Abstract
Background
There is lack of evidence regarding safety, effectiveness and applicability of prehabilitation on cardiac surgery population, particularly in patients candidates to cardiac valve replacement. The aim of the study is to assess and compare the effect of a multimodal prehabilitation program on functional capacity in patients with severe aortic stenosis (AoS) and severe mitral regurgitation (MR) proposed for valve replacement surgery.
Methods
Secondary analysis from a randomised controlled trial whose main objective was to analyze the efficacy of a 4–6 weeks multimodal prehabilitation program in cardiac surgery on reducing postoperative complications. For this secondary analysis, only candidates for valve replacement surgery were selected. The primary outcome was the change in endurance time (ET) from baseline to preoperative assessment measured by a cycling constant work-rate cardiopulmonary exercise test.
Results
68 patients were included in this secondary analysis, 34 (20 AoS and 14 MR) were allocated to the prehabilitation group and 34 (20 AoS and 14 MR) to control group. At baseline, patients with AoS had better left systolic ventricular function and lower prevalence of atrial fibrillation compared to MR (p = 0.022 and p = 0.035 respectively). After prehabilitation program, patients with MR showed greater improvement in ET than AoS patients (101% vs. 66% increase from baseline). No adverse events related to the prehabilitation program were observed.
Conclusions
A 4–6 week exercise training program is safe and overall improves functional capacity in patients with severe AoS and MR. However, exercise response is different according to the cardiac valve type disfunction, and further studies are needed to know the factors that predispose some patients to have better training response.
Trial registration
The study has been registered on the Registry of National Institutes of Health ClinicalTrials.gov (NCT03466606) (05/03/2018).
Similar content being viewed by others
Background
Exercise training plays a key role to improve functional capacity in patients with heart disease after an event such as acute coronary syndrome, stent placement or cardiac surgery (rehabilitation), or as a preparatory intervention before major surgery (prehabilitation) [1, 2]. Previous data on ischemic patients suggest that prehabilitation improves physical fitness [3], but little is known of the specific response to exercise training in patients with severe valve disease. Physiopathology, hemodynamic repercussion, and cardiac remodeling of the two most frequent valve disease types in adults, aortic stenosis (AoS) and mitral regurgitation (MR), are known to behave differently from other cardiac dysfunctions. As a result, response to increased hemodynamic load and catecholamine release caused by endurance training may cause significant distinct effects in those specific populations. Fear of worsening not only symptoms and pulmonary hypertension but also the possible adverse cardiac effects including myocardial ischemia and arrhythmias may have prevented patients with moderate to severe valve disease from the benefit of an exercise training program. Moreover, no consensus exists on the effectiveness, exercise intensity, and duration of a prehabilitation program in patients with severe valvular disease and its potential benefit before and after valve replacement. Many factors may contribute to exercise response variability. Hence, the identification and selection of patients who may strongly benefit from intervention can help to improve efficiency, sustainability, and adherence to this preventive intervention.
Therefore, our objective is to assess and compare the effect of a 4–6 week multimodal prehabilitation program including high-intensity interval training, on functional capacity in patients with severe AoS and severe MR proposed for valve surgery.
Materials and methods
Design and population
The study is a secondary analysis from a single-centre, randomised, open-label, controlled trial to determine if a multimodal prehabilitation program (including supervised high-intensity endurance and resistance training, psychological and nutritional support) reduces the rate of postoperative complications after elective cardiac surgery compared to standard care (control group). Characteristics of the study design and exclusion criteria have been reported previously in the study protocol [4]. Patients scheduled for coronary artery bypass grafting (CABG) and/or valve surgery, with an expected waiting time before surgery of 6 weeks or more, were invited to participate. The trial was carried out accomplishing all ethical standards (Hospital Clinic of Barcelona Ethics Committee approval-HCB/2017/0708).
For this secondary analysis, we restricted to the subset of candidates to valve replacement surgery due to severe AoS or severe MR, defined according to the latest guidelines [5]. To analyze the response to exercise training, we only included the individuals who completed the program. All participants underwent a baseline medical and functional assessment: (i) clinical history and physical examination including the American Society of Anesthesiologists status, Charlson Comorbidity Index [6] and European System for Cardiac Operation Risk Evaluation (Euroscore II) [7]; (ii) functional capacity evaluation by an incremental cardiopulmonary exercise test (CPET) and endurance time (ET) measured by a cycling constant work-rate exercise test performed at a load equivalent of 80% of the peak workload (PWR) the patient could tolerate on the incremental CPET [8] (Ergoline 900, Ergoline, Bitz, and Ergocard Professional, Medisoft), hand grip strength test (Jamar Hydraulic Hand Dynamometer; Sammons Preston), 6-minute walk test (6MWT) [9], 30-second Sit To Stand test (STS) [10], and estimation of functional capacity by the Duke Activity Status Index (DASI) [11] questionnaire; and (iii) assessment of physical activity level, measured by the Yale Physical Activity Survey (YPAS) [12]. Patients were reassessed after the program using the same tests, one week before undergoing cardiac surgery (pre-surgery assessment). During the exercise training program, the attendance to the sessions, the progression of the training, and the occurrence of adverse events were recorded.
The primary outcome was the change in ET after completing the prehabilitation program. The magnitude of change in the ET (from baseline to pre-surgery assessments) was compared between groups (severe AoS vs. severe MR patients) and between intervention vs. control group. Secondary outcomes were the effects on functional capacity assessed by a submaximal exercise test (6MWT), hand grip strength test and STS; and differences in physical activity level, measured by the YPAS.
Intervention
Participants in the intervention group underwent a 4–6 week personalized multimodal prehabilitation program in addition to standard care. The main components of the program consisted in:
-
a)
Supervised high-intensity interval training program: 1 h per session, 2 sessions per week at the hospital outpatient gym. It consisted of at least 5 sets combining 2 min. of moderate to high-intensity exercise (starting at 70% of PWR and progressing to 90-100% of PWR throughout the program) interspersed with 2 min. of active recovery periods at a lower intensity (40% of PWR), performed on a stationary bike. Likewise, they performed a resistance strength training of 2–3 upper and lower limb exercises based on 2–3 sets of 8–15 repetitions, avoiding Valsalva maneuvers. During the training session, patients were continuously monitored with a 2 electrode non-invasive wearable electrocardiogram (ECG) system (Nuubo ECG). Side effects attributed to the exercise training were registered: hypotension, arrhythmia, chest pain, cardiac arrest, syncope, angina and/or myocardial infarction.
-
b)
Promotion of physical activity and healthy lifestyle: as described elsewhere [4].
-
c)
Incentive spirometer exercising. Patients were instructed in daily breathing exercises (chest expansions, diaphragmatic breaths and deep inspirations) with a 4000mL volumetric incentive spirometer (Airlife, Vyaire Medical Inc), to perform at least twice a day, 1–2 sets of 10–15 breaths for each exercise.
-
d)
Nutrition counselling on a well-balanced cardioprotective Mediterranean diet and protein supplementation for an intake of 1.2–1.5 g/kg/day.
-
e)
Weekly mindfulness sessions. Participants were invited to attend 1 hourly session per week of Mindfulness-Based Stress Reduction led by a registered psychologist.
Participants in the control group followed the standard preoperative protocol that included physical activity recommendations, nutritional and smoking cessation advice. Moreover, patients suffering from iron-deficiency anemia received intravenous iron infusion.
The detailed characteristics of the intervention have been previously reported in the study protocol [4].
Statistical analysis
Accepting an alpha risk of 0.05 in a two-tailed test with 34 in the prehabilitation group and 34 in the control group, the statistical power was 100% to recognize as statistically significant a difference of means of 250 s in the endurance time [13].
Data were presented as mean and standard deviation or absolute frequency. Group means were compared using Student’s t-test, Mann-Whitney or ANOVA tests as appropriate. All analyses were performed using STATA 14.0 (Stata Comp, College Station, Tx, USA).
Results
The inclusion period of the randomised controlled trial (RCT) spanned from March 2018 to June 2021. The results from the primary outcome of the clinical trial were submitted for publication.
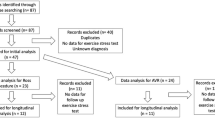
From a total of 160 patients included in the RCT, we selected 68 patients with severe valve disease for this secondary analysis. Among them, 20 and 14 patients with severe AoS and MR, respectively, were allocated to the prehabilitation group. On the other hand, 20 and 14 patients with severe AoS and MR, respectively, were allocated to the control group.
Baseline clinical characteristics and demographics of patients included in the prehabilitation group are summarized in Table 1. Patients with AoS had better left systolic ventricular function and a lower prevalence of atrial fibrillation compared to MR group. Although non-statistically significant, patients with AoS showed better related physical function parameters (i.e., DASI, VO2peak, ET, and hand-grip) and were less sedentary (YPAS) compared to MR patients.
Comparing prehabilitation vs. control groups, there were no differences at the time of inclusion (Table 1S-Supplementary Material).
The mean adherence to prehabilitation program was over 80% (i.e., percentage of completion of the scheduled training sessions) and mean duration of the prehabilitation program was 7 weeks. The impact of the prehabilitation program on functional capacity was measured for patients with severe AoS (Table 2) and severe MR (Table 3). After completing the prehabilitation program, the functional capacity in both patients with severe AoS and severe MR was improved, showing a significant increase in ET (Tables 2 and 3, respectively). Overall, patients with MR showed greater improvement in ET than AoS patients (101% vs. 66% increase from baseline).
ET individual changes for patients reassessed prior to surgery are shown in Fig. 1. 90% of MR patients showed a positive response to exercise (established as > 30% ET increase), while in AoS group, 62% showed the mentioned increase.
Patients with severe MR involved in prehabilitation also showed significant improvement in other functional parameters such as 6MWT, hand grip, or STS-test (Table 3).
Regarding patients allocated to control group, those with severe AoS remained functionally unchanged during surgery waiting-list time, whereas not prehabilitated patients with severe MR, showed a trend to functional decline during this time (ET 21% decrease from baseline) (Fig. 1).
No patients had any adverse events or complications attributable to the physical training, neither during the sessions nor during the program period.
Discussion
The results from this exploratory study support that a supervised high-intensity exercise training program as part of multimodal prehabilitation overall enhances preoperative functional capacity. Likewise, this intervention seems feasible and safe in patients with severe valve disease candidates to valve replacement. Prehabilitation may even prevent the significant functional deterioration that occurred in these patients while being in the waiting list before surgery, especially in MR patients. However, the effect on aerobic capacity appears to be heterogeneous among these valve diseases. Patients with severe MR seem to benefit more from intervention in terms of functional capacity, patients with AoS showed more modest benefit but patients in the control group presented a deterioration in hand grip.
Since cardiorespiratory fitness response to exercise presumably determines the benefit of prehabilitation programs on the postoperative outcomes, identifying patients that will response to exercise is crucial to be efficient. ET has gained popularity over the years in evaluating the effect of exercise interventions in different populations because of its higher sensitivity in comparison to other tests [14]. Although there is no consensus, a 20 to 30% increase in ET is widely accepted in other populations to define a positive response to an exercise program [15]. We assumed the ET to be a valid variable to assess response to exercise training and in our study, the proportion of patients fulfilling this score was differential between severe MR and AoS patients (92 vs. 65%). We could see a less intense response in 6-WMT and in fact, patients with AoS in control group presented a significant increase after the intervention, one possible explanation would be learning or hawthorne effect.
Many factors may contribute to this functional capacity response variability. Some are related to unmodifiable factors, such as age, genetics, subjacent comorbidities and baseline fitness; while others to modifiable factors, such as training characteristics.
The profound impact of the valve lesion on cardiac performance and the severity of the valve lesion, which are key points when deciding for surgical intervention, might also affect the exercise response. Different reactions to exercise training in MR may be related to specific responses to catecholamine release and hemodynamic profile during exercise. In fact, patients with MR presented a worse ejection fraction than those with AoS, even when ejection fraction may be a poor marker of left ventricular (LV) function in MR and may underestimate the extent of LV systolic dysfunction. Because of adrenergic release, tachycardia may be beneficial in the acute setting of exercise, thanks to a decrease in diastole time and regurgitation volume. Volume overload seen in MR can be handled in a ventricle with preserved systolic function, and response to exercise may enhance forward flow without significant pulmonary congestion. Patients with severe MR and those with end-stage heart failure, specifically those with severely depressed LV systolic function may share some characteristics, such as volume overload and pulmonary edema, and possibly offer a good substrate for improvement with endurance training. Patients with severe AoS traditionally present with preserved LV systolic function but suffer from diastolic dysfunction due to hypertrophy and non-compliant chambers. Ventricular stiffness and decreased compliance may harden ventricular filling and stroke volume increase needed during exercise whereas tachycardia decreases diastole and further impairs ventricular filling. In our study, exercise training in patients with AoS modestly improved ET and prevented from deterioration.
It is also worth mentioning that greater improvements in functional capacity have been previously reported after prehabilitation programs in less fit patients compared to patients with higher baseline fitness [16]. Thus, the higher benefit observed in MR patients after prehabilitation may be partially explained by their significant trend to lower baseline fitness compared to AoS patients.
The modality, intensity, and volume of exercise, as well as the specific training program all influence the individual training response. There is little evidence regarding the dose and specific exercise training characteristics of the prehabilitation intervention, especially in cardiac surgery patients. To our knowledge, no other training programs such as continuous exercise or moderate-intensity interval training have been tested and it is possible that one size does not fit all these particular patients. The effects of a given dose of exercise training on cardiorespiratory fitness response are not uniform at the individual level, while some individuals gain large improvements, others show small or even no improvement. Indeed, studies specifically designed to assess the individual variation in exercise response demonstrate substantial variability in functional capacity response [17, 18].
The evidence in this specific population (i.e., patients with severe MR and AoS who fulfilled criteria for valve replacement surgery) is scarce likely because recommendations for exercise training in people with severe valve disease preclude them from extenuating exercise, due to the risk of complications and even previously reported sudden death. This applies specifically to patients with severe AoS and those patients with severe MR and pulmonary hypertension, ventricular dilatation, or systolic dysfunction. Traditionally and based on expert opinions, low-intensity aerobic exercise is usually recommended in patients with valve disease to improve functional capacity and general well-being [19]. A very recent randomised controlled trial in elective cardiac surgery including about 50% of patients undergoing valve replacement, revealed that adverse event rates during prehabilitation were higher than in the standard care group [20]. However, the authors recognized that only a minority were related to the intervention. Moreover, they did not state if any monitoring during the exercise was performed. On the contrary, our setting included a supervised and indoor monitored exercise training as a part of the prehabilitation program. Intensity of highly demanding exercise in athletes and highly active individuals is radically different from exercise training during prehabilitation [21]. Exercise training in prehabilitation should not be considered as the bottom line, as it aims to improve functional capacity, to avoid sarcopenia and has a pivotal role to improve frailty in surgical patients in a short period of time. In our study, all patients safely concluded the program with no adverse effects associated to the intervention.
This study has some limitations that we must address. First, it consists of a secondary analysis derived from a randomised controlled trial whose main objective was to analyze the feasibility and efficacy of a multimodal prehabilitation program in cardiac surgery in terms of reduction of postoperative complications. Therefore, the sample size used might not be adequate for the performed analysis and our findings should be cautiously considered as exploratory. Even so, the sample size is adequate to assess the response to exercise in patients with severe valvular heart disease. Further studies with wider samples are needed in order to confirm these findings. In addition, some data at the pre-surgery assessment were missing as a result of COVID-19 pandemic restrictions. Second, CPET with incremental load was only performed at baseline to plan the exercise program. After intervention, change in ET was used to assess functional capacity before surgery. ET correctly reflects functional capacity, but more data could have been obtained from maximal oxygen consumption (VO2) and anaerobic threshold. Moreover, ET has been validated to assess exercise response but more studies are needed to assess response to prehabilitation. Third, there is some evidence that cardiac remodeling can be obtained after short interventions [22]. Unfortunately, we didn’t perform any measurement of cardiac geometry or function immediately before and after the intervention and we can’t correlate improvement of endurance time with cardiac remodeling. Fourth, multimodal prehabilitation is a more complex intervention, including in our case other components like nutritional and psychological support that could have played a role in the positive results. Finally, only the preoperative period is analyzed but we have no long-term data. It would be desirable to know long-term adherence to life-style changes and its implication in postoperative functional status.
Conclusions
In summary, patients with severe AoS or MR seem to benefit from a preoperative exercise training program of at least 4–6 weeks with no complications associated. However, exercise response may be different depending on the type of valve dysfunction, and patients may benefit differently according to their baseline fitness, comorbidities, and cardiovascular reserve. This complex multifactorial nature of functional capacity response variability poses a significant challenge to our understanding. Further studies are needed to know the factors that predispose some patients to have a better training response in order to design a tailored approach to every patient and better use of resources in clinical practice.
Data availability
Data is provided within the manuscript or supplementary information files and are also available from the corresponding author on reasonable request.
Abbreviations
- AoS:
-
Aortic stenosis
- CABG:
-
Coronary artery bypass grafting
- CPET:
-
Cardiopulmonary exercise test
- DASI:
-
Duke Activity Status Index
- ECG:
-
Electrocardiogram
- ET:
-
Endurance time
- LV:
-
Left ventricular
- MR:
-
Mitral regurgitation
- PWR:
-
Peak workload
- 6MWT:
-
6-minute walk test
- STS:
-
Sit to Stand test
- YPAS:
-
Yale Physical Activity Survey
- VO2 :
-
Oxygen consumption
References
McCann M, Stamp N, Ngui A, Litton E. Cardiac Prehabilitation. J Cardiothorac Vasc Anesth. 2019;33(8):2255–65.
Drudi LM, Tat J, Ades M, et al. Preoperative Exercise Rehabilitation in Cardiac and vascular interventions. J Surg Res. 2019;237(514):3–11.
Sawatzky JAV, Kehler DS, Ready AE, et al. Prehabilitation program for elective coronary artery bypass graft surgery patients: a pilot randomized controlled study. Clin Rehabil. 2014;28(7):648–57.
Coca-Martinez M, Lopez-Hernandez A, Montane-Muntane M et al. Multimodal prehabilitation as strategy for reduction of postoperative complications after cardiac surgery: a randomised controlled trial protocol. BMJ Open. 2020;10(12).
Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–44.
Levett DZH, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth. 2018;120(3):484–500.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of Lower Body Strength in community-residing older adults. Res Q Exerc Sport. 1999;70:2:113–9.
Struthers R, Erasmus P, Holmes K, Warman P, Collingwood A, Sneyd JR. Assessing fitness for surgery: a comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br J Anaesth. 2008;101(6):774–80.
Donaire-Gonzalez D, Gimeno-Santos E, Serra I, et al. Validation of the Yale Physical Activity Survey in chronic obstructive pulmonary disease patients. Arch Bronconeumol. 2011;47(11):552–60.
Van der Molen MC, Slebos DJ, Augustijn SWS, Kerstjens HAM, Hartman JE. The minimal important difference of the constant work rate cycle test in severe COPD. Respir Med 2023; 215.
Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–60.
Puente-Maestu L, Villar F, de Miguel J, et al. Clinical relevance of constant power exercise duration changes in COPD. Eur Respir J. 2009;34(2):340–5.
Minnella EM, Awasthi R, Gillis C, et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery. 2016;160(4):1070–9.
Ross R, de Lannoy L, Stotz PJ. Separate Effects of Intensity and Amount of Exercise on Interindividual Cardiorespiratory Fitness Response. Mayo Clin Proc. 2015; 90(11):1506-14.
Pandey A, Swift DL, McGuire DK, et al. Metabolic effects of Exercise Training among Fitness-nonresponsive patients with type 2 diabetes: the HART-D Study. Diabetes Care. 2015;38(8):1494–501.
Chatrath N, Papadakis M. Physical activity and exercise recommendations for patients with valvular heart disease. Heart. 2022;108(24):1938–44.
Akowuah EF, Wagnild JM, Bardgett M et al. A randomised controlled trial of prehabilitation in patients undergoing elective cardiac surgery. Anaesthesia. 2023 Jul 4. Epub ahead of print.
Bonow RO, Nishimura RA, Thompson PD, Udelson JE. Eligibility and disqualification recommendations for competitive athletes with Cardiovascular abnormalities: Task Force 5: Valvular Heart Disease: A Scientific Statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e292–7.
Eser P, Trachsel LD, Marcin T, Herzig D, Freiburghaus I, De Marchi S, et al. Short- and Long-Term effects of high-intensity interval training vs. moderate-intensity continuous training on left ventricular remodeling in patients early after ST-Segment Elevation myocardial infarction-the HIIT-EARLY randomized controlled trial. Front Cardiovasc Med. 2022;9:869501–869501.
Acknowledgements
We thank the patients participating in this trial. We also thank the high-quality work carried out by the Hospital Clinic de Barcelona Prehabilitation Group.
Funding
Grant from the Spanish Public Government, Fondos de Investigación en Salud (FIS) Instituto Carlos III (PI17/00852).
Author information
Authors and Affiliations
Contributions
ALH, EGS, RNR, MJA, GMP contributed to trial design and conception.MJA, GMP co-lead investigators, obtained ethics approval.ALH, EGS, RNR, GMP contributed to manuscript conception and drafting.ALH, EGS, RNR, MJA, MLB, MSG, GMP contributed to manuscript revision and edition.All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of Clinical investigation of Hospital Clinic de Barcelona (HCB/2017/0708) and all participants have signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
López-Hernández, A., Gimeno-Santos, E., Navarro-Ripoll, R. et al. Differential response to preoperative exercise training in patients candidates to cardiac valve replacement. BMC Anesthesiol 24, 280 (2024). https://doi.org/10.1186/s12871-024-02671-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-024-02671-x