Abstract
Background
Neurolymphomatosis (NL) is an extremely rare disease and tracheal asphyxia due to NL has not been previously reported.
Case Presentation
A 54-year-old Chinese woman with a history of diffuse large B-cell lymphoma in her first complete remission developed peripheral neuropathy and tracheal asphyxia. Neurolymphomatosis involving the right brachial plexus and the right vagus nerve was demonstrated by PET/CT, but not by MRI. She underwent urgent tracheotomy and impact chemotherapy using rituximab combined with high dose methotrexate and involved field radiotherapy. She achieved a second complete remission.
Conclusion
PET/CT plays valuable role in differentiating NL from other neuropathies in patients with lymphoma. Complete remission can be achieved in NL due to large B-cell lymphoma.
Similar content being viewed by others
Background
Neurolymphomatosis (NL) is an extremely rare disease that can be caused by T cell, B cell or NK cell lymphoma, but most commonly B cell lymphoma. It is characterized by lymphoma cells directly infiltrating cranial nerve, peripheral nerve, nerve root and nerve plexus. NL may occur primary in the peripheral nervous system or occur as a part of systemic lymphoma [1–3]. NL may involve different parts of the peripheral nervous system, but simultaneous involvement of brachial plexus nerve and vagus nerve has not been reported in the literature. Here we report one such a case of NL simultaneously involving brachial plexus nerve and vagus that caused a medical emergency of vocal cord paralysis and trachea asphyxia.
Case presentation
-
A 54 years old female first presented to hematology department of West China Hospital in November 2012 with numbness and pain of right upper extremity lasting for 2 months. She denied any past medical history. A 5 × 5 cm mass in upper outer quadrant of right breast was palpated during the physical examination. A neurological examination showed diminished flexion and extension of right fingers. Electromyogram (EMG) showed peripheral neurogenic damage of right upper extremity. Fine needle aspiration of the mass revealed some atypical cells scattered or focally infiltrated in the breast stroma. Immunohistochemistry of the atypical cells showed diffuse large B cell lymphoma (DLBCL). CT scan of chest and bone marrow studies were negative. Cerebrospinal fluid (CSF) analysis showed no nucleated cells, protein 0.56 g/L, normal chlorides and glucose. A flow cytometry examination showed no abnormal cells. She received 2 courses R-hyperCVAD(rituximab + scheme A: cyclophosphamide + vindesine + doxorubicin + dexamethasone/scheme B: methotrexate + cytarabine), 2 courses R-CHOP (rituximab + cyclophosphamide + vindesine + liposomal doxorubicin + prednisone) and 4 times intrathecal preventive chemotherapy (methotrexate + cytarabine + dexamethasone). Protein level of CSF fluctuated between 0.54-0.61 g/L and no abnormal cell was detected by flow cytometry. She was considered in complete remission. The patient was stable during regular follow-ups.
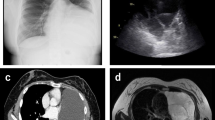
In February 2014, right upper extremity numbness reoccurred and developed nonproductive cough, and shortness of breath which worsened at night. She was hospitalized. Tactile hypoesthesia and hypoalgesia of right forearm and dorsal ulnar side of right palm were also noted again. Her lungs were clear by auscultation and a chest CT scan was normal. She was given an empirical treatment using antibiotics and glucocorticoid. Her symptoms deteriorated. She developed marked numbness and severe pain in the right arm, also strength reduction and limited activities. She showed paroxysmal choke to cough and hoarse voice. Twenty days after admission she developed acute respiratory distress and stridor. An urgent laryngoscopy revealed bilateral vocal cord paralysis and laryngeal obstruction. An emergency tracheotomy was performed due to the trachea asphyxia. Enhanced MRIs of head neck and thorax were unremarkable (Fig. 1). PET/CT, however, showed significant abnormally in the area of the right vagus nerve running zone and right brachial plexus running zone, indicating tumor invasion (Figs. 2 and 3). CSF protein level was 0.52 g/L and no abnormal cell was detected by flow cytometry. Considering the patient’s medical history of lymphoma, symptoms and signs as well as the PET/CT results, a diagnosis of peripheral neurolymphomatosis (involving the right brachial plexus nerve, vagus nerve) and extranodal relapse of DLBCL was established after other factors, such as chemotherapy, infections, tumor compression and paraneoplastic neuropathy had been taken into account.
PET/CT images before treatment showing involvement of right vagus nerve and brachial plexus (detailed images). a, b, c show abnormally high glucose metabolism area of the right vagus nerve running zone; d, e, f show abnormally high glucose metabolism area of right brachial plexus running zone; a, d are coronal sections; b, e are sagittal sections; c,f are cross sections; white arrows direct at lesions in nerves
The patient was given five courses R-DHAP(rituximab + cisplatin + cytarabine + dexamethasone) and high dose methotrexate impact chemotherapy. The patient received 30-35Gy involved field radiotherapy. All her symptoms resolved and her tracheostomy tube was removed. A follow-up PET/CT showed complete remission of the tumor involvement in the right vagus and right brachial plexus nerve (Fig. 4). She remains stable as of now.
Discussion
Neurolymphomatosis (NL) is a rare condition with lymphoma infiltrating parts of the peripheral nerve system including cranial nerves. Only a few cases have been reported and the exact incidence is unclear. It was estimated that NL may occur in 0.2 % of all non-Hodgkin’s lymphoma (NHL) [1]. NHL is responsible for over 90 % of NL and NL rarely occurs in Hodgkin’s lymphoma or lymphoid leukemia. It has diverse clinical manifestations depending on the location of involvement. Delayed diagnosis or misdiagnosis is common and there is significant variations in treatment response and prognosis [1–4].
NL may affect the peripheral nerves, nerve roots and cranial nerves. Multiple nerve involvement is more common than single nerve involvement. Lumbosacral plexus nerve, horsetail nerve and sciatic nerve are common sites of involvement [5–7]. In this case the NL occurred during her large B-cell lymphoma relapse and manifested as brachial plexus and vagus nerve. The tumor infiltration in the peripheral nervous system caused life-threatening bilateral vocal cord paralysis and acute upper and real obstruction, which resolved after chemo and radiation therapy of her underlying large B-cell lymphoma. To our knowledge, such an acute airway obstruction as a complication of NL has not been previously reported in the literature.
The differential diagnosis of neurological symptoms in patients with lymphoma includes inflammatory neuropathy, drug-induced neuropathy, tumor compression and paraneoplastic syndrome [8]. It is also important to include NL in the differential diagnosis. Electromyography is sensitive to detecting peripheral neuropathy, but it is not specific for NL. Direct nerve biopsy is considered the the gold standard for the diagnosis [7]. However, it is invasive and false negative results occur due to sampling errors. Ramirez-Zamora [9] reported 1 case of NL with negative nerve biopsy that was later confirmed by autopsy. NL is easily misdiagnosed or missed due to its rarity and complex clinical manifestations. An earlier retrospective study showed that about half of the cases were only diagnosed by autopsy [1]. Casselberry [10] reported one 64-year-old NL patient presented with aggravating neurological dysfunction that had been misdiagnosed as Guillain-Barré syndrome and inflammatory demyelination. The patient died after 3 months of ineffective treatment and was later found to have NL by autopsy.
About 60 % of patients with NL per reported to have elevated protein level of CSF [2]. Repeated CSF examinations of our case found slightly elevated protein as well. However this is nonspecific and is sensitive. Enhanced MRI is a very useful tool in neurology practice. However it appears to have limited role in detecting NL peripheral nerve involvement, as demonstrated in our case report. PET/CT is far more superior than MRI. It is generally believed that MRI has lower sensitivity than PET/CT depending on the thickening of involved nerve, nerve root/bundle with or without contrast enhancement [1, 2]. PET/CT has higher sensitivity and specificity for NL and the abnormally high glucose metabolism can be used to follow therapeutic response [11–13]. We feel that a PET/CT should be performed in all patients suspected of NL. If a convincingly positive test result is obtained, the treatment for the underlying lymphoma should be instituted immediately without a nerve biopsy.
The prognosis of NL varies greatly among different patients. It’s reported that the median survival time was ten months and 3 year overall survival rate was 24 % [2]. One case was reported to have survived over 31 years [14]. Currently high dose methotrexate plus involved field radiotherapy is the treatment of choice for NL [1–3, 7]. Rituximab, hematopoietic stem cell transplantation and plasma exchange have also been tried to treat NL with variable success [3, 11, 15]. We used 5 cycles of rituximab combined with high dose methotrexate impact chemotherapy for this patient who achieved complete clinical remission based on her symptoms and follow-up PET/CT studies. However her long-term survival needs further observation.
Conclusion
NL is a rare complication of lymphoma with highly variable clinical manifestations depending on the locations of peripheral nerve involvement. Early diagnosis can be challenging. EMG and CSF protein level may be helpful but nonspecific. Enhanced MRI is of limited value. PET/CT appears to be sensitive and specific for NL diagnosis. Complete remission of lymphoma can still be achieved despite the development of NL.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
References
Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro-Oncology. 2003;5(2):104–15.
Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115(24):5005–11.
Kamiya-Matsuoka C, Shroff S, Gildersleeve K, Hormozdi B, Manning JT, Woodman KH. Neurolymphomatosis: a case series of clinical manifestations, treatments, and outcomes. J Neurol Sci. 2014;343(1–2):144–8.
Lagarde S, Tabouret E, Matta M, Franques J, Attarian S, Pouget J, et al. Primary neurolymphomatosis diagnosis and treatment: a retrospective study. J Neurol Sci. 2014;342(1–2):178–81.
Roncaroli F, Poppi M, Riccioni L, Frank F. Primary non-Hodgkin’s lymphoma of the sciatic nerve followed by localization in the central nervous system: case report and review of the literature. Neurosurgery. 1997;40(3):618–21. discussion 621–612.
Chamberlain MC, Fink J. Neurolymphomatosis: a rare metastatic complication of diffuse large B-Cell lymphoma. J Neuro-Oncol. 2009;95(2):285–8.
Baehring JM, Batchelor TT. Diagnosis and management of neurolymphomatosis. Cancer J (Sudbury, Mass). 2012;18(5):463–8.
Hughes RA, Britton T, Richards M. Effects of lymphoma on the peripheral nervous system. J R Soc Med. 1994;87(9):526–30.
Ramirez-Zamora A, Morales-Vidal S, Chawla J, Biller J. Autopsy proven peripheral nervous system neurolymphomatosis despite negative bilateral sural nerve biopsy. Front Neurol. 2013;4:197.
Casselberry JN, Kritz AD. Neurolymphomatosis: a case study of diffuse large B-cell lymphoma. Clin J Oncol Nurs. 2012;16(6):636–7.
Gan HK, Azad A, Cher L, Mitchell PL. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro-Oncology. 2010;12(2):212–5.
Cheung C, Lopes D, Hung KN, Chan T, Chan KW, Kwong YL. Neurolymphomatosis: role of positron emission tomography in diagnosis. Ann Hematol. 2012;91(8):1313–4.
Zhou WL, Wu HB, Weng CS, Han YJ, Wang M, Huang S, et al. Usefulness of 18 F-FDG PET/CT in the detection of neurolymphomatosis. Nucl Med Commun. 2014;35(11):1107–11.
Borit A, Altrocchi PH. Recurrent polyneuropathy and neurolymphomatosis. Arch Neurol. 1971;24(1):40–9.
Ghobrial IM, Buadi F, Spinner RJ, Colgan JP, Wolanskyj AP, Dyck PJ, et al. High-dose intravenous methotrexate followed by autologous stem cell transplantation as a potentially effective therapy for neurolymphomatosis. Cancer. 2004;100(11):2403–7.
Acknowledgements
This work was supported by grant from the Sichuan provincial Science and Technology program 2009FZ0068. The funder had no role in study design, date collection and analysis, decision to publish, or manuscript preparation. We thank Rong Tian for the image description. And thank Fengming Tian in the collecting of the medical records. We also thank Jiantao Mou for correcting the English writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZFL collected the clinic information and drafted the manuscript. JT helped with data modification and drafted the manuscript. NH helped with image modification and drafted the manuscript. YQJ reviewed the draft and made critical modification. All authors have read and approved the final manuscript.
Zuofeng Liu and Tao Jiang are co-first authors.
Zuofeng Liu and Tao Jiang contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, Z., Jiang, T., Hou, N. et al. Peripheral neurolymphomatosis with tracheal asphyxia: a case report and literature review. BMC Neurol 15, 149 (2015). https://doi.org/10.1186/s12883-015-0405-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-015-0405-2