Abstract
Background
Though on-line intermittent hemodiafiltration (OL-IHDF) is a routine therapy for chronic dialysis patients, it is not yet widespread used in critically ill patients. This study was undergone to evaluate efficiency and tolerance of OL-IHDF and to appreciate inflammatory consequences of its use in intensive care unit (ICU)-acute kidney injury (AKI) patients.
Methods
In this prospective cohort study conducted in a medical academic ICU in France, 30 AKI patients who underwent OL-IHDF were included. OL-HDF used an ultrapure water production: AQ 1250 line with double reverse osmosis, a generator 5008 with a 1.8m2 dialyzer with Polysulfone membrane (Fresenius Medical Care). Tolerance and efficiency of OL-IHDF were evaluated as well as its inflammatory risk by the measurement of plasma concentrations of proinflammatory (Interleukin 6, IL1β, IL8, Interferon γ) and anti-inflammatory (IL4, IL10) cytokines, Epidermal growth factor (EGF), Vascular Endothelial growth factor (VEGF) and Macrophage Chemoattractive Protein-1 (MCP-1) before and after sessions.
Results
Intradialytic hypotensive events were observed during 27/203 OL-IHDF sessions accounting for a mal-tolerated session’s rate at 13.3%. Mean delivered urea Kt/V per session was 1.12 ± 0.27 with a percentage of reduction for urea, creatinine, β2-microglobulin and cystatine C at 61.6 ± 8.8%, 55.3 ± 6.7%, 51.5 ± 8.7% and 44.5 ± 9.8% respectively. Production of superoxide anion by leukocytes, mean levels of pro- and anti-inflammatory cytokines and plasmatic concentrations of EGF, VEGF and MCP-1 did not differ before and after OL-IHDF sessions. We observed however a significant decrease of mean TNFα plasmatic concentrations from 8.2 ± 5.8 to 4.8 ± 3.5 pg/ml at the end of OL-IHDF.
Conclusions
OL-IHDF was not associated with an increase in pro and anti-inflammatory cytokines, oxidative stress or EGF, VEGF and MCP-1 in AKI patients and seems therefore a secure and feasible modality in ICUs.
Similar content being viewed by others
Background
In intensive care units (ICUs), around 5% of patients suffering from acute kidney injury (AKI) require renal replacement therapy (RRT) [1]. Intermittent conventional hemodialysis (IHD) and continuous venovenous hemo(dia)filtration are the 2 principal modalities used for RRT. However, the ideal renal replacement method for intensive care patients remains under scrutiny [2, 3]. It should combine the advantages of continuous RRT (CRRT) with those of IHD, be simple to implement and induce minimal work with limited cost. On-line dialysis fluids preparation may fit these conditions since it offers favorable technical possibilities and highly flexible procedures to apply various forms of cost-effective high efficiency hemodiafiltration (HDF) modalities in intermittent or sustained mode [4,5,6,7].
On-line HDF (OL-HDF) is a RRT based on cold sterilization of dialysis fluid to prepare the infusate which is readily administered into the extracorporeal bloodstream. It necessitates a fully microbiological integrity of on-line produced dialysis fluids. In chronic dialysis facilities, this technique became a routine and safe modality of RRT and now represents the most effective dialysis therapy [8,9,10]. A few reports exist about its use in ICUs and its routine application is mainly restricted to ICU facilities working with a trained nephrological team [4,5,6,7, 11,12,13,14,15,16,17]. The limited use of this technique by intensivists is largely related to the technical complexity with water treatment and HDF machines and to the potential infectious risk of on-line produced fluids infusion especially in septic patients who represent the majority of admissions in ICUs. We have previously reported our experience of OL-HDF use in ICU [11]. A regular bacteriological control of dialysis fluids showed that this technique is safe and well tolerated [11]. Whether OL-HDF modulates plasma cytokine concentration and oxidative stress production is still not investigated. The combined use of synthetic biocompatible membrane and ultrapure dialysis fluid may limit the additional inflammatory risk induced by OL-HDF. However, this potential acute inflammatory risk induced by OL intermittent HDF (OL-IHDF) which remains possible in ICU-AKI has never been evaluated. We designed therefore a study to determine whether OL-IHDF would induce an overproduction of oxidative stress, cytokines and growth factors in critically ill patients.
Methods
This observational prospective study was carried out at the Medical ICU of Lapeyronie University Hospital at Montpellier and was approved by the Ethics Research Committee of our hospital; PHRC régional: N° 2006-A00510–51.
Patients
Since 2004, we exclusively used OL-HDF besides continuous therapies to treat our critically ill patients. During one-year period, we consecutively enrolled all patients admitted to the ICU with AKI requiring RRT support and who underwent OL-IHDF. Exclusion criteria included pregnancy, age < 18 years old, previous chronic renal failure, and severe neutropenia. Epidemiological data and severity of patients assessed by the Simplified Acute Physiologic (SAPS) II [18] and Sepsis-related Organ Failure Assessment (SOFA) scores [19] were collected. Decisions regarding the initiation, management, and discontinuation of RRT were made by the referring physician according to the KDIGO recommendations [20]. The choice of RRT modality was depending on patient hemodynamic stability and was daily re-evaluated. Patients with hemodynamic instability or severe fluid overload were preferentially treated with continuous venovenous hemodiafiltration and with OL-IHDF when they had or recovered hemodynamic stability. Only OL-IHDF sessions were investigated. Outcome was assessed at ICU discharge.
On-line intermittent hemodiafiltration
On-line intermittent hemodiafiltration: Description, disinfection procedures
OL-IHDF was performed using a RRT generator (Fresenius 5008, Fresenius Medical Care, Bad Homburg, Germany) with a standard 1.8 m2 Polysulfone hemodiafilter HF80 (Fresenius Medical Care, Bad Homburg, Germany). Countercurrent dialysate flow (QD) was routinely set at 500 mL/min, on-line infusate flow in pre-dilution mode (Qi) at 100 mL/min and blood flow (QB) at 300 mL/min. Dialysate and infusate temperature were adjusted to 36 °C and the sodium dialysate concentration at 145 mmol/L. The net-ultrafiltration rate was adapted to the hemodynamic parameters and extracellular volume status of each patient. Our ICU water production and distribution system was identical to that routinely used in chronic dialysis facilities performing on-line therapies [21] as previously described [11]. Dialysate and infusate purity has been also validated previously [9, 11, 22] and was ensured by regular endotoxin and microbiological testing. Vascular access was obtained through double lumen jugular catheter, with unfractionated heparin for anticoagulation whenever needed.
On-line intermittent hemodiafiltration: Clinical tolerance
OL-IHDF clinical tolerance was investigated by collecting the following intradialytic parameters at baseline and every 30 min: pulse, temperature, mean arterial pressure (MAP). A pyrogenic reaction was defined as the onset of objective chills and an increase in temperature of more than 1 °C in a patient who had no recorded signs or symptoms of infection before RRT [23]. An intradialytic hypotensive event was defined by a 20% reduction of MAP or by an initiation or/and increase in vasoconstrictive agents’ dose.
On-line intermittent hemodiafiltration solute control and inflammatory mediators’ evaluation
Biological plasma parameters
Blood solutes including urea, creatinine, β2-microglobulin (β2-M) and cystatine C (CyC) were routinely monitored at the beginning and the end of each OL-IHDF session. Blood samples were collected at the end of the treatment by standard stop-flow technique [24] and after the first hour of treatment, simultaneously at the arterial and venous ports after a temporary net ultrafiltration cessation. Urea, creatinine, β2-M and CyC removals per session were evaluated by the percentage of solute reduction ratios according to: RR = [(Cpre–Cpost)/Cpre]*100 where Cpre and Cpost are respectively pre-treatment (baseline) and post-treatment concentrations [25]. Kt/V were determined by using Daugirdas second generation, single pool urea kinetic model equation: Kt/V = −ln (T-0.008 * time duration session in minutes) + (4–3.5*T)*(UF/weightpostsession) where T represents plasma ureapostsession/ureapresession [26]. Instantaneous whole blood (KW.B) and plasma water solutes clearances (KP.W.) were estimated as follows: KW.B = QB*[(Cart-Cven)/Cart] where QB is effective blood flow, Cart and Cven are solute concentration in arterial and venous blood line; KP.W. = KW.B *(1–0.00107*Tp)*[(SPC*Ht) + (1 - Ht)] where Ht is the patient’s predialysis hematocrit level, Tp the average of total protein level in arterial and venous blood line (Tp = [Tpart + Tpven]/2) and solute partition coefficient: 0.86 for urea, 0.73 for creatinine, and 0 for β2-M and CyC [27].
Determination of superoxide (O2 °-) anion production by whole blood
O2 °- anion production was measured in blood samples before and after OL-IHDF sessions. It was determinated in 200 μL of fresh whole blood (treated immediately after collection) diluted in 820 μl of DMEM medium and 200 μL of lucigenin (1.5.10−4 mol/L) (Sigma Chemical, Saint Quentin Fallavier, France) [28]. After a 20-min incubation at 37 °C under gentle agitation, whole blood was stimulated by using Phorbol 12-Myristate 13-Acetate (PMA) (10−7 M) and the luminescence was immediately recorded at 37 °C by means of a Victor Wallac luminometer (Perkin Elmer, Turku, Finland). Luminescence intensity was normalized to leukocyte count. Response of PMA-free whole blood (basal O2 °- production) incubated simultaneously was used as control and considered as equal to 100%. To rule out autoproduction of O2 °- by lucigenin or by plasma compounds, O2 °- production was determined in whole blood, de-leukocyted blood, plasma and culture medium. Imprecision studies of O2°- production measure were as follows: intra-assay CV = 3.5% (basal O2°- production) and 3.9% (PMA-stimulated O2°- production); interassay CV = 5.0% (basal O2°- production) and 9.7% (PMA-stimulated O2°- production).
Determination of plasmatic cytokines, growth factors, and advanced oxidation protein products, measurements
Pre-and post-OL-IHDF sessions blood samples were immediately centrifuged at 1000 g for 10 min at 4 °C and stored at −80 °C until use. A panel of cytokines was determined on frozen plasma using a proteomic approach on an Evidence Investigator® biochip system (Randox, Mauguio, France). This proteomic method allows the simultaneous determination of IL1β, IL4, IL6, IL8, IL10, interferonγ, Epidermal growth factor (EGF), Vascular Endothelial growth factor (VEGF), Tumor Necrosis factor α (TNFα) and Macrophage Chemoattractive Protein-1 (MCP-1) levels. After addition of a sample (100 μl) to the biochip, the degree of binding of each analyte to its specific ligand is determined using a chemiluminescence light source and quantified using a super-cooled charge-coupled camera and an image-processing software [29].
Plasma Advanced Oxidation Protein Products (AOPP) levels (μM/l) were measured in pre- and post-OL-IHDF sessions blood samples by spectrophotometry [30, 31].
Statistical analysis
Statistical analysis was performed using SAS Entreprise Guide version 4.1. We first performed a descriptive analysis by computing frequencies and percents for categorial data, means, standard deviations, quartiles and extreme values for continuous data. For every patient included, 2 or more OL-IHDF sessions were investigated. The session that induced the highest post treatment cytokine increase was solely analyzed per patient. We checked for normality of continuous data distribution (O2 °- anion production, cytokines and proinflammatory mediators measurements), using Shapiro-Wilk’s tests. To analyze differences between before and after treatment measurements, univariate analysis was performed using two-tailed Student t-test, or two-tailed Mann-Whitney-Wilcoxon’s test (signed Rank Statistic) when appropriate. Kinetic of oxidative stress and cytokine release before and after OL-IHDF was also investigated in all included sessions using a linear mixed model. A value of p < 0.05 was considered significant.
Results
Patient demographic data
During the study period, 34 of 51 patients admitted to our ICU for severe AKI treated by RRT were included in the study. Main reasons for non-inclusion were: contra-indication (8 patients for aplasia, 2 for non-consent, and 2 for life expectancy less than 48 h), and 5 patients treated only with continuous RRT. In addition, 4 patients were excluded because of missing data. Thus, 30 patients were enrolled in the study and completely analyzed. Age, gender, cause of AKI, severity scores are listed in Table 1. The cause of AKI was septic in approximately ¾ of the cases. All patients were anuric, treated by vasoactive agents and most of them ventilated. The ICU mortality rate was 26.7%.
Parameters, clinical and tolerance evaluation of on-line intermittent hemodiafiltration sessions.
Ol-IHDF duration time ranged from 4 to 6 h with a median time at 4.8 h. Sessions parameters were as follows: blood flow: 285(326–185) ml/mn, dialysate flow: 468 (442–489) ml/mn, predilution infusate flow: 89 (110–85) ml/mn with convection volume at 28 (20–36) l per session. Venous recirculation was less than 5% in all sessions.
Among the 203 OL-IHDF sessions (>3/patient) evaluated, mean arterial pressure (MAP) increased from 86.2 ± 16 to 90 ± 16 mmHg after treatment (p < 0.05) with a significant increase of MAP after 119/203 (58%) sessions. An intradialytic hypotensive event was observed during 27 sessions accounting for a mal-tolerated session’s rate at 13.3% (Table 2). Cessation of ultrafiltration was sufficient to restore hemodynamic stability in 23 sessions while a fluid challenge or a vasopressor support was necessary during the remaining sessions (Table 2). No pyrogenic reactions occurred among all OL-IHDF sessions performed.
On-line intermittent hemodiafiltration efficiency and solutes control
Evaluation for efficiency and inflammatory consequences of OL-IHDF was achieved for 76 out of 203 (37.4%) OL-IHDF sessions accounting for at least one session per patient (choosing the session with the highest cytokine increase after treatment according to Statistical analysis). Mean delivered urea Kt/V session was 1.12 ± 0.27. RR for urea, creatinine, β2-M and CyC were respectively 61.6 ± 8.8%, 55.3 ± 6.7%, 51.5 ± 8.7% and 44.5 ± 9.8%. KW.B. and KP.W. were 239.2 ± 22.3 and 213.4 ± 20.7 mL/min for urea, 197 ± 22 and 168.6 ± 20.5 mL/min for creatinine, 58.9 ± 17.3 and 38.5 ± 10.9 mL/min for CyC, 77.8 ± 29.6 and 50.3 ± 17.4 mL/min for β2-M. Albuminemia increased from 27.5 ± 4.0 g/L to 28.5 ± 4.3 g/L (p < 0.05).
On-line intermittent hemodiafiltration and inflammatory mediators
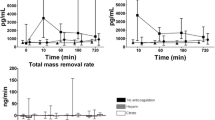
Mean basal and PMA-stimulated production of O2°- anion by leukocytes did not differ before and after OL-IHDF sessions (Fig. 1a). The activation rate of PMA-stimulated O2°- production reached 294% ± 273 at the initiation and 372% ± 415 at the end of OL-IHDF, but differences were not statistically significant (Fig. 1b). However, OL-IHDF sessions provided a slight but significant decrease in AOPP (p = 0.008) (Fig. 1c).
Cytokines plasmatic measurements showed a significant heterogeneity between individuals before initiation of OL-IHDF but their variations after OL-IHDF sessions remained totally similar. Thus, mean levels of pro- (IL6, IL10, IL8, interferon γ) and anti- (IL4, IL10) inflammatory cytokines were not significantly different before and after OL-IHDF sessions (Figs. 2 and 3). We observed however a significant decrease of mean TNFα plasmatic concentrations from 8.2 ± 5.8 to 4.8 ± 3.5 pg/mL after OL-IHDF sessions, a reduction ratio at 41.4% (Fig. 3). Analysis of plasmatic concentrations of EGF, VEGF and MCP-1, before and after OL-IHDF sessions showed no significant differences (Fig. 4).
Discussion
The present study demonstrated that OL-IHDF achieved an adequate dialysis dose with a fair hemodynamic tolerance in critically ill patients, and was not associated with an increased inflammatory risk. It did not induce an overproduction of oxidative stress and of pro- and anti-inflammatory cytokines but a significant decrease of TNFα and AOPP plasmatic levels.
On-line substitution fluid preparation is a standard feature of modern dialysis devices for chronic treatments [32]. In ICU settings, on-line RRT modalities are however rarely used and their routine application is mainly restricted to ICU facilities working with a trained nephrological team [4,5,6,7, 9, 11,12,13,14,15,16,17]. The carefulness of intensivist to use OL-IHDF is related to the potential infectious risk and deleterious effects of on-line produced fluid infusion especially in septic patients who represent the majority of those admitted to ICUs. On-line preparation is not accompanied by on-line control of the microbiological quality and inadequate infusate would be administered directly without prior product check and release. In a previous work, we have evaluated, over a 7 year-period, the purity of on-line produced ultrapure water and dialysis fluids by a weekly bacterial control monitoring in ICU settings: more than 90% of samples showed negative bacterial growth and undetectable levels of endotoxin indicating an overall compliance rate of 99% [11]. However, water and dialysis fluids may still contained cytokines-inducing substances other than endotoxins like breakdown products of microorganisms, peptidoglycans and β-glycans [33]. They have the potential capability of penetrating ultrafiltration and dialysis membranes with subsequent blood exposure and intradialytic cytokine and oxidative stress induction [34]. We aimed therefore to assess the inflammatory risk for critically ill patients receiving OL-HDF by the quantification of cytokines, oxidative stress and growth factors potentially produced during OL-IHDF.
Critically ill patients with AKI have higher circulating plasma concentration of inflammatory biomarkers implicated in RRT dependence and mortality than those without AKI [35, 36]. This inflammatory process is partly due to the generation of oxidative stress which is mainly of multifactorial origin including sepsis, and accumulation of uremic toxins in case of AKI but may be also related to RRT modalities. Indeed, online therapy itself can exacerbate oxidative stress production through leukocytes activation induced by dialysis and substitution fluids. Herein, we found that OL-IHDF did not alter superoxide anion production by leukocytes either basal or after stimulation by PMA. Our AKI patients treated by RRT have an increased levels of AOPPs [37,38,39], varying from 35 to 120 μmol/L as previously reported by Du et al. [39] but lower than those observed by Lentini et al. [38]. We observed that OL-IHDF provided a slight but still significant decrease in AOPP plasma concentrations. Of note, it has been suggested earlier that AOPPs contribute to the progression of renal failure and that higher AOPP levels are associated with poor renal recovery [40]. Cytokine induction has been also considered as the trigger of the inflammatory response and a critical parameter of dialysis biocompatibility during RRT [41]. Moreover, high levels of pro-inflammatory cytokines have been associated with increased mortality in AKI [35, 42]. We found that both pro- and anti-inflammatory cytokines plasma levels did not increase after OL-IHDF suggesting that it might not alter the balance of cytokines production. Other studies reported, like us, the lack of cytokines reduction by hemodiafiltration [43]. Substance clearance is dependent on its molecular size but also on ultrafiltration rate and on whether the substitution fluid is administered before and after the filter. In our study, we used a predilution modality of HDF diluting the blood before filter passage and convection volumes were less than 30 l per session explaining, at least for a part, the observed non significative reduction of plasmatic cytokines.
Last, OL-IHDF sessions were not associated with an increased production of VGEF, EGF and MCP-1 in our patients. Enhanced production of growth factors after endothelial activation has been reported in critical conditions especially sepsis-related AKI leading to increased capillary permeability, systemic vasodilatation and multi-organ failure [44, 45]. Chancharoenthana W [46] reported however that OL-HDF provided a significant removal of VEGF and was associated with better renal outcome as compared to high-flux hemodialysis.
Furthermore, on-line IHDF was best tolerated and achieved an adequate urea reduction rate. We did not observe any pyrogenic reactions whereas data on chronic on-line HDF reported an incidence at 0.04% [47]. A low incidence of intradialytic hypotensive events was also repertoried (13.3%) lower than recently reported (18.7%) (47). However, we may not state that OL-IHDF lead to a better hemodynamic tolerance than other modalities especially continuous therapies since this study was only observational.
We must acknowledge some limitations to the study. First, our work shares the limitations of single-center studies. Our unit is indeed familiar with online therapies which are not the general rule in ICU settings. Second, the number of patients included may be considered small but a high number of OL-HDF sessions were analyzed and secure our observations. Third, our RRT patients exhibited a cellular reactivity as reflected by inflammatory and oxidative stress parameters with a medium intensity thanks to the biocompatibility membranes. An evaluation of more sensitive biomarkers like isoprostanes may evaluate more thoroughly oxidative stress. Nevertheless, most of the tested parameters were not modified during the on-line sessions performed, a result that represents our primary hypothesis in this work. Last, outcome was not studied in this study since our concern was focused on feasibility and potential risk of this technique in critically ill patients.
Conclusions
In conclusion, our study shows that OL-IHDF does not induce additional inflammatory risks in critically ill patients with AKI and may be used securely in these settings. On-line production of ultrapure water seems to be very useful for ICU acute renal failure as it gives possibilities for a large scale of dialysate and infusate rate prescription. Further studies should however investigate its effect on all-cause mortality in comparison to other RRT modalities.
Abbreviations
- AKI:
-
Acute kidney injury
- Cpost:
-
Post-treatment concentration
- Cpre:
-
Pre-treatment concentration
- CRRT:
-
Continuous renal replacement therapy
- CyC:
-
Cystatine C
- EGF:
-
Epidermal growth factor
- HDF:
-
Hemodiafiltration
- ICU:
-
Intensive care unit
- IHD:
-
Intermittent conventional hemodialysis
- KP.W. :
-
Plasma water solutes clearances
- Kt/V:
-
Clearance adjusted for total body volume
- KW.B. :
-
Instantaneous whole blood solutes clearances
- MCP-1:
-
Macrophage Chemoattractive Protein-1
- O2°-:
-
Superoxide anion
- OL-HDF:
-
On-line hemodiafiltration
- OL-IHDF:
-
On-line intermittent hemodiafiltration
- PMA:
-
Phorbol 12-myristate 13-acetate
- QB:
-
Blood flow
- QD:
-
Dialysate flow
- Qi:
-
Infusate flow
- RR:
-
reduction ratio
- RRT:
-
Renal replacement therapy
- SAPS II:
-
Simplified Acute Physiologic II score
- SOFA:
-
Sepsis-related Organ Failure Assessment score
- TNF α:
-
Tumor Necrosis factor alpha
- VEGF:
-
Vascular Endothelial growth factor
- β2-M:
-
β2-microglobulin
References
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8.
Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–85.
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154–63.
Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, et al. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–7.
Marshall MR, Ma T, Galler D, Rankin APN, Williams AB. Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: towards an adequate therapy. Nephrol Dial Transplant. 2004;19:877–84.
Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y, et al. Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artif Organs. 2010;34:331–8.
Kron J, Kron S, Wenkel R, Schuhmacher H-U, Thieme U, Leimbach T, et al. Extended daily on-line high-volume haemodiafiltration in septic multiple organ failure: a well-tolerated and feasible procedure. Nephrol Dial Transplant. 2012;27:146–52.
Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A, Strippoli GF. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic revierw of randomized controlled trials. Am J Kidney Dis. 2014;63:954–67.
Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A, Strippoli GF. Haemodiafiltration, haemofiltration and haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev. 2015;5:CD006258.
Morena M, Jaussent A, Chalabi L, Leray-Moragues H, Chenine L, Debure A, Thibaudin D, et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;17:30040–6.
Klouche K, Amigues L, Serveaux-Delous M, Machado S, Delabre J-P, Laydet E, et al. Implementing on-line hemodiafiltration as a renal replacement therapy for ICU acute renal failure: a single-center report of feasibility, safety and hemodynamic tolerance over a seven-year period. Blood Purif. 2012;34:10–7.
Pettilä V, Tiula E. Intermittent hemodiafiltration in acute renal failure in critically ill patients. Clin Nephrol. 2001;56:324–31.
Laurent I, Adrie C, Vinsonneau C, Cariou A, Chiche J-D, Ohanessian A, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. 2005;46:432–7.
Kawanishi H. On-line hemodiafiltration in critical care. Ther Apher. 2002;6:199–203.
Kawanishi H. On-line continuous hemodiafiltration in sepsis. Transfus Apher Sci. 2006;35:265–9.
Kumar VA, Yeun JY, Depner TA, Don BR. Extended daily dialysis vs. continuous hemodialysis for ICU patients with acute renal failure: a two-year single center report. Int J Artif Organs. 2004;27:371–9.
Kielstein JT, Kretschmer U, Ernst T, Hafer C, Bahr MJ, Haller H, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis. 2004;43:342–9.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. 1993;270:2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10.
KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1–138.
Canaud B, Bosc JY, Leray H, Stec F, Argiles A, Leblanc M, et al. On-line haemodiafiltration: state of the art. Nephrol Dial Transplant. 1998;13(Suppl 5):3–11.
Canaud B, Bosc JY, Leray H, Stec F. Microbiological purity of dialysate for on-line substitution fluid preparation. Nephrol Dial Transplant. 2000;15(Suppl 2):21–30.
Pegues DA, Oettinger CW, Bland LA, Oliver JC, Arduino MJ, Aguero SM, et al. A prospective study of pyrogenic reactions in hemodialysis patients using bicarbonate dialysis fluids filtered to remove bacteria and endotoxin. J Am Soc Nephrol. 1992;3:1002–7.
NKF-DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1997;30:suppl2 S38-S42.
Bergström J, Wehle B. No change in corrected beta 2-microglobulin concentration after cuprophane haemodialysis. Lancet. 1987;1:628–9.
Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–13.
Badiou S, Dupuy AM, Descomps B, Cristolead JP. Comparison between the enzymatic vitros assay for creatinine determination and three other methods adapted on the Olympus analyzer. J Clin Lab Anal. 2003;17:235–40.
Ventura E, Durant R, Jaussent A, Picot M-C, Morena M, Badiou S, et al. Homocysteine and inflammation as main determinants of oxidative stress in the elderly. Free Radic Biol Med. 2009;46:737–44.
Dupuy AM, Lehmann S, Cristol JP. Protein biochip systems for the clinical laboratory. Clin Chem Lab Med. 2005;43:1291–302.
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13.
Delample D, Durand F, Severac A, Belghith M, Mas E, Michel F, et al. Implication of xanthine oxidase in muscle oxidative stress in COPD patients. Free Radic Res. 2008;42:807–14.
Canaud B, Barbieri C, Marcelli D, Bellocchio F, Bowry S, Mari F, et al. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int. 2015;88:1108–16.
Tsuchida K, Takemoto Y, Yamagami S, Edney H, Niwa M, Tsuchiya M, et al. Detection of peptidoglycan and endotoxin in dialysate, using silkworm larvae plasma and limulus amebocyte lysate methods. Nephron. 1997;75:438–43.
Schindler R, Krautzig S, Lufft V, Lonnemann G, Mahiout A, Marra MN, et al. Induction of interleukin-1 and interleukin-1 receptor antagonist during contaminated in-vitro dialysis with whole blood. Nephrol Dial Transplant. 1996;11:101–8.
Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–65.
Chawla LS, Seneff MG, Nelson DR, Williams M, Levy H, Kimmel PL, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30.
Selmeci L, Seres L, Antal M, Lukács J, Regöly-Mérei A, Acsády G. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: a simple, fast and inexpensive automated technique. Clin Chem Lab Med. 2005;43:294–7.
Lentini P, de Cal M, Cruz D, Chronopoulos A, Soni S, Nalesso F, et al. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J Crit Care. 2010;25:605–9.
Du S, Zeng X, Tian J, Ai J, Wan J, He J. Advanced oxidation protein products in predicting acute kidney injury following cardiac surgery. Biomarkers. 2015;20:206–11.
Li HY, Hou FF, Zhang X, Chen PY, Liu SX, Feng JX, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol. 2007;18:528–38.
Vaslaki L, Weber C, Mitteregger R, Falkenhagen D. Cytokine induction in patients undergoing regular online hemodiafiltration treatment. Artif Organs. 2000;24:514–8.
Kadiroglu AK, Sit D, Atay AE, Kayabasi H, Altintas A, Yilmaz ME. The evaluation of effects of demographic features, biochemical parameters, and cytokines on clinical outcomes in patients with acute renal failure. Ren Fail. 2007;29:503–8.
Tamme K, Maddison L, Kruusat R, Ehrlich HE, Viirelaid M, Kern H, Starkopf J. Effects of high volume haemodiafiltration on inflammatory response profile and microcirculation in patients with septic shock. BioMed Research International. 2015; 125615. doi: 10.1155/2015/125615.
van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–8.
Pickkers P, Sprong T, van Eijk L, van der Hoeven H, Smits P, van Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. 2005;24:508–12.
Chancharoenthana W, Tiranathanagul K, Srisawat N, Susantitaphong P, Leelahavanichkul A, Praditpornsilpa K, et al. Enhanced vascular endothelial growth factor and inflammatory cytokine removal with online hemodiafiltration over high-flux hemodialysis in sepsis-related acute kidney injury patients. Ther Apher Dial. 2013;17:557–63.
The VA/NIH Acute Renal Failure Trial Network. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20.
Acknowledgements
None.
Funding
None.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KK, LA and JPC designed the study. KK, LA, AMD, MM, VB, NB, DD, JPC analyzed and interpreted the data. AMD, MM and JPC performed the biological sample analysis and were contributor in writing the manuscript. AJ and MCP made the statistical analysis. All authors participated in drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This observational prospective study was carried out at the Medical ICU of Lapeyronie University Hospital at Montpellier and was approved by the Ethics Research Committee of our hospital; PHRC régional: N° 2006-A00510–51. A written informed consent was obtained from the patients. If the patient has not the capability to give consent, a written informed consent was obtained from its closest relative. All the patients confirmed their consent by writing later on.
Consent for publication
Not applicable.
Competing interests
The authors’ declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Klouche, K., Amigues, L., Morena, M. et al. On-line hemodiafiltration did not induce an overproduction of oxidative stress and inflammatory cytokines in intensive care unit-acute kidney injury. BMC Nephrol 18, 371 (2017). https://doi.org/10.1186/s12882-017-0785-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-017-0785-1