analyses were performed for the entire cohort (Table 2). In survival and univariable analysis, almost all variables including tumor location (Median OS, 27 vs. 39 months; HR, 0.74; 95% CI, 0.62–0.88; P < 0.001; head vs. body/tail), non-initiation (Median OS, 35 vs. 16 months; HR, 2.10; 95% CI, 1.74–2.54; P < 0.001) and non-completion (Median OS, 53 vs. 17 months; HR, 3.08; 95% CI, 2.61–3.64; P < 0.001) of adjuvant chemotherapy were identified as significant prognostic factors. However, multivariable analysis adjusting for confounding variables revealed that the tumor location was not the independent prognostic factor (HR, 0.94; 95% CI, 0.77–1.14; P = 0.511; body/tail compared with head). Regarding adjuvant chemotherapy, non-initiation (HR, 0.92; 95% CI, 0.70–1.21; P = 0.556) of treatment was not identified as a prognostic factor, whereas non-completion (HR, 3.16; 95% CI, 2.59–3.86; P < 0.001) of treatment was identified as the most powerful prognostic factor among several variables.
Clinical characteristics of genomic cohort
The baseline characteristics of the genomic cohort stratified by tumor location are summarized in Table 3. Out of the 153 patients, 88 (57.5%) had pancreatic head cancer and 65 (42.5%) had pancreatic body/tail cancer. There were no significant differences in demographics, including age, sex, and preoperative physical status between the two groups. In addition, the completion rates of adjuvant chemotherapy, which was the most powerful prognostic factor in the entire cohort, were almost the same between patients with pancreatic head cancer (46.6%) and those with pancreatic body/tail cancer (47.7%; P > 0.99). Even when compared to the entire cohort, there were no discernible differences in the frequencies of prognostic variables including pathological stage and adjuvant chemotherapy within the genomic cohort.
Mutational landscape
The mutational status of the genomic cohort is shown in Fig. 2. The four most common genomic alterations were KRAS (targeted sequencing), TP53, CDKN2A, and SMAD4, which are known to be the main driver gene alterations, in patients with both pancreatic head and body/tail cancers. There were no significant differences in the frequency of common genomic alterations between the two groups (Table 4).
Comparison based on gene expression
While molecular subtypes associated with poor prognosis, such as the squamous subtype described by Bailey et al. (2016) and basal-like subtype described by Moffitt et al. (2015), demonstrated higher ratios in patients with pancreatic body/tail cancer than in those with pancreatic head cancer, these findings were not statistically significant (Fig. 3A) [8, 9]. Considering these molecular subtypes, survival analyses according to tumor location were performed (Fig. 3B–C). There were no significant differences in OS between patients with pancreatic head cancer and those with pancreatic body/tail cancer in almost all subgroups stratified by molecular subtype, except the squamous subtype.
Immune cell infiltration
Using Tumor Immune Estimation Resource 2.0, the infiltration levels of B cells, CD4 + T cells, CD8 + T cells, neutrophils, macrophages, and myeloid dendritic cells were retrieved from patients with pancreatic head and body/tail cancers. There were no significant differences in the infiltration levels of the six immune cell types between the two groups (Fig. 4).
Discussion
Pancreatic head and body/tail cancers show differences in both embryonic genesis and clinical characteristics. It is known that pancreatic body/tail cancer is associated with a poor prognosis; nevertheless, several investigations have shown inconsistent results regarding the prognostic power of tumor location [24]. This study presents the outcomes of patients with PDAC who underwent upfront surgery according to tumor location and genomic characteristics with clinical details often overlooked in many other studies. We found that tumor location was not an independent prognostic factor in patients with PDAC, and that there were no genomic differences between pancreatic head and body/tail cancers with similar clinical characteristics, including demographics and pathological stages.
Previous clinical studies have reported inconsistent outcomes regarding the effect of tumor location on the survival of patients with PDAC. Lee et al. (2020) reported that tumor location was not an independent prognostic factor, and the better survival outcomes of patients with pancreatic head cancer compared to those with body/tail cancer were associated with higher resection rates [25]. In contrast, a meta-analysis by Tomasello et al. (2019) reported that tumor location in the head of pancreas at the time of diagnosis is a significant predictor of better outcomes compared to that in body/tail (HR, 0.96; 95% CI, 0.92–0.99; P = 0.02) [24]. However, this study targeted a total of 93 studies, and most of the individual studies reported that tumor location was not a significant prognostic factor, and there was also a large degree of variability among the studies (I2 = 68%). In this study, which included 800 patients who underwent surgery, tumor location in the body/tail of the pancreas was identified as a significant predictor of better survival compared to that in the head in the univariate analysis but not in the multivariate analysis. We think that more frequent lymphatic and perineural invasion in pancreatic head cancer compared to pancreatic body/tail cancer may have added to the prognostic significance of tumor location in the univariate analysis. But the most important thing that caused the prognostic relevance of tumor location in the univariate analysis may be the difference in the completion rates of adjuvant chemotherapy, which was the strongest predictive factor in the multivariate analysis. The differences in surgical load between the two groups, which are supported by differences in operation type, operation time, estimated blood loss, and post-operative complication rates, may be responsible for the disparity in the completion rates of adjuvant chemotherapy.
Several studies have reported that PDAC exhibits considerable heterogeneity with a wide range of genomic alterations and gene expression [8, 9, 26]. Subsequently, some studies have reported differences in tumor biology according to tumor location in PDAC; however, these results were not consistent. Sun et al. (2022) and Zhang et al. (2021) reported that pancreatic body/tail cancer has significantly more mutations involved in main driver gene alterations, such as KRAS, TP53, and SMAD4 [10, 11]. Mutant KRAS drives PDAC development and promotes tumor cell proliferation via altered metabolic pathways or activation of Wnt and MAPK pathways [27]. There are also several studies about the prognostic effect of mutant KRAS status [7]. Maddalena et al. (2021) reported that TP53 missense mutations may contribute to worse PDAC prognosis by promoting a more aggressive tumor microenvironment and reducing CD8 + T cell infiltration [28]. As such, alterations in main driver genes play important roles not only in tumor initiation but also in clonal expansion processes and are also associated with worse prognosis. However, in studies conducted by Sun et al. (2022) and Zhang et al. (2021), there were significantly more patients with later stage (stage III or IV) pancreatic body/tail cancer than those with head cancer [10, 11]. Because of these differences in clinical stage, it may be challenging to interpret whether genomic differences are actually caused by the location of the tumor or its clinical severity.
In addition to somatic mutations, Dreyer et al. (2018) reported the association of tumor location in the pancreatic body/tail with squamous subtype defined by Bailey et al. (2016) [9, 29]. Abdelrahim et al. (2022) reported that patients with pancreatic body/tail cancer showed significantly lower infiltration of immune cells, including B cells, CD8 + T cells, NK cells, and neutrophils [30]. However, in Abdelrahim et al. (2022), there were significantly more distant metastasis in patients with pancreatic head cancer than in those with pancreatic body/tail cancer [30]. These findings imply that pancreatic head cancer is comparatively more susceptible to immune checkpoint inhibitors and that pancreatic body/tail cancer is less responsive to cytotoxic chemotherapy, indicating the importance of timely surgery for prognosis. Although many clinical trials on the efficacy of neoadjuvant chemotherapy and chemotherapy regimens, including immunotherapy, have been carried out in patients with PDAC, no noteworthy subgroup analysis results based on tumor location have been provided. This study, with its strength of similar clinical characteristics between pancreatic head and body/tail cancer and minimal confounding factors, such as batch effects, demonstrated that there were no significant differences in the mutational landscape, transcriptomic profiling, and infiltration of immune cells between the two groups. These findings eventually show that rather than tumor biology, disparities in prognosis according to tumor location can be induced by clinical variations, such as the timing of diagnosis and surgical loads.
We acknowledge that our study had a few limitations. First, this was a retrospective cohort study, which may have introduced a potential selection bias. Second, although deconvolution analysis with transcriptomic data from bulk tissue was used to estimate the infiltration of immune cells, transcriptome information containing a single cell unit and spatial information should be produced for more precise analysis. Third, although it was pertinent to the objective of this study, we only included patients who underwent curative pancreatectomy. Although, there were no significant differences in the proportion of patients who underwent palliative resection between patients with pancreatic head cancer (5.8%, 31/531) and those with pancreatic body/tail cancer (7.7%, 25/325), it would be preferable to include patients who did not undergo surgery in follow-up studies to lessen selection bias.
Conclusion
In summary, we could not find differences in tumor biology and prognosis depending on tumor location in PDAC. Therefore, it is challenging to develop a treatment plan based solely on the location of the tumor, and various examinations and efforts are still needed for precision medicine in patients with PDAC.
Data availability
The whole exome and RNA sequencing data generated in this study are already available in dbGaP (https://www.ncbi.nlm.nih.gov/gap/; accession ID: phs002347.v1.p1). The clinical datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PDAC:
-
Pancreatic ductal adenocarcinoma
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48.
Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270(1):248–60.
Luo G, Jin K, Cheng H, Guo M, Gong Y, Fan Z, Yang C, Huang Q, Ni Q, Liu C, et al. Prognosis of distal pancreatic cancers controlled by stage. Exp Ther Med. 2020;20(2):1091–7.
Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford). 2008;10(5):371–6.
Winer LK, Dhar VK, Wima K, Morris MC, Lee TC, Shah SA, Ahmad SA, Patel SH. The impact of Tumor Location on Resection and Survival for pancreatic ductal adenocarcinoma. J Surg Res. 2019;239:60–6.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6.
Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, Welch MW, Brais LK, Da Silva A, Li T, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 2018;4(3):e173420.
Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–78.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52.
Sun K, Mylavarapu C, Crenshaw A, Zhang Y, Hsu E, Xu J, Niravath M, Jones SL, Ordonez A, Abdelrahim M. Pancreatic head vs pancreatic body/tail cancer: are they different? World J Gastrointest Oncol. 2022;14(3):716–23.
Zhang X, Feng S, Wang Q, Huang H, Chen R, Xie Q, Zhang W, Wang A, Zhang S, Wang L, et al. Comparative genomic analysis of head and body/tail of pancreatic ductal adenocarcinoma at early and late stages. J Cell Mol Med. 2021;25(3):1750–8.
Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail pancreatic carcinomas are not the same tumors. Cancers (Basel) 2019, 11(4).
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26(5):589–95.
Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 2013, 43(1110):11 10 11–11 10 33.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–9.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164.
Cancer Genome Atlas Research Network. Electronic address aadhe, Cancer Genome Atlas Research N: Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185–203. e113.
Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323.
Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–3.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, Li B, Liu XS. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–14.
Tomasello G, Ghidini M, Costanzo A, Ghidini A, Russo A, Barni S, Passalacqua R, Petrelli F. Outcome of head compared to body and tail pancreatic cancer: a systematic review and meta-analysis of 93 studies. J Gastrointest Oncol. 2019;10(2):259–69.
Lee M, Kwon W, Kim H, Byun Y, Han Y, Kang JS, Choi YJ, Jang JY. The role of location of Tumor in the prognosis of the pancreatic Cancer. Cancers (Basel) 2020, 12(8).
Hyeon DY, Nam D, Han Y, Kim DK, Kim G, Kim D, Bae J, Back S, Mun DG, Madar IH, et al. Proteogenomic landscape of human pancreatic ductal adenocarcinoma in an Asian population reveals tumor cell-enriched and immune-rich subtypes. Nat Cancer. 2023;4(2):290–307.
Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39(2):91–100.
Maddalena M, Mallel G, Nataraj NB, Shreberk-Shaked M, Hassin O, Mukherjee S, Arandkar S, Rotkopf R, Kapsack A, Lambiase G et al. TP53 missense mutations in PDAC are associated with enhanced fibrosis and an immunosuppressive microenvironment. Proc Natl Acad Sci U S A 2021, 118(23).
Dreyer SB, Jamieson NB, Upstill-Goddard R, Bailey PJ, McKay CJ, Australian Pancreatic Cancer Genome I, Biankin AV, Chang DK. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg. 2018;105(2):e183–91.
Abdelrahim M, Kasi A, Baca Y, Xiu J, Walker P, Korn WM, Lou E, Shields AF, Weinberg BA. Comparative molecular profiling of pancreatic ductal adenocarcinoma (PDAC) of the head (H) versus body/tail (B/T) and the tumor immune microenvironment (TIME). In.: American Society of Clinical Oncology; 2022.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2022R1A2C2011122). This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (no. HI20C1234).
Author information
Authors and Affiliations
Contributions
Study design: Yun WG, Jang JY; Data collection and management: Yun WG, Kim D, Lee M, Han Y, Chae YS, Jung HS, Cho YJ, Kwon W, Park JS, Park D, Jang JY; Data analysis and interpretation: Yun WG, Kim D, Park D, Jang JY; Manuscript writing: Yun WG; Manuscript editing: Yun WG, Kim D, Park D, Jang JY; Study supervision: Jang JY. All authors have read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective cohort study was approved by the Institutional Review Board of Seoul National University Hospital (H-2309-159-1471) and waived the requirement for written informed consent for clinical analysis. However, for genomic analysis, 153 patients provided informed consent about collecting tumor tissue and blood samples and generating next-generation sequencing for research purposes before surgery. To protect anonymity, we omitted non-essential identifying details, such as in genetic pedigrees, etc.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yun, WG., Kim, D., Lee, M. et al. Comparing clinical and genomic features based on the tumor location in patients with resected pancreatic cancer. BMC Cancer 24, 1048 (2024). https://doi.org/10.1186/s12885-024-12795-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12795-5