Abstract
Background
To explore the overall prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae(S. pneumoniae) among healthy children.
Methods
A search for pneumococcal nasopharyngeal carriage studies including children published up to July 31th, 2016 was conducted to describe carriage in China. The review also describes antibiotic resistance in and serotypes of S. pneumoniae and assesses the impact of vaccination on carriage in this region. Summary measures for overall prevalence, antibiotic resistance, and serotype distributions extracted from the analyzed data were determined with 95% confidence intervals (CIs) using random-effects models. Heterogeneity was assessed using I 2 test statistics.
Results
Thirty-seven studies were included in this review, and the majority of studies (64.9%) were located in the pre-introduction period of 7-valent pneumococcal conjugate vaccine (PCV7) in China. The pooled prevalence of S. pneumoniae nasopharyngeal carriage was 21.4% (95% CI: 18.3–24.4%). Carriage was highest in children attending kindergartens [24.5%, (19.7–29.3%)] and decreased with increasing age. Before the introduction of PCV7 into China, the prevalence of S. pneumoniae nasopharyngeal carriage was 25.8% (20.7–30.9%), the pooled carriage of S. pneumoniae sharply dropped into the 14.1% (11.3–16.9%) by PCV7 vaccination period (P < 0.001). Before the pneumococcal conjugate vaccine (PCV) was introduced in China, the penicillin resistance rate in S. pneumoniae isolated from healthy children was 31.9% (21.2–42.6%); however, this rate sharply decreased after the introduction of PCV7 in China [21.6%, (7.4–35.9%)], and the difference between the rates during these two time periods was statistically significant (P value <0.05). Serotypes 19F, 6A and 23F were the most commonly isolated. Meta-analysis of data from young children showed a pooled rate estimate of 46.6% (38.8–54.4%) for PCV7 vaccine coverage and 66.2% (58.6–73.8%) for PCV13 vaccine coverage.
Conclusions
The prevalence of nasopharyngeal carriage among children was high in China. PCV7 immunization was found to be associated with reduction of nasopharyngeal colonization of S. pneumoniae. Conjugate vaccination coverage was slightly affected by the introduction of PCV7 into China because of low vaccination rate. The government should implement timely adjusted conjugate vaccination strategies based on our findings.
Similar content being viewed by others
Background
Streptococcus pneumoniae (S. pneumoniae) is a major pathogen that can cause invasive pneumococcal disease (IPD) and respiratory tract infections and result in high morbidity and mortality. The World Health Organization has reported that nearly 500,000 children under 5 years of age are infected by S. pneumoniae annually, and the vast majority of these infections occur in developing countries [1]. Asymptomatic nasopharyngeal carriage of S. pneumoniae is an essential element of the transmission of pneumococcal disease [2], a prerequisite for the occurrence of invasive pneumococcal disease, and a known risk factor for subsequent acute and recurrent otitis media [3, 4].
The prevalence of nasopharyngeal pneumococcal carriage has been found to vary in different countries and regions [5]. Because S. pneumoniae carriage is more common than the S. pneumoniae disease, it is important to investigate carriage status to evaluate the effect of new pneumococcal vaccines [6]. When the 7-valent pneumococcal vaccine was introduced in mainland China, the invasive pneumococcal disease burden decreased sharply, especially disease caused by the vaccine type (VT) serotypes; this decrease was accompanied by an increase in non-vaccine type (NVT) serotype, particularly serotype 19A, as previously seen in Europe [7, 8].
This systematic review was conducted to describe the nasopharyngeal carriage status of S. pneumoniae in healthy children, describe the major serotypes of S. pneumoniae, and evaluate the impact of pneumococcal vaccination on the coverage of PCV7.
Methods
Literature search
The following databases were searched for relevant articles through July 31, 2016 without language limitations: PubMed, Web of Science, EMBASE, CNKI, and WANFANG database. Keywords used for this search were: (“China” OR “Chinese”), (“nasal” OR “nasopharyngeal” OR “oropharyngeal”), (“children” OR “pediatric” OR “paediatric”), (“carriage” OR “colonization” OR “colonisation”) “Streptococcus pneumoniae”, “serotypes”, “pneumococcal vaccine”.
Inclusion and exclusion criteria
Studies were required to meet the following criteria for inclusion in this meta-analysis: (1) subjects were healthy children, (2) samples were collected from nasopharyngeal or oropharyngeal swabs, (3) studies focused on non-vaccination group and (4) sufficient information was provided to compute positive carriage rates and their 95% confidence intervals (CIs). Exclusion criteria were as follows: (1) if a study included both adults and children, only children data were enrolled, (2) studies reporting clinical infectious diseases caused by S. pneumoniae, (3) if studies included both vaccinated and non-vaccinated children, only non-vaccinated data were enrolled, (4)studies with a lack of sufficient baseline information to compute carriage rates and their 95CIs, (5) review studies, or conference studies or newspaper articles, (6) studies determining antibiotic resistance rates without carriage data, or studies were referred to infections rather than colonization, and (7) duplicate reports.
Data extraction
Two reviewers (LW and JF) independently identified and extracted the following data: first authors, sample year, study location, study population, number of participants, number of participants with pneumococcal carriage, pre/post vaccination period, vaccination history, type of swabs, immediately incubated into plates or not, transportation period, culture plates, culture into the 5% CO2 or not, identification methods, serotyping methods, storage medium, rates of antibiotic resistance, and prevalence of S. pneumoniae serotypes and their corresponding 95% CIs.
Quality assessment
The quality of included studies was assessed in accordance with the STROBE statement [9], studies with scores <8 were excluded from the systematic review.
Statistical analysis
STATA version 10.0 was used to perform the statistical analyses. DerSimonian and Laird random-effects models (REM) were used to pool the data. Funnel plots were used to examine publication bias, which was further assessed using Egger’s test, with P < 0.10 indicating potential bias [10]. Stratified analyses were carried out to assess the heterogeneity across subgroup defined by age and PCV7 vaccination period.
Results
Characteristic of included studies
The flow chart in Fig. 1 depicts the selection process for the included studies. Overall, 614 studies were written in Chinese, and 21 studies were written in English. By reviewing the titles and abstracts, 487 articles were excluded; by using the inclusion/exclusion criteria, 37 articles were selected for further investigation that included a total of 18,881 children. They were all cross-sectional studies. The main characteristics of the studies are listed in Table 1. The first study of nasopharyngeal carriage of S. pneumoniae in healthy children was conducted in two kindergartens in Beijing in 1999. All samples were from nasopharyngeal and nasal swabs. The ages of the healthy children included in the studies ranged from 0 to 14 years.
Nasopharyngeal carriage rates of S. pneumoniae in healthy children
A total of 37 studies including 19,120 healthy children reported nasopharyngeal carriage of S. pneumoniae. Among them, 4 children from Di [24] and 235 children from Gao [35] reported a vaccination history, were all excluded. Finally, only 3511 colonization were reported among 18881non-vaccination children, The lowest prevalence was reported by XH Cao [46], which was 2.6% (0.9–4.3%); the highest prevalence was reported by ZG Lai [38], which was 38.4% (33.2–43.5%). The pooled prevalence of nasopharyngeal carriage of S. pneumoniae in healthy children was 21.4% (18.3–24.4%) (Fig. 2).
Identification and confirmation of S. pneumoniae with different methods
Table 2 summarizes the methods used to identify and confirm the S. pneumoniae strains. Three different methods, including PCR, optochin disk with bile solubility and latex agglutination were used. There was no impact on the prevalence of S. pneumoniae when using three different identification methods, see Fig. 3.
Nasopharyngeal carriage of S. pneumoniae by age
Figure 4 summarizes the prevalence of nasopharyngeal carriage of S. pneumoniae in healthy children in different age groups. Six studies [11, 14, 17, 34, 39, 42] reported the prevalence of nasopharyngeal carriage of S. pneumoniae among children younger than 2 years of age. Among the 6656 healthy children in this age group, a total of 847 were identified to be positive for nasopharyngeal carriage of Streptococcus pneumoniae; thus, the pooled prevalence was 11.7% (9.1–14.2%). Twenty-seven studies [12,13,14,15,16, 18, 21,22,17,23,24,25,26,27,28,29,30,31,32,33, 36,37,38, 41, 44,45,46,47] including 10,480 kindergarten children (2–5 years of age) investigated the prevalence of nasopharyngeal carriage of S. pneumoniae. Within these studies, a total of 2437 children were identified to be positive for S. pneumoniae carriage, and the pooled prevalence was 24.5% (19.7–29.3%). Among the 1122 healthy children who were older than 5 years of age [15, 19, 37], 104 were identified as S. pneumoniae carriers; therefore, the prevalence of nasopharyngeal carriage was 8.8% (6.0–11.5%) in this age group. The prevalence of nasopharyngeal carriage of S. pneumoniae varied between the three age groups, with the highest rate reported in kindergarten children (P = 0.002).
PCV7 and S. pneumoniae nasopharyngeal carriage
The 7-valent pneumococcal conjugate vaccine was introduced to China in October 2008, but it has not yet been included in the Chinese Expanded Program on Immunizations (EPI) [48]. Unlike the vaccination in Chinsed EPI schedule, the PCV7 vaccine was not free to the public and the coverage was estimated as 9.91% [49].
Before the PCV7 was introduced in mainland China, 24 studies [12, 16, 18,19,20,21,22,23, 25,26,27,28,29,30,31, 33, 36, 40, 41, 43,44,45, 47] had reported the prevalence of nasopharyngeal carriage of S. pneumoniae; within these studies, the pooled prevalence was 25.8% (20.7–30.9%), Fig. 5. The prevalence of nasopharyngeal carriage sharply declined following the introduction of PCV7, with a pooled prevalence of 14.1% (11.3–16.9%) identified in studies conducted post-PCV7 introduction [11, 13,14,15, 17, 24, 32, 34, 35, 37, 39, 42, 46]. There was a highly significance differences in the prevalence between these two time periods (P < 0.001). In kindergarten children, before the pcv7 vaccination period, the pooled prevalence was 27.2% (21.3, 33.2%) and 16.6% (9.5, 23.7%) in the post vaccination period (P < 0.001).
Overall heterogeneity and publication bias
Stratified analyses were carried out to assess the heterogeneity across subgroups defined by age, PCV7 introduction period and PCV7 introduction period within kindergarten children groups. The sensitivity analysis indicated that the pooled prevalence of S. pneumoniae carriage had only slight variations by stratified studies into pre/post vaccination period when individual studies were omitted one by one. The prevalence estimates ranged from 13.4% (10.6, 16.1%) to 14.8% (12.5, 17.1%) in post vaccination period and from 25.2% (20.7, 31.1%) to 26.3% (21.1, 31.6%) in pre-vaccination period, suggesting that the results were stable.
Slight publication bias was noted from the statistical tests (Egger’s test, P = 0.011; Begg’s test, P = 0.01). After stratified the pooled prevalence of S. pneumoniae by PCV7 vaccination period, the potential publication bias was adjusted as no significant (Egger’s test, P = 0.134; Begg’s test, P = 0.602) in pre-vaccination period and (Egger’s test, P = 0.353; Begg’s test, P = 0.125) in post vaccination period.
Antibiotic resistance profiles of the isolates
A total of 20 studies [11, 12, 15, 16, 18, 20, 21, 23, 25,26,27,28, 31, 33, 34, 36, 37, 40,41,42] were identified that reported antibiotic resistance in S. pneumoniae. The rate of pneumococcal resistant to levofloxacin was 2.5% (0.3–4.6%), which was the lowest rate of antibiotic resistance identified. The highest resistant rate was reported against tetracycline antibiotics; for this class of antibiotics, a pooled resistance rate of 67.1% (33.8–96.4%) was identified. The pneumococcal resistance rate to penicillin was 28.9% (20.4–37.4%). Before the introduction of PCV7 [12, 16, 18, 20, 23, 25,26,27,28, 31, 33, 36, 40, 41], the pooled resistant rate to penicillin was 31.9% (21.2–42.6%). This rate decreased by 21.6% (7.4–35.9%) following the introduction of PCV7 [11, 15, 21, 34, 37, 42]. The penicillin resistant rate varied significantly between the pre- and post-PCV7 time periods (P < 0.001) (Table 3). The results of subgroup analysis indicated that the heterogeneity of resistant to penicillin may came from pre/post vaccination period, while the rest of them may came from different age groups. A slightly publication bias was found in Chloromycetin resistant rate, no publication bias was found in the rest of the antibiotics.
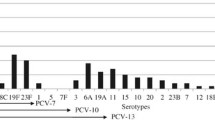
Serotypes and S. pneumoniae nasopharyngeal carriage
Nine studies [17, 18, 29, 30, 33, 35, 41, 43, 44] reported the serotypes of Streptococcus pneumoniae. In the 1626 isolates evaluated, 11 different serotypes were identified, and the predominant serotype was 19F. The pooled prevalence of serotype 19F was 19.1% (12.2–26.0%). The least prevalent serotype was 18C, which was identified in 3.2% (0.1–6.3%) of isolates (Fig. 6, Table 4). Of the 1626 isolates, 755 were identified as serotypes included in the coverage of PCV7, and 1059 were identified as serotypes included in the coverage of PCV13. The serotype coverage rates were 46.6% (38.8–54.4%) for PCV7 and 66.2% (58.6–73.8%) for PCV13. >Before PCV7 was introduced in mainland China [16, 27, 28, 31, 38, 41], the serotype coverage rates of PCV7 and PCV13 were 43.9% (34.1–53.6%) and 66.8% (56.1–76.0%), respectively. These rates changed to 52.1% (37.3–66.9%) and 66.3% (50.6–81.9%) for PCV7 and PCV13, respectively, following the introduction of PCV7 [17, 39, 42].
Heterogeneity was detected in the serotype distributions of 23F, 6A, 19F, 18C, 15, 19A and PCV7, PCV13 vaccine coverage rate (all P values were <0.05), although after sequential exclusion of each study, the conclusion was not affected by the exclusion of any specific study.
Discussion
This systematic review analyzed the prevalence and serotype distributions of nasopharyngeal carriage of S. pneumoniae, antibiotic resistant rates in S. pneumoniae, and the rates corresponding the serotype coverage provided by PCV7 and PCV13.
Since the serotypes distribution of and antibiotic resistance in S. pneumoniae isolates have been found to vary from region to region, the prevalence of S. pneumoniae has also been found to vary in different populations. The prevalence of nasopharyngeal carriage of S. pneumoniae was found to be 60% in infants under 2 years of age in Greenland [49], while the prevalence of nasal carriage was only identified as 9.8% in elderly populations in Italy [50]. In Hong Kong, the prevalence of nasopharyngeal carriage S. pneumoniae was identified as 13.5% in children younger than 5 years of age who had never received any pneumococcal vaccines, 14.1% in children who received at least one dose of PCV13, and 15.3% in children who received at least 3 doses of the PCV13 vaccine [51]. In Taiwan, the prevalence of nasopharyngeal carriage of S. pneumoniae identified in children younger than 5 years of age was 14.1%, similar to that identified Hong Kong [52]. However, data collected in mainland China have differed from data collected in Taiwan and Hong Kong. The pooled prevalence of nasopharyngeal carriage of S. pneumoniae was determined to be 21.4% (18.3–24.4%) among children in China.
A variety of studies have confirmed that colonization by S. pneumoniae begins in infanthood and early childhood. It has been reported that carriage of this pathogen is acquired within the first 6 months of life and, the prevalence of the epidemic appeared to peak in children of pre-school age [53]. A study conducted by Ueno M [53] showed that prevalence of nasopharyngeal carriage of S. pneumoniae increased with age within pediatric age groups, with rates of 19 and 23% identified in infants younger than 1 years-old and children 2 to 3 years old, respectively. The highest prevalence has been identified during the pre-school period. Our data were consistent with the findings of Ueno M [53], suggesting that carriage trends differed with age. The prevalence was 12.8% (10.0–15.6%) in children younger than 2 years old; the prevalence increased with age and reached a peak at 24.7% (19.7–29.7%) in children aged 2 to 5 years and then decreased to 8.8% (6.0–11.5%) in children aged 5 years and older. It is well known that attending kindergarten has been identified as a risk factor [52, 53] for colonization by opportunistic pathogens, such as S. pneumoniae, due to poor hygiene, confined physical environmental conditions and frequent interaction with other children. Nasopharyngeal carriage of S. pneumoniae in kindergarten children results in this population serving as an asymptomatic reservoir that spreads this pathogen into community. Since the PCV7 was introduced into China in October 2008, the studies conducted between 2009 to 2012 in age 2 to 5 years-old children were the coverage and the active population of getting shot by PCV7 vaccine, which leads to a reduction of prevalence of nasopharyngeal carriage of S. pneumoniae.
Unlike the GAVI Alliance [54] in the world and EPI in China, the PCV7 is available at immunization clinics for a fee during 2008–2015, these clinics designated as “point of vaccination” centers, children at 2, 4, 6 months will get shot of one dose of PCV7 and at 1 years old will get the fourth shot of does to enhance the immunity after purchase the vaccine [54]. Because of the high price of PCV7, the PCV7 coverage level was not as many other countries [8, 9]. According to a survey of children age 1 to 2 years selected from 31 provinces throughout China conducted in 2012, 9.9% of children had received one dose of PCV7 [49]. Another study from Shanghai reported a similar PCV7 coverage level at 11.4% [55]. We observed a slightly change of PCV7 coverage level from 43.9% (34.1, 53.6%) to 52.1% (37.3, 66.9%) between pre/post vaccination period because of the limited herd immunity from low vaccine rate of pneumococcal conjugate vaccination.
High antibiotic resistance rates in S. pneumoniae may facilitate transmission of this pathogen among young children. Crowding and barriers to maintaining quality hygiene facilities could accelerate the transmission of highly antibiotic resistant S. pneumoniae in the kindergarten environment [56]. Our pooled data indicated that the rates of erythromycin, clindamycin, trimethoprim- sulfamethoxazole and tetracycline resistance among isolates were all more than 60%. High-level resistance to the aforementioned antibiotics has also been identified in previous studies [57]. Macrolides and lincosamides have been reported to be the first-line empirical antibiotic therapy for pneumococcal infections in China, and the use of these agents has led to a high rate of antibiotic resistance in S. pneumoniae [42, 57]. Previous studies have demonstrated that the penicillin-non-susceptible pneumococci (PNSP) rate varied in different regions. The prevalence of nasopharyngeal carriage of S. pneumoniae in Brazilian and Korean children who attended day care centers were identified as 26.0 and 31.3%, respectively [58, 59]. A marked modification in pneumococcal antibiotic susceptibility rates was observed after the introduction of pneumococcal conjugate vaccines. The PNSP rate was 47.1% before the introduction of PCV13 in France, and this rate rapidly decreased to 39% 3 years after PCV13 was introduced [60]. The pooled data in this study were consistent with results identified in France. The proportion of pneumococcal isolates resistant to penicillin identified in this study decreased from 31.9% (21.2–42.6%) to 21.6% (7.4–35.9%) after the introduction of PCV7.
A remarkable decrease in the incidence and mortality of invasive pneumococcal disease has been observed following the introduction of pneumococcal conjugate vaccines into pediatric immunization programs [61]. With the introduction of these PCVs and further reductions in the prevalence of nasopharyngeal carriage of S. pneumoniae in pediatric groups. Our data demonstrated that the prevalence of nasopharyngeal carriage of S. pneumoniae was 25.8% (20.7–30.9%) among healthy children before the introduction of PCV7. The prevalence dropped sharply to 14.1% (11.3–16.9%) following the introduction of PCV7 in China, indicating that the impact of PCV7 introduction on disease prevalence can be determined by assessing the nasopharyngeal carriage of S. pneumoniae in healthy children.
Conclusions
Pneumococcal carriage was identified to occur at generally high prevalence among children in China. PCV7 immunization was associated with a reduction in the rate of penicillin resistance among nasopharyngeal carriage isolates of S. pneumoniae. The distribution of serotypes identified in the nasopharynx was only slightly modified following the introduction of the PCV7 vaccination because of the low PCV7 immunization rates. The Centers for Disease Control and Prevention should timely adjust PCV vaccination strategies based on these findings to reduce the incidence and morbidity of pneumococcal invasive disease in pediatric populations.
Abbreviations
- CIs:
-
Confidence intervals
- IPD:
-
Invasive pneumococcal disease
- NVT:
-
Non-vaccine type
- PCV:
-
Pneumococcal conjugate vaccine
- PNSP:
-
Penicillin-non-susceptible pneumococci
- REM:
-
Random-effects model
- S. pneumonia :
-
Streptococcus pneumonia
- VT:
-
Vaccine type
References
World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008.
Bogaert D, De Groot R, Hermans PWS. Pneumoniaecolonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54.
Syrjanen RK, Kilpi TM, Kaijalainen TH, Herva EE, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae in Finnish children younger than 2 years old. J Infect Dis. 2001;184:451–9.
HT V, Yoshida LM, Suzuki M, Nguyen HA, et al. Association between nasopharyngeal load of Streptococcus Pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–8.
Abdullahi O, Nyiro J, Lewa P, Slack M, et al. The descriptive epidemiology of Streptococcus Pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi District, Kenya. Pediatr Infect Dis J. 2008;27:59–64.
Rinta-Kokko H, Dagan R, Givon-Lavi N, Auranen K. Estimation of vaccine efficacy against acquisition of pneumococcal carriage. Vaccine. 2009;27:3831–7.
Hauser C, Kronenberg A, Allemann A, Mühlemann K, et al. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus Pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016;21(21):30239.
Horácio AN, Silva-Costa C, Diamantino-Miranda J, Lopes JP, et al. Portuguese Group for the Study of streptococcal infections. Population structure of Streptococcus Pneumoniae causing invasive disease in adults in Portugal before PCV13 availability for adults: 2008-2011. PLoS One. 2016;11(5):e0153602.
von Elm E, Altman DG, Egger M, Pocock SJ, et al. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Ping G, Liu Z, Wang Q, Li Q, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae, Haemophilus influenzae and Moraxella catarrhalis among healthy infants in Xuanwu district. J Modern Med Health. 2013;29(21):3245–6.
Liu Y, Sun Y. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy infants in Wuhan City. J Xiangnan University. 2006;8(1):19–22.
Kang Y, Zheng X, Zhang L. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy infants. Chronic Pathematol J. 2010;12(6):576.
Zhang L, Li H, Yuan D, Huang S, et al. Study on the population carriage of Streptococcus Pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in children at the age of 12-18 months in Dongguan city. Chin J Health Lab Technol. 2011;21(2):496–8.
Yang H, Jiang J, Li G, Jiang Y, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy population in Hubei. J Public Health Prev Med. 2014;25(3):20–3.
Yang F, Zhang Y, McGee L, Wasas A, et al. Nasopharyngeal carriage of 222 Streptococcus Pneumoniae and its characteristic among 791 healthy children in shanghai. National Med J China. 2001;81(10):589–92.
Liu Y, Zhang J, Zhao C, Zhang F, et al. The serotypes and their antibiotic resistance of common pathogens nasopharyngeal carried by healthy young children under 2 years old in China. J Appl Clin Pediatr. 2012;27(22):1723–7.
Li H, Cheng J, Wang Z, Wang S, et al. Detection and analysis of penicillin-resistant Streptococcus Pneumoniae among community healthy children. Chin J Epidemiol. 2002;23(5):409.
Zhao X, Zhao Z, Lin P, Li X, et al. A survey of throat flora among elementary healthy school children. Chin J Misdiagnostics. 2005;5(2):378–9.
Hou A, Liu Y, Xin D, Li J, et al. The clinical characteristics of common pathogens nasopharyngeal carried by healthy young children. Chin J Pediatr. 2002;40(1):45–9.
Liu J. The distribution of throat flora among healthy pre-school children. Chin J Public Health Eng. 2009;8(1):31.
Li F, Shao Y, Wang L, Wang G. Nasopharyngeal carriage of Streptococcus Pneumoniae among healthy children in Zhangjiakou City. J Hebei North University. 2005;22(4):54.
Liang J, Luo X, Gu Y, Gao S, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae among 186 healthy children in Zhongshan City. Guangdong J Health Epidemic Prev. 2003;29(5):39–40.
Di M, Huang H, Lv M, Zhang Y, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae among 221 1-12 years old healthy children in Beijing Dongcheng District. Disease Surveillance. 2012;27(8):595–8.
He Y, Liu Y, Zhang L, Lai Z, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy children in Dongguan City. Int J Clin Biochem Lab Sec. 2005;26(12):865–6.
Hua C, Zhao L, Song P, Xu S, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae among healthy pre-school children. Chin J Epidemiol. 2004;25(10):915–6.
Sangjie Y, Wang J, Li J, Li Y, et al. The antibiotic resistance and serotype distribution of Streptococcus Pneumoniae and the characteristic of occult strains resistant clones. Chin J Pediatr. 2000;38(7):424–7.
Sun Z, Zhang J, Li L, Zhu X, et al. The epidemiology of nasopharyngeal carriage of Streptococcus Pneumoniae under 5 years old healthy children. Chin J Pediatr. 2007;45(5):382–6.
Zhou H, Deng L, Ye Q, Chen S, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy children. Chin J Lab Med. 2002;25(1):52–3.
Zhang L, Liu Y, Lai Z, Zhu X, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae, Haemophilus influenzae and their antibiotic resistance among healthy pre-school children in Dongguan. China Med Industry. 2005;2(21):3–4.
Wang H, Chen M. Molecular epidemiology and antibiotic resistance of Streptococcus Pneumoniae in Beijing. National Med J China. 1999;79(4):253–6.
Chen H, Huang L, Huang R, Yuan M. Epidemiology and antibiotic resistance of Streptococcus pneumoniae among healthy population in Shenzhen, Guangdong. Disease Surveillance. 2010;25(5):351–3.
Zhang J, Sun Z. Nasopharyngeal carriage of Streptococcus Pneumoniae among healthy children. Chin J Infect Chemother. 2007;7(6):96–9.
Bai A, Zheng W, Yang Y, Liu Z, et al. Epidemiology and antibiotic resistance of Streptococcus Pneumoniae among 1-2 years old of healthy pediatric population in Jinan, 2010. Preventive Med Tribune. 2013;20(1):29–31.
Gao Z, Zhao X, Li C. Nasopharyngeal carriage of Streptococcus Pneumoniae and vaccine effectiveness of 7-valent pneumococcal conjugate among 2-5 years old healthy children in Huairou, Beijing. Capital J Public Health. 2014;8(1):13–7.
Wu B, Tang Y, Zhu J, Tan S, et al. Epidemiology of Streptococcus Pneumoniae among 2-6 years old of kindergarten children in Guangzhou. Chin J Contemp Pediatr. 2001;3(5):529–31.
Jiang L, Tan L, Hou Y, Jinjian F. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy children in Liuzhou. Modern Prev Med. 2015;42(22):4091–3.
Lai Z, Zhang L, Liu Y, Zhu X, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy children in Dongguan. Jiangxi J Med Lab Sci. 2006;24(1):27–8.
Zhao D, Hu Q, Xiong Y, Quan Y, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae and its serotype distributions among healthy children in Wuhan. Modern Prev Med. 2012;39(9):2166–8.
Zeng Y. Nasopharyngeal carriage of Streptococcus Pneumoniae and its antibiotic resistance among healthy children in Chongqing. J Practical Med Techniques. 2003;10(8):832–3.
Lee NY, Song JH, Kim S, Peck KR, et al. Carriage of antibiotic- resistant pneumococci among Asian children : a multinational surveillance by the Asian network for surveillance of resistant pathogens (ANSORP). Clin Infect Dis. 2001;32(10):1463–9.
Hu J, Sun X, Huang Z, Wagner AL, et al. Streptococcus Pneumoniae and Haemophilus influenzae type b carriage in Chinese children aged 12-18 months in shanghai, China: a cross-sectional study. BMC Infect Dis. 2016;16:149.
Luo X, Gu Y, Liang J, Gao S, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae among healthy people in Zhongshan. Chin J Microecol. 2002;14(4):223–4.
Liu Y, Zhang L, Lai Z, He Y. Nasopharyngeal carriage of Streptococcus Pneumoniae, Haemophilus influenzae and their antibiotic resistance among healthy children in Dongguan, Guangdong. Chin J Antibiotics. 2006;31(11):697–8.
Liu Y, Hou S. Distribution of throat flora among healthy preschool children. Chin J Misdiagnostics. 2007;7(1):204.
Cao X, Cunguo G. A survey of nasopharynx flora among healthy children in alpine region. Int J Lab Med. 2012;33(7):804–5.
Chen D, Wang D, Chen Y, Zhang X. Distribution of nasopharynx flora among healthy preschool children in Beijing. Chin J Pediatr. 1999;37(8):502.
Burkitt L. Pfizer to cease vaccine sales business in China. Wall Str J. 2015. Available: http://www.wsj.com/articles/pfizer-to-cease-vaccine-salesbusiness-in-china-1427965438; Accessed 9 October 2017
Zheng J, Cao L, Guo S, A K, Wang L, et al. Survey of the current situation of category 2 vaccines in Chinese children aged 1 to 2 years. Chin J Vaccines Immunization 2012; 18: 233–237.
Esposito S, Mari D, Bergamaschini L, Orenti A, et al. Pneumococcal colonization in older adults. Immun Ageing. 2016 Jan 12;13:2.
Ho PL, Chiu SS, Law PY, Chan EL, et al. Increase in the nasopharyngeal carriage of non-vaccine serogroup 15 Streptococcus Pneumoniae after introduction of children pneumococcal conjugate vaccination in Hong Kong. Diagn Microbiol Infect Dis. 2015 Feb;81(2):145–8.
Kuo CY, Hwang KP, Hsieh YC, Cheng CH, et al. Nasopharyngeal carriage of Streptococcus Pneumoniae in Taiwan before and after the introduction of a conjugate vaccine. Vaccine. 2011 Jul 18;29(32):5171–7.
Ueno M, Ishii Y, Tateda K, Anahara Y, et al. Prevalence and risk factors of nasopharyngeal carriage of Streptococcus Pneumoniae in healthy children in Japan. Jpn J Infect Dis. 2013;66(1):22–5.
Yu H, Yang W, Varma JK. To save children’s lives, china should adopt an initiative to speed introduction of pneumonia vaccines. Health Aff. 2012;31(11):2545–53.
Boulton ML, Ravi NS, Sun X, Huang Z, et al. Trends in childhood pneumococcal vaccine coverage in shanghai, China, 2005-2011: a retrospective cohort study. BMC Public Health. 2016;16:109.
Lee EK, Jun JK, Choi UY, Kwon HJ, et al. Nasopharyngeal carriage rate and serotypes of Streptococcus Pneumoniae and antimicrobial susceptibility in healthy Korean children younger than 5 years old: focus on influence of pneumococcal conjugate vaccination. Infect Chemother. 2013 Mar;45(1):76–84.
Yao KH, Yang YH. Streptococcus Pneumoniae diseases in Chinese children. Past, present and future. Vaccine. 2008;26:4425–33.
Poulakou G, Katsarolis I, Matthaiopoulou I, Tsiodras S, et al. Nationwide surveillance of Streptococcus Pneumoniae in Greece: patterns of resistance and serotype epidemiology. Int J Antimicrob Agents. 2007;30(1):87–92.
Falup-Pecurariu O, Bleotu L, Zavarache C, Peled N, et al. Streptococcus Pneumoniae nasopharyngeal colonization in children in Brasov, Central Romania: high antibiotic resistance and coverage by conjugate vaccines. Pediatr Infect Dis J. 2011;30(1):76–8.
Angoulvant F, Cohen R, Doit C, Elbez A, et al. Trends in antibiotic resistance of Streptococcus Pneumoniae and Haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2015;15:236.
Myint TT, Madhava H, Balmer P, Christopoulou D, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Adv Ther. 2013;30(2):127–51.
Acknowledgements
Not applicable.
Funding
This manuscript was funded by Guangxi Medical and Health Self-funding Project (No Z2014379) and Liuzhou Science and Technology Bureau Project (No 2014 J030422). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
We declare that the data supporting the conclusions of this article are fully described within the article, and provided as Additional file 1.
Author information
Authors and Affiliations
Contributions
LW and ZL designed the study and drafted an outline. LW and JF participated in data analysis, JF draft of initial manuscript, JC revised the manuscript and all of authors approved the final content off this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Liuzhou Maternity and Child Healthcare Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Data base. (XLSX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, L., Fu, J., Liang, Z. et al. Prevalence and serotype distribution of nasopharyngeal carriage of Streptococcus pneumoniae in China: a meta-analysis. BMC Infect Dis 17, 765 (2017). https://doi.org/10.1186/s12879-017-2816-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-017-2816-8