Abstract
Aims
This study aims to evaluate the presence of EBV, HCMV, and BKV genomic sequences in the plasma samples (active infection/viremia) of kidney transplant recipients suspected of rejection and to investigate host and risk factors related to the activation of these viruses in these patients.
Methods
In this cross-sectional single-center study, plasma samples were collected from 98 suspected kidney transplant rejection patients at Labafinejad Hospital, Tehran, Iran, between December 2022 and June 2023. Quantitative real-time PCR assays for HCMV, EBV, and BK were performed using GeneProof Real-time PCR kits. ROC curve analysis was used to determine the viral load cutoff point for each virus.
Findings
HCMV active viremia was detected in 18 (18.36%) recipients, EBV active viremia in 7 (7.14%), and BKV active viremia in 5 (5.10%). ROC results indicated viral load cutoff points of 778, 661, and 457 points for HCMV, EBV, and BKV, respectively. The duration of time after transplantation significantly differed between active viremia and no viremia groups (120.5 vs. 46 months, P = 0.014). In the BKV active viremia group, the increase in creatinine compared to baseline creatinine was significantly higher than in the no viremia group (2.7 vs. 0.8, P = 0.017). The odds ratio of HCMV active viremia in patients taking tacrolimus was 2.84 times higher, and the odds of HCMV active viremia in patients taking antithymocyte globulin was 3.01 times higher than in patients not taking these drugs.
Conclusion
Rapid and timely diagnosis of viral active infections in kidney transplant patients is crucial for effective disease management and implementation of appropriate treatment strategies. Identifying potential risk factors, including host and treatment-related factors that influence transplantation, can facilitate the development of suitable preventive strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A growing number of people worldwide are suffering from end-stage renal disease (ESRD) [1]. People with ESRD are often treated with renal transplantation as the first-line treatment modality [2]. Every year 24 transplants per million people are performed in Iran, resulting in 1700 kidney transplants per year [3]. Infections are a major cause of morbidity and mortality in kidney transplant recipients (KTR). The main threats for KTRs are BK virus (BKV), human cytomegalovirus (HCMV), and Epstein-Barr virus (EBV). These viruses are widespread in the healthy population and exist in latent asymptomatic states. In immunocompromised individuals, their reactivation can cause various systemic diseases [4, 5]. HCMV is a ubiquitous double-stranded DNA (ds DNA) virus belonging to the herpesviridae family with a seroprevalence rate of 70–90% in the general adult population [4, 6,7,8]. It transmits through contact with infectious body fluids such as blood, saliva, urine, tears, seminal fluid, cervical secretions, breast milk, as well as solid organ and stem cell transplantation. Reactivation occurs in solid organ transplant recipients (SOTR) and individuals on immunosuppressive therapy that triggers latent virus activation [9, 10]. Reactivation of this virus in SOTRs can cause direct effects including fever, leukopenia, thrombocytopenia, raised liver enzymes, meningitis, colitis, pneumonia, hepatitis, and retinitis. Indirect effects of the virus on the host’s immune response include acute allograft rejection, reduced graft function, chronic allograft nephropathy, increased risk of other opportunistic infections, decrease in graft and patient survival rate [4, 7, 11,12,13]. The detection of HCMV-DNA in plasma indicates active viral replication and active viremia is the most sensitive and precise marker of active HCMV infection [9, 14].
Epstein-Barr virus (EBV) is a double-stranded DNA virus with a seroprevalence of over 90% by age 5 in developing countries and 50% in affluent countries [2, 15]. It is transmitted via oropharyngeal secretions and latency occurs within the B lymphocytes without active infection in the majority of hosts [4, 6]. In immunocompromised individuals and kidney transplant recipients, it can manifest as asymptomatic active viremia, fever, lymphadenopathy, hepatosplenomegaly, infectious mononucleosis syndrome, pneumonia, encephalitis, hepatitis, myocarditis, and pancreatitis. The most important complication of EBV is post-transplant lymphoproliferative disorder (PTLD) [6, 15]. EBV DNAemia has been reported in up to 40% of patients in the first year after transplantation [6]. BK virus (BKV) is a ubiquitous, non-enveloped, and circular dsDNA virus belonging to the polyomaviridae family [16, 17]. BK polyomavirus infection is common in healthy adults with a seroprevalence of 80–90% [2, 6]. BK virus active infection can manifest as hemorrhagic cystitis, ureteric stenosis, nephritis, tubulointerstitial nephritis, and BKV nephropathy (BKVN) [4, 16]. Risk factors for causing infection or reactivation of these viruses in KTRs may include donor and recipient serostatus mismatch (D+, R-), donor and recipient age, gender, living or deceased donor, human leukocyte antigen mismatch (HLA), underlying diseases, immunosuppressive drugs [4, 11, 12, 16, 18].
Taken together, BKV, HCMV, and EBV are known to be the main pathogens causing complications in KTR. Therefore, timely diagnosis of reactivation of these viruses in kidney transplant patients is crucial for effective management of the patient [19]. It should be noted that the presence of concurrent active infection of these three viruses, along with a wide range of host factors and treatment for patients suspected of kidney transplant rejection, has not been comprehensively investigated. Therefore, this study aims to evaluate the presence of active BKV, HCMV, and EBV infections in the blood samples of patients suspected of kidney transplant rejection. In addition, host and a wide range of risk factors related to the activation of these viruses including age, the time interval between receiving the transplant and the onset of infection symptoms, blood creatinine level, estimated glomerular filtration rate (eGFR), use of antiviral, corticosteroid, and immunosuppressive drugs, and HCMV and EBV IgG antibody serostatus of recipient and donor before receiving a kidney transplant (D + R+, D + R-, D-R+, D-R-) were investigated.
Material and methods
Setting and study population
In this cross-sectional single-center study, we collected plasma samples from 98 patients suspected of kidney transplant rejection in Labafinejad Hospital, Tehran, Iran, between December 2022 and June 2023. The criteria for the inclusion of samples in the study were as follows: (I) Blood samples from kidney transplant recipients suspected of transplant rejection based on medical diagnosis. (II) Post-transplant complications include fever for more than a day, dysuria, edema in the hands and feet and around the eyes, sudden pain in the transplanted kidney, vomiting and diarrhea, shortness of breath, hematuria, strangury, dizziness, cellulitis, jaundice, creatinine elevation, proteinuria, and oliguria. The occurrence of at least two of these complications was the criterion for participation in this study. (III) The study group consisted of adults over 18 years old. Patients with incomplete medical records were excluded. Data regarding demographics (including age, gender, HCMV-EBV donor and recipient serostatus, blood creatinine level, and GFR), immunosuppressive regimens, underlying diseases, donor type, and history of kidney transplant rejection were collected from the patient’s medical records. In this study, we checked the creatinine increase rate and GFR reduction rate of the patients. “Creatinine increase rate” means the increase in creatinine of kidney transplant recipients when they were suspected of kidney transplant rejection and were admitted to the hospital compared to the baseline creatinine before hospitalization. Also, the “GFR reduction rate” means the reduction of GFR in kidney transplant recipients when they were suspected of kidney transplant rejection and were admitted to the hospital compared to the baseline GFR before hospitalization. eGFR was calculated using the Cockroft equation (Cockcroft-Gault CrCl, mL/min = (140 – age) × (weight, kg) × (0.85 if female) / (72 × Serum Cr, mg/dL).
The project was approved by the ethical committee of Tarbiat Modares University (IR.MODARES.REC.1402.059).
Sampling and quantitative real-time PCR
98 plasma samples free of cells and cell debris were stored at -80 °C and used to quantify and detect the HCMV, EBV, and BKV. DNA was extracted from 200 μl of plasma using ROJE DNall Plus Kit (ROJE, Iran) according to the manufacturers’ instructions The optical absorption ratio of extracted DNA at 260/280 nm wavelength was measured by nanodrop device, which ratio should be 1.8-2.0. Detection and quantification of HCMV, EBV, and BKV were performed in a StepOne plus real-time PCR system (Applied Biosystems International, Foster City, CA, USA). Quantitative real-time PCR assays were performed using the GeneProof Real-time PCR kits (Videnska, Czech Republic). The HCMV kit is designed based on the identification of the coding region 4 IE, the EBV kit is based on the identification of the EBNA region 1, and the BKV kit is based on the T-Ag gene region. The characteristics of GeneProof real-time PCR kits are outlined in Table 1. The internal standard (IS) included in the kits was used to check the quality of the extracted DNA and to evaluate potential inhibition of the PCR reaction. The primer, probe, and MasterMix are designed in one vial. The kits contain four calibrators at concentrations of 101, 102, 103, and 104 IU/μL. First, 1 μL of IS was mixed with 10 μL of the extraction products. Samples were assayed in a 40 μL reaction mixture consisting of 30 μL of MasterMix and 10 μL of extraction products mixed with IS. The cycling steps were set as follows: initial denaturation at 95 °C for 10 min, followed by 45 cycles of 95 °C for 5 s, 60 °C for 40 s, and 72 °C for 20 s, at the end of which fluorescence was read. Real-time PCR amplification data were analyzed with software provided by the manufacturer. The number of HCMV, EBV, and BKV copies was calculated from the standard curve. Data were expressed as copies of viral DNA per milliliter (Copy/ml) of plasma samples for EBV and as an international unit (IU) per milliliter (IU/ml) for HCMV and BKV.
Statistical analysis
The probable predictive factors for HCMV, EBV, and BKV replication were analyzed using the statistical software package SPSS version 26. The Shapiro-Wilk test was used to assess whether data were distributed normally. The result was reported as mean (standard deviation) or frequency (percentage) for numerical or qualitative data. Baseline characteristics were compared using a t-test or chi-square test. We used the chi-square test to compare the frequency of qualitative variables according to the presence of HCMV, EBV, and BKV in plasmas. Also, an independent t-test was used to assess the numerical variables in groups with and without the HCMV, EBV, and BKV. The association between HCMV, EBV and BKV was assessed by the chi-square test. A 2-tailed P < 0.05 was regarded as statistically significant. The univariate logistic regression (ENTER method) and multivariate analysis using the BACKWARD model were performed to assess the effect of factors with a potential impact on HCMV, EBV, and BKV replication development.
Results
Demographic and clinical characteristics of patients suspected of kidney transplant rejection
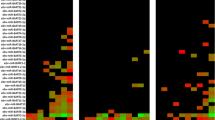
During the study period, 98 patients including 57 (58.2%) males and 41 (41.8%) females with a mean age of 50.7 ± 16.7 years were included. The most underlying diseases in the studied population included hypertension (71.4%), diabetes mellitus (23.5%), polycystic kidney (23.5%) and glomerulonephritis (20%). Other baseline characteristics of studied patients are presented in Table 2. The analyses of clinical signs and symptoms are shown in Fig. 1.
Real-time PCR of active viremia status
We used the real-time PCR test to detect HCMV, EBV, and BKV in patients suspected of kidney transplant rejection. In our study population, HCMV active viremia was detected in 18 (18.36%) recipients, EBV active viremia in 7 (7.14%) recipients, and BKV active viremia in 5 (5.10%) (Fig. 2). The median viral load in the HCMV active viremia group was 24,914 IU/ml (136523 − 9196.75), in the EBV active viremia group was 1715 copies/ml (10110 − 791.5), and in the BKV active viremia group was 9000 IU/ml (37000-924).
Receiver operating characteristic (ROC) curve analysis
Since there is no agreed cut-off point for HCMV, EBV, and BKV, we used ROC analysis to determine the viral load cut-off points for HCMV, EBV, and BKV and to divide the samples into active viremia and no active viremia. Finally, based on past studies and ROC results, 778, 661, and 457 points were considered for HCMV, EBV, and BKV, respectively.
Comparison of host factors in patients with and without active viremia for HCMV, EBV, and BK
Tables 3, 4 and 5 show the results of the variable comparison in patients with and without active viremia for HCMV, EBV, and BKV. The quantitative variables in the mentioned tables are all normal, except for the creatinine level and its increase. Therefore, an independent t-test was used for normal variables and a Mann-Whitney test for non-normal comparisons. Table 3 shows that in the HCMV active viremia group, there is a statistically significant difference between the two groups in the “duration of time after transplantation”, so this value is 120.5 and 46 months in the active viremia group and the non-viremia groups, respectively (P = 0.014). Table 6 shows that the odds ratio (OR) of HCMV active viremia in the group that used tacrolimus was 2.84 times higher than the OR of active viremia in the group that did not use tacrolimus (OR:2.84, 95% C.I:0.97–8.35, P = 0.057) and it was significant at the 10% level. The OR of HCMV active viremia in the group that used anti-thymocyte globulin (ATG) was 3.01 times higher than the OR of active viremia in the group that did not use ATG (OR:3.01,95% C.I:0.91–9.94, P = 0.071). According to Table 5, in the BKV active viremia group, the increase in creatinine (2.7) was significantly higher than the baseline creatinine (0.8) (P = 0.017).
There was no significant association between HCMV, EBV and BKV active viremia with factors such as age, underlying diseases, post-transplant diseases, number of transplants, smoking, BMI, type of transplant donor, HLA mismatch, pre-transplant HCMV and EBV IgG serostatus (D + R+, D + R-, D-R+, D-R-) and biochemical parameters (P > 0.05) (Tables 3 and 4).
The median time interval from the time of transplantation to active viremia was 13 months as the shortest time interval for BKV active viremia and 120.5 months as the longest time interval for HCMV active viremia. EBV active viremia occurred at a median of 75 months after transplantation (Tables 3, 4 and 5).
94.4% of HCMV-active viremic recipients received their transplant from an HCMV- IgG positive donor, regardless of status, whereas only 5.5% of HCMV-viremic recipients received a transplant from an HCMV- IgG negative donor. Among transplant recipients with detected active HCMV viremia, none were virus-negative before transplantation and received a kidney transplant from a virus-negative donor (D-R-) (Table 4). Also, 85.7% of recipients with EBV active viremia received their transplant from an EBV-IgG positive donor, regardless of their status, and 14.2% of recipients with EBV active viremia received a transplant from an EBV-IgG negative (D-R-) donor (Table 5).
Concurrent presence of HCMV, EBV, and BKV
22.4% of patients suspected of kidney transplant rejection had an active infection of one of the viruses and 4% had a concurrent active infection of several viruses. There was no significant association between active viremia of one or more viruses simultaneously in patients and association with host factors. Of a total of 4 active co-infection patients, 3 patients had HCMV/BKV active co-infection and 1 patient had HCMV/EBV active co-infection. Table 7 presents the results of the variable comparison among patients based on active mono and co-infection.
Discussion
Viruses are one of the most common opportunistic infectious agents in transplant recipients, so pre-transplant screening, preventive antiviral treatments, and tracking the presence of the virus after the transplant are important [19]. According to previous studies for the detection of viruses, Real-Time PCR is recognized as a valuable tool in identifying viral agents due to its high sensitivity and specificity and its capacity to measure both qualitative and quantitative aspects. The test result serves as a valuable tool for epidemiological studies and disease management [19,20,21].
In this study, 3 out of 4 active co-infections exhibited concurrent HCMV-BKV active viremia and there is no significant difference in the time of occurrence of active mono-infection and active co-infection post-transplantation. (Table 7). The lack of statistical significance regarding these factors may be attributed to the limited number of individuals with active viremia in each viral group in our study. Co-infection with DNAemia may arise from the heightened risk faced by transplant recipients as immunosuppressive individuals leading to the reactivation of multiple viruses and the potential predisposition of the patient to additional infection [22, 23] In active co-infections, kidney transplant performance is worse compared to uninfected patients or patients infected with a single virus [24]. In the group with BKV active viremia, the increase in creatinine (2.7) was significantly higher than the increase in the group without active viremia (0.8) (P = 0.017) and the average baseline creatinine in the group with BKV active viremia increased from 1.5 mg/dl to 2.7 mg/dl (Table 5). Based on previous studies, serum creatinine levels greater than 2 mg/dl were identified as risk factors for BK nephropathy and graft loss [25]. A decrease in GFR slope was observed at the time of active viremia compared to patients without viremia. The slope of GFR reduction in patients with BKV active viremia was higher than EBV and HCMV active viremia. Also, the slope of GFR reduction in patients with active co-infection was higher than that of active mono-infection and this suggests that lower kidney function is associated with a weaker immune response because decreased kidney function causes a decrease in the number of B and T lymphocytes, impaired monocyte function, decreased memory cell production, and insufficient antibody production [26]. However, in none of these groups, there was no significant difference between the decrease in GFR slope and the incidence of active viremia (Tables 3, 4, 5 and 7).
In the study by Kairi Pullerits et al., in the UK, the median time to active viremia after transplantation was reported as 9 months for HCMV, 45 months for EBV, and 13–15 months for BKV and JCV active infections [4]. However, additional research has shown that the mean time to detect BKV active infection is around four months after transplantation, with significant active viremia occurring approximately five months after transplantation [27]. Furthermore, a study following patients for 6 years post-transplant reported that BK active viremia cleared in 96% of patients after a median of 137 days [28]. These findings highlight the variability in the onset of viremia for different viruses post-transplantation and the importance of long-term monitoring for viral active infections in transplant recipients. In the present study, the median time interval from the time of transplantation to the occurrence of HCMV, EBV, and BKV active viremia was 120.5 months, 75 months, and 13 months, respectively (Tables 3, 4 and 5). In the HCMV active viremia group, “time after transplantation” has a statistically significant difference, so this amount is 120.5 and 46 months in the active viremia and no active viremia groups, respectively (P = 0.014) (Table 3). One of the important side effects of the prophylaxis treatment method is the occurrence of late HCMV disease, which can lead to the failure of the transplanted organ and death. Therefore, following up with these patients using quantitative molecular tests and initiating antiviral treatment is necessary [29].
In the present study, more than half of the patients suspected of kidney transplant rejection who experienced HCMV active viremia had received treatment with ganciclovir and valganciclovir (83.3%) in addition to reduced immunosuppression. Furthermore, 57.1% and 60% of individuals diagnosed with EBV or BKV active viremia, respectively were treated with ganciclovir and valganciclovir (Tables 4 and 5). An interesting study in this field reported that the relative risk of PTLD was reduced by 38% for every 30 days of ganciclovir use after kidney transplantation. However, the main treatment options for PTLD include reducing the immunosuppressive regimen, rituximab, chemotherapy radiation therapy, or a combination of these [30]. Universal valganciclovir prophylaxis may have a higher risk of BKV active viremia than valacyclovir prophylaxis, according to a study by Tomas Reischig et al. Several studies have shown that ganciclovir can directly suppress T-cell proliferation and activation by inhibiting DNA synthesis, so ganciclovir may suppress BKV-specific T-cell immunity, thereby contributing to the high risk of BKV active viremia [31]. The chance of HCMV active viremia in the tacrolimus-treated group was 2.84 times that of the non-tacrolimus group (OR: 2.84, 95% C.I: 0.97–8.35, P = 0.057) which was significant at the 10% level (Table 6). The reduction of TNF-α, IFN-γ, and IL-2 by tacrolimus is implicated in increasing the risk of infection reactivation in these patients [32, 33].
Also, the chance of HCMV active viremia in the anti-thymocyte globulin (ATG) treated group was 3.01 times than that of the non-ATG group (OR: 3.01, 95% C.I: 0.91–9.94, P = 0.071) (Table 6). ATG causes T-lymphocyte depletion and suppresses the T-lymphocyte-mediated specific immune response against HCMV [34, 35]. Further research is needed to determine the optimal strategies for balancing the immunosuppressive effects of ATG with the risk of viral infections’ reactivation in this population.
Based on the studies conducted in this field and the use of the sensitive PCR method, the differences observed between different studies may be attributed to several factors, including the varying sample size, the duration of the studies, and the specific target regions of the viral genome in molecular investigations. Additionally, the heterogeneity of the studied population in terms of the time interval between transplantation and the onset of clinical symptoms may contribute to the differences in observed outcomes. The use of sensitive molecular techniques and the careful characterization of study populations are essential for accurately assessing the impact of viral infections on transplant outcomes and for informing preventive and therapeutic strategies in this population. The number of patients in this study was relatively small, and the follow-up period was limited. Further studies with a larger sample size and a longer duration are necessary to assess the role of factors influencing the incidence of active viremia after transplantation. The interval between the time of transplantation and the reactivation of the viruses in our studied patients varied from several months to several years therefore, it is recommended to compare the results of patients with suspected kidney transplant rejection who experience re-infection during the first year after transplantation with the findings of the present study.
As a future direction to exclude contamination of plasma DNA by cellular DNA, a housekeeping gene PCR should be performed, yielding negative results for the human DNA PCR. This is important for accurately determining active viremia, as the DNA of the aforementioned viruses may be present in blood cells during the latent phase of infection.
Conclusion
Quick and timely diagnosis of viral active infections in kidney transplant patients is crucial for effective disease management and implementation of appropriate treatment strategies. In this regard, molecular assays are useful due to their high sensitivity in measuring viral load and rapid diagnosis of infection’s reactivation. Also identifying potential risk factors, including host and treatment-related factors that influence transplantation can facilitate the development of suitable preventive strategies. According to this study, among the risk factors affecting the occurrence of active viremia in kidney transplant recipients, we can mention the increase of creatinine in the occurrence of BKV active viremia and the effect of ganciclovir prophylaxis in the occurrence of late HCMV active viremia, as well as the effect of anti-thymocyte globulin and tacrolimus on the occurrence of HCMV active viremia.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ghonemy TA, Farag SE, Soliman SA, El-Okely A, El-Hendy Y. Epidemiology and risk factors of chronic kidney disease in the El-Sharkia Governorate, Egypt. Saudi J Kidney Dis Transplantation. 2016;27(1):111–7.
Scaggs Huang FA, Danziger-Isakov L. Infectious disease risks in pediatric renal transplantation. Pediatr Nephrol. 2019;34:1155–66.
Shaeerbaf E, Shams sF, Lotfi Z, Sheikhi M, Shakeri S, Bahrami A. Cytomegalovirus infection among kidney transplant recipients one year after transplantation. J Ilam Uni Med Sci. 2018;26(2):172–9.
Pullerits K, Garland S, Rengarajan S, Guiver M, Chinnadurai R, Middleton RJ, et al. Kidney transplant-associated viral infection rates and outcomes in a single-centre cohort. Viruses. 2022;14(11):2406.
Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, Bauer C, Wolk K, Sabat R, et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: results from a large multi-centre study. EBioMedicine. 2018;34:113–21.
Agrawal A, Ison MG, Danziger-Isakov L. Long-term infectious complications of kidney transplantation. Clin J Am Soc Nephrol. 2022;17(2):286–95.
Afshari A, Yaghobi R, Golshan M. Cytomegalovirus microRNAs level determination in kidney recipients post transplantation. Virol J. 2022;19(1):147.
JABBARI M, Sabahi F, KHANSARI NB, Shirkoohi R, SABERI H, Parvin M, Ahmadi E. Human cytomegalovirus infection in tumor specimens of Iranian patients with glioma. Progress Biol Sci. 2016;6(1):11–8.
Eslami Kojidi M, Shatizadeh Malekshahi S, Jabbari MR. Assessment of human cytomegalovirus viral load in kidney transplant recipients in Tehran, Iran. IEM. 2023;9(4):323–30.
Fowler K, Mucha J, Neumann M, Lewandowski W, Kaczanowska M, Grys M, et al. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health. 2022;22(1):1659.
Higdon LE, Tan JC, Maltzman JS. Infection, rejection, and the connection. Transplantation. 2023;107(3):584–95.
Herrera S, Bernal-Maurandi J, Cofan F, Ventura P, Marcos MA, Linares L, et al. BK virus and cytomegalovirus coinfections in kidney transplantation and their impact on allograft loss. J Clin Med. 2021;10(17):3779.
Jabbari MR, Soleimanjahi H, Hajiabdolbaghi M, Sarraf-Shirazi M, Malekshahi SS. Cytomegalovirus end organ disease in Iranian HIV-1 infected patients with CD4 + cell counts less than 100 cells/mm3. IRCMJ. 2019;21(11):1–5.
RANGBAR KR, Sharifi Z, MAHMOODIAN SM, Mousavi K. Quantitative analysis of CMV-DNA load in renal transplant recipients using real-time PCR. Iran J Virol. 2011;5(1):28–30.
Fishman J. Infection in organ transplantation. Am J Transpl. 2017;17(4):856–79.
Shatizadeh Malekshahi S, Soleimanjahi H, Dorostkar F, Salimi V, Farahmand M. Survey of BK virus in renal transplant recipients in Iran: a systematic review and meta-analysis. Intervirology. 2021;64(1):27–35.
Alalawi F, Alnour H, Kossi ME, Jenkins JR, Halawa A. BK virus infection in adult renal transplant recipients; risk factors and their impact on allograft survival. Trends Transplantation. 2020;13(2).
Randhawa P, Ho A, Shapiro R, Vats A, Swalsky P, Finkelstein S, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42(3):1176–80.
Ashouri Saheli Z, Shenagari M, Harzandi N, Monfared A. Evaluation of prevalence of BKV and JCV DNAs in renal allograft recipients in Guilan Province using real-time PCR, during 2010–2016. J Ardabil Univ Med Sci. 2019;19(2):149–60.
Caliendo AM, St. George K, Kao S-Y, Allega J, Tan B-H, LaFontaine R, et al. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J Clin Microbiol. 2000;38(6):2122–7.
Jabbari MR, Soleimanjahi H, Shatizadeh Malekshahi S, Gholami M, Sadeghi L, Mohraz M. Frequency of cytomegalovirus viral load in Iranian human immunodeficiency virus-1-infected patients with CD4 + counts < 100 cells/mm3. Intervirology. 2021;64(3):135–9.
Toyoda M, Puliyanda DP, Amet N, Baden L, Cam V, Radha R, et al. Co-infection of polyomavirus-BK and cytomegalovirus in renal transplant recipients. Transplantation. 2005;80(2):198–205.
Rahbar M, Amiri M, Poormand G, Poortahmasebi V, Karkhaneh MM, Jazayeri A, Jazayeri SM. Simultaneous detection of opportunistic viral infections among renal transplant patients from Sina Hospital, Tehran. Future Virol. 2019;14(6):419–26.
Jehn U, Schütte-Nütgen K, Bautz J, Pavenstädt H, Suwelack B, Thölking G, Reuter S. Clinical features of BK-polyomavirus and cytomegalovirus co-infection after kidney transplantation. Sci Rep. 2020;10(1):22406.
Mohamed M, Parajuli S, Muth B, Astor B, Panzer S, Mandelbrot D, et al. In kidney transplant recipients with BK polyomavirus infection, early BK nephropathy, microvascular inflammation, and serum creatinine are risk factors for graft loss. Transpl Infect Dis. 2016;18(3):361–71.
Chukwu CA, Mahmood K, Elmakki S, Gorton J, Kalra PA, Poulikakos D, Middleton R. Evaluating the antibody response to SARS-COV-2 vaccination amongst kidney transplant recipients at a single nephrology centre. PLoS ONE. 2022;17(3):e0265130.
Abeywardana K, Rajamanthri R, Wazil A, Nanayakkara N, Muthugala M. Longitudinal viral kinetic study of BK virus in renal transplant patients-A single-center study in Sri Lanka. J Clin Virol Plus. 2022;2(4):100125.
Bischof N, Hirsch HH, Wehmeier C, Amico P, Dickenmann M, Hirt-Minkowski P, et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transpl. 2019;34(7):1240–50.
Torre-Cisneros J, Aguado J, Caston J, Almenar L, Alonso A, Cantisán S, et al. Management of cytomegalovirus infection in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transpl Rev (Orlando). 2016;30(3):119–43.
Neuwirt H, Rudnicki M, Schratzberger P, Pirklbauer M, Kronbichler A, Mayer G. Immunosuppression after renal transplantation. memo-Magazine Eur Med Oncol. 2019;12:216–21.
Reischig T, Kacer M, Hes O, Machova J, Nemcova J, Lysak D, et al. Cytomegalovirus prevention strategies and the risk of BK polyomavirus viremia and nephropathy. Am J Transpl. 2019;19(9):2457–67.
Fuhrmann S, Lachmann R, Streitz M, Hetzer R, Volk HD, Lehmkuhl H, Kern F. Cyclosporin A and tacrolimus reduce T-cell polyfunctionality but not interferon‐γ responses directed at cytomegalovirus. Immunology. 2012;136(4):408–13.
Tedesco-Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes-Freitas T, et al. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transpl. 2015;15(10):2655–64.
Páez-Vega A, Gutiérrez-Gutiérrez B, Agüera ML, Facundo C, Redondo-Pachón D, Suñer M, et al. Immunoguided discontinuation of prophylaxis for cytomegalovirus disease in kidney transplant recipients treated with antithymocyte globulin: a randomized clinical trial. Clin Infect Dis. 2022;74(5):757–65.
Romao EA, Yamamoto AY, Gaspar GG, Garcia TMP, Muglia VA, Nardin MEP et al. editors. Significant increase in cytomegalovirus (CMV) infection in solid organ transplants associated with increased use of thymoglobulin as induction therapy? Transpl Proc. 2023;55(9):2035–40.
Acknowledgements
The authors of the present study are grateful to Dr. Batoul Khoundabi (Iran Helal Institute of Applied-Science and Technology, Research Center for Health Management in Mass Gathering, Red Crescent Society of the Islamic Republic of Iran, Tehran, Iran) for her help with data analysis.
Funding
The results presented in this paper were part of a student thesis, which was supported by a grant number (no. Med- 417945) from the Research Deputy of Tarbiat Modares University, Faculty of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
SSM designed and supervised the work. MEK collected the samples and performed the laboratory tests. SSM & MEK wrote the manuscript. MRJ contributed in sample collection.
Corresponding author
Ethics declarations
Ethical approval and consent for publication
Ethical approval of the study was obtained from the medical ethics committee of Tarbiat Modares Univesity (IR.MODARES.REC.1402.059). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eslami Kojidi, M., Shatizadeh Malekshahi, S. & Jabbari, M. The simultaneous presence of active BK, Epstein Barr, and human cytomegalovirus infection and their correlation by host factors in patients suspected of kidney transplant rejection. BMC Infect Dis 24, 937 (2024). https://doi.org/10.1186/s12879-024-09821-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09821-z