Abstract
Purpose
The purpose of the current study is to analyze the difference of short-term and oncologic outcomes between younger and older colorectal cancer (CRC) patients who underwent primary CRC surgery using a propensity score matching (PSM) analysis.
Methods
We retrospectively collected CRC patients who underwent primary surgery in a single clinical database from Jan 2011 to Jan 2020. The short-term and oncologic outcomes were compared between younger aged group and older aged group.
Results
A total of 4599 patients were included in this study, and there were 4196 patients in older aged group and 403 patients in younger aged group. After 1:1 ratio PSM, there were 401 patients in each group. No significant difference was found in terms of baseline information after PSM (p>0.05). Younger aged group had larger retrieved lymph nodes before (p<0.001) and after PSM (p=0.001) than older aged group. In multivariate analysis, younger age was an independent predictor of better overall survival (OS) (p<0.001, HR=2.303, 95% CI=1.658-3.199) and disease-free survival (DFS) (p=0.008, HR=1.425, 95% CI=1.098-1.850). In terms of different tumor stage after PSM, younger aged group had better OS than older group in stage II (p<0.001) and stage IV (p=0.028) CRC, and younger aged group had better DFS than older group in stage II (p=0.016) CRC.
Conclusion
Younger CRC patients had larger retrieved lymph nodes and better prognosis than older CRC patients after primary CRC surgery.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the most common cancers with approximate 185 million cases worldwide [1], in addition, CRC is one of the most common causes of cancer-related deaths, with nearly 700,000 deaths every year [2]. Radical surgery is the cornerstone of CRC treatment [3,4,5].
The American Cancer Society updated the CRC screening guidelines recently. A major change was related to the start of screening age which was recommended from 50 to 45 years old [6]. Based on the recent data, although the incidence of CRC decreased, the incidence of younger CRC patients was increasing [7].
It remained controversial whether younger CRC patients were related to the prognosis [8,9,10,11]. Some studies reported that younger CRC patients had better prognosis [8], however, other studies reported younger CRC patients were associated with poorer prognosis [9,10,11]. Furthermore, there was only one study which analyzed the difference between younger and older CRC patients using a propensity score matching (PSM) analysis, however, this study focused on cancer-specific survival [12]. Therefore, the purpose of this study is to analyze the difference of short-term outcomes, overall survival (OS) and disease-free survival (DFS) between younger and older CRC patients who underwent primary CRC surgery using PSM.
Methods
Patients
We retrospectively collected CRC patients who underwent primary surgery in a single clinical database from Jan 2011 to Jan 2020. The study was approved by the ethics committee of our institution (The First Affiliated Hospital of Chongqing Medical University, 2021-517), and all patients signed informed consent forms. This study was conducted in accordance with the World Medical Association Declaration of Helsinki as well.
Inclusion and exclusion criteria
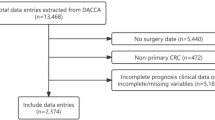
Patients who underwent primary CRC surgery and diagnosed by pathology were included in this study (n=5473). The exclusion criteria were as follows: 1, Patients with incomplete clinical medical data (n=849); and 2, Non-R0 resection (n=25). Finally, a total of 4599 patients were included in this study.
Surgery management and follow-up
The radical CRC surgery was according to the principles of oncology. Total mesorectal excision or complete mesocolic excision was performed, and the pathology confirmed R0 resection. Patients were followed up regularly three months for three years and six months for the following two years.
Definitions
The tumor stage was diagnosed according to the AJCC 8th Edition [13]. The younger aged group was defined as the age was ≤ 45 years old, the older aged group was defined as the age was > 45 years old. The complications were defined according to the Clavien-Dindo classification [14], and major complications were defined as ≥ III classification complications. OS was defined as the time from CRC surgery to death or last follow-up. DFS was defined as the time from CRC surgery to recurrence, death or last follow-up.
Data collection
The perioperative and follow-up information were collected through the inpatient system, outpatient system and telephone interviews. The baseline information included sex, age, smoking, drinking, hypertension, type 2 diabetes mellitus (T2DM), coronary heart disease (CHD) and tumor location. The surgical information included operation time and blood loss. The pathologic information included tumor stage and retrieved lymph nodes. The postoperative information included complications and postoperative hospital stay.
PSM
PSM was conducted between younger aged group and older aged group to minimize the bias of baseline information. Nearest neighbor matching was performed without replacement at a 1:1 ratio and a caliper width with a 0.01 standard deviation was specified. The matched baseline information was as follows: sex, BMI, drinking, smoking, T2DM, hypertension, CHD, tumor location and tumor stage.
Statistical analysis
Continuous variables are expressed as the mean ± SD and independent-sample t test was used to analyze the difference between younger aged group and older aged group. Frequency variables are expressed as n (%), and Chi-square test or Fisher's exact test was used. The Kaplan-Meier curve was conducted to compare the age (younger/ older) on different tumor stage, and cox regression analyses were performed to identify independent predictive factors for OS and DFS. Data were analyzed using SPSS (version 22.0) statistical software. A bilateral p value of <0.05 was considered statistically significant.
Results
Patients
A total of 4599 patients were included in this study according to the inclusion and exclusion criteria, and there were 4196 patients in older aged group and 403 patients in younger aged group. After 1:1 ratio PSM, there were 401 patients in each group (Fig 1).
Baseline information
The baseline information was compared between the two groups. The age was 39.4 ± 5.4 years old in younger aged group and 65.1 ± 10.0 years old in older aged group. Younger group had higher portion of hypertension (p<0.001), T2DM (p<0.001) and CHD (p<0.001) before PSM. After 1:1 ratio PSM, there were 401 patients in each group, and no significant difference was found in terms of baseline information (p>0.05) (Table 1).
Short-term outcomes
The short-term outcomes included operation time, blood loss, retrieved lymph nodes, postoperative hospital stay and complications. We compared the difference between younger aged group and older aged group in terms of short-term outcomes. Younger aged group had larger retrieved lymph nodes before (p<0.001) and after PSM (p=0.001) than older aged group (Table 2).
Univariate and multivariate analysis of OS and DFS
Univariate analysis was conducted to find potential factors for predicting OS and DFS, and multivariate analysis was conducted to identify independent predictors of OS and DFS.
In terms of OS, age (p<0.001, HR=2.303, 95% CI=1.658-3.199), tumor stage (p<0.001, HR=2.141, 95% CI=1.940-2.363), overall complications (p<0.001, HR=1.607, 95% CI=1.363-1.893) and major complications (p<0.001, HR=2.303, 95% CI=1.658-3.199) were independent predictors of OS (Table 3).
As for DFS, age (p=0.008, HR=1.425, 95% CI=1.098-1.850), tumor stage (p<0.001, HR=2.100, 95% CI=1.921-2.295), overall complications (p<0.001, HR=1.504, 95% CI=1.293-1.750) and major complications (p<0.001, HR=2.015, 95% CI=1.461-2.776) were independent predictors of DFS (Table 4).
The effect of younger aged group and older aged group on different tumor stages
Before PSM, younger aged group had better OS than older aged group in terms of stage II (p<0.001), stage III (p<0.001) and stage IV (p<0.001) CRC (Fig 2). Younger aged group had better DFS than older aged group in terms of stage II (p=0.010), stage III (p=0.010) and stage IV (p=0.010) CRC as well (Fig 3).
After PSM, younger aged group had better OS than older aged group in terms of stage II (p<0.001) and stage IV (p=0.028) CRC. (Fig 4) Younger aged group had better DFS than older aged group in terms of stage II (p=0.016) CRC (Fig 5).
Discussion
A total of 4599 patients were included in this study and there were 4196 patients in older aged group and 403 patients in younger aged group. After 1:1 ratio PSM, there were 401 patients in each group. No significant difference was found in terms of baseline information after PSM. Younger aged group had larger retrieved lymph nodes before and after PSM. In multivariate analysis, younger age was an independent predictor of better OS and DFS. In terms of different tumor stages after PSM, younger aged group had better OS than older aged group in stage II and stage IV CRC, and younger aged group had better DFS than older aged group in stage II CRC.
The impact of age on the prognosis of CRC was still controversial [8,9,10,11], Zhao L [9]. et al reported a poor prognosis for CRC in younger patients, but Yang Z [15]. et al reported that the prognosis of younger patients was similar to that of older patients. However, another study reported a better prognosis for younger patients [8]. We summarized the previous studies which reported the age on the outcomes of CRC patients in Table 5 [8,9,10,11,12, 15,16,17,18]. As was shown in the table, OS, DFS, CSS (cancer-specific survival) and short-term outcomes were the main indicators. Moreover, short-term outcomes were reported in two studies, which were relatively small [12, 17]. Furthermore, no previous studies reported the age on the survival outcomes of specific tumor stages. PSM analysis was method to reduce the selection bias of the baseline information, which could benefit precise results when there was no difference in baseline information [19, 20]. Therefore, the current study aimed to explore the specific impact of age on CRC including short-term outcomes, OS and DFS in different tumor stages using PSM.
However, the cut-off age of younger CRC patients was different in previous studies, and the cut-off age included 30, 35, 40, 45 and 50 years old [8,9,10,11,12, 15,16,17,18]. In this study, we used 45 years old as the cut-off of younger and older CRC patients, which was according to the recommended screening age and previous studies [6, 8, 12, 15]. In addition, PSM was used in this study to analyze the different outcomes between younger aged group and older aged group.
There were many factors that could affect the prognosis of CRC patients including tumor stage, BMI, T2DM, age and complications [3, 21,22,23,24]. In this study, tumor stage and complications were independent predictors of CRC patients which was similar with previous studies [3, 21, 22]. In addition, younger aged patients were associated with better prognosis than older aged patients, which meant that after radical CRC surgery, age was an important factor affecting the prognosis. Therefore, in order to explore the effect of age on each specific tumor stage, Kaplan-Meier was used and we found that younger aged group had better OS than older aged group in stage II and stage IV CRC, and younger aged group had better DFS than older aged group in stage II CRC. The mechanism was unclear, and it was found in a previous study that younger patients had better prognosis in stage III CRC [12], so more researches are needed on specific tumor stage in the future.
In addition to prognosis, previous studies rarely reported age on short-term outcomes. Only one study reported the shot-term outcomes of after CRC surgery [12]. Wang L [12]. et al reported that there was no difference between younger aged group and older aged group, however, the information of retrieved lymph nodes was missing and they failed to match the baseline information. In this study, younger CRC patients had more retrieved lymph nodes than older CRC patients. The possible reasons might be as follows: First, younger CRC patients might have better anatomy, which was more convenient to harvest lymph nodes; Second, surgeons might be more inclined to operate on younger CRC patients with a larger range. Furthermore, less retrieved lymph nodes might contribute to poor prognosis in older CRC patients [25,26,27].
To our knowledge, this study analyzed the short-term outcomes of younger and older CRC patients with the largest amount of data and compared the retrieved lymph nodes between younger and older CRC patients for the first time. Furthermore, PSM was used, OS and DFS were compared for specific tumor stage. However, some limitations exited in this study. First, this was a retrospective single center study; Second the follow-up time was relatively short; Third, the definition of younger age was not the same in previous studies, we chose the cut-off of 45 years old based on the recommended CRC screening age and published studies. Thus, multi-center prospective randomized controlled trials with comprehensive perioperative information should be performed in the future.
In conclusion, younger CRC patients had larger retrieved lymph nodes and better prognosis than older CRC patients after primary CRC surgery.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017 Apr;66(4):683–91. https://doi.org/10.1136/gutjnl-2015-310912.
Peng D, Liu XY, Cheng YX, et al. Improvement of Diabetes Mellitus After Colorectal Cancer Surgery: A Retrospective Study of Predictive Factors For Type 2 Diabetes Mellitus Remission and Overall Survival. Front Oncol. 2021;6(11):694997. https://doi.org/10.3389/fonc.2021.694997.
Watanabe T, Momosaki R, Suzuki S, et al. Preoperative rehabilitation for patients undergoing colorectal cancer surgery: a retrospective cohort study. Support Care Cancer. 2020;28(5):2293–7. https://doi.org/10.1007/s00520-019-05061-z.
Ji X, Zhao Y, Zhu X, et al. Outcomes of Stereotactic Body Radiotherapy for Metastatic Colorectal Cancer With Oligometastases, Oligoprogression, or Local Control of Dominant Tumors. Front Oncol. 2021;29(10):595781. https://doi.org/10.3389/fonc.2020.595781.
Yeo H, Betel D, Abelson JS, et al. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin Colorectal Cancer. 2017;16(4):293–9. e6. https://doi.org/10.1016/j.clcc.2017.06.002.
Dwyer AJ, Murphy CC, Boland CR, et al. A Summary of the Fight Colorectal Cancer Working Meeting: Exploring Risk Factors and Etiology of Sporadic Early-Age Onset Colorectal Cancer. Gastroenterology. 2019;157(2):280–8. https://doi.org/10.1053/j.gastro.2019.04.049.
Nakayama Y, Kobayashi H, Kawamura H, et al. The long-term outcomes in adolescent and young adult patients with colorectal cancer -A multicenter large-scale cohort study. J Cancer. 2020;11(11):3180–5. https://doi.org/10.7150/jca.36721.
Zhao L, Bao F, Yan J, et al. Poor prognosis of young patients with colorectal cancer: a retrospective study. Int J Colorectal Dis. 2017;32(8):1147–56. https://doi.org/10.1007/s00384-017-2809-5.
Fu JF, Huang YQ, Yang J, et al. Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol. 2013;19(44):8078–84. https://doi.org/10.3748/wjg.v19.i44.8078.
Shida D, Ahiko Y, Tanabe T, et al. Shorter survival in adolescent and young adult patients, compared to adult patients, with stage IV colorectal cancer in Japan. BMC Cancer. 2018;18(1):334. https://doi.org/10.1186/s12885-018-4241-9.
Wang L, Hirano Y, Heng G, et al. Better Cancer-specific Survival in Younger Patients With Stage III Colorectal Cancer: A Propensity Score Matching Study From Japan. Anticancer Res. 2020;40(8):4365–72. https://doi.org/10.21873/anticanres.14439.
Weiser MR. AJCC 8th Edition: Colorectal Cancer. Ann Surg Oncol. 2018;25(6):1454–5. https://doi.org/10.1245/s10434-018-6462-1.
Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2.
Yang Z, Kang L, Wang L, et al. Characteristics and long-term survival of colorectal cancer patients aged 44 years and younger. Clin Transl Oncol. 2012;14(12):896–904. https://doi.org/10.1007/s12094-012-0876-1.
Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol. 2007;14(10):2759–65. https://doi.org/10.1245/s10434-007-9465-x.
Schellerer VS, Merkel S, Schumann SC, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int J Colorectal Dis. 2012;27(1):71–9. https://doi.org/10.1007/s00384-011-1291-8.
Wong SW, Ling DY, Yeow RQ, et al. Clinicopathological patterns and survival outcomes of colorectal cancer among young adults in Malaysia: an institutional cohort study. Singapore Med J. 2021. https://doi.org/10.11622/smedj.2021051.
Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249–64.
Matsunaga T, Ishiguro R, Miyauchi W, et al. Appraisal of long-time outcomes after curative surgery in elderly patients with gastric cancer: a propensity score matching analysis. BMC Surg. 2021;21(1):33. https://doi.org/10.1186/s12893-021-01046-0.
Peng D, Cheng YX, Cheng Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed Res Int. 2021;1(2021):5541613. https://doi.org/10.1155/2021/5541613.
Chiu CC, Ho CH, Hung CM, et al. Correlation of Body Mass Index with Oncologic Outcomes in Colorectal Cancer Patients: A Large Population-Based Study. Cancers (Basel). 2021;13(14):3592. https://doi.org/10.3390/cancers13143592.
Miyamoto Y, Hiyoshi Y, Tokunaga R, et al. Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: A propensity-score analysis. J Surg Oncol. 2020;122(2):344–9. https://doi.org/10.1002/jso.25961.
Azar I, Al Masalmeh N, Esfandiarifard S, et al. The impact of primary tumor sidedness on survival in early-onset colorectal cancer by stage: A National Veterans Affairs retrospective analysis. Cancer Med. 2021;10(9):2987–95. https://doi.org/10.1002/cam4.3757.
Ogino S, Nosho K, Irahara N, et al. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. 2010;105(2):420–33. https://doi.org/10.1038/ajg.2009.578.
Altintas S, Bayrak M. Assessment of Factors Influencing Lymph Node Count in Colorectal Cancer. J Coll Physicians Surg Pak. 2019;29(12):1173–8. https://doi.org/10.29271/jcpsp.2019.12.1173.
Arnold A, Kloor M, Jansen L, et al. The association between microsatellite instability and lymph node count in colorectal cancer. Virchows Arch. 2017;471(1):57–64. https://doi.org/10.1007/s00428-017-2150-y.
Acknowledgments
We acknowledge all the authors whose publications are referred in our article.
Funding
This study was supported by Chongqing key diseases Research and Application Demonstration Program (Colorectal Cancer Prevention and Treatment Technology Research and Application Demonstration [No. 2019ZX003]).
Author information
Authors and Affiliations
Contributions
All authors contributed to data collection and analysis, drafting or revising the manuscript, have agreed on the journal to which the manuscript will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and informed consent
The study was approved by the ethics committee of our institution (The First Affiliated Hospital of Chongqing Medical University, 2021-517), and all patients signed informed consent. This study was conducted in accordance with the World Medical Association Declaration of Helsinki as well.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, XY., Kang, B., Cheng, YX. et al. The short-term and oncologic outcomes of younger VS older colorectal cancer patients undergoing primary surgery: a propensity score matching analysis. BMC Cancer 22, 153 (2022). https://doi.org/10.1186/s12885-022-09246-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09246-4