Abstract
Background
Iron is essential for the growth and development of trace elements in plants, and iron deficiency can lead to leaf chlorosis. Ammonium and nitrate are the major forms of nitrogen present in soils. Ammonium nitrate alleviates the chlorosis of leaves caused by iron deficiency, but the mechanism is not clear in pear.
Results
Ammonium nitrate induced the increase of nitric oxide (NO) under iron deficiency. We further analyzed the effect of NO by exogenous NO treatment. The results showed that ammonium nitrate and NO increased the activity of ferric chelate reductase. NO induced the expression of multiple IRT genes and promoted the transmembrane transport of irons. Ammonium nitrate and NO promoted the activity of nitrogen assimilation-related enzymes and the nitrogen absorption capacity, and they also increased glutamine synthetase activity. Finally, ammonium nitrate and NO increased chlorophyll synthesis, with subsequent increase in the photosynthetic capacity of plants and accumulation of biomass.
Conclusion
Ammonium nitrate indirectly alleviates the symptoms of plant yellowing by promoting the increase of NO, which increases the response of iron transporters. Both substances increase the nitrogen accumulation in plants. This study demonstrates a new option for minimizing Fe deficiency by regulating the balance between nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Iron (Fe) is an essential micronutrient and a universal cofactor that plays a vital role in photosynthesis, respiration, hormone biosynthesis, morphogenetic cellular enzymatic reactions, and the growth and developmental processes of plants [1,2,3]. Although iron is the element with the fourth-highest abundance in the Earth’s crust, most of it exists in the form of hydroxides with low bioactivity. Fe deficiency is a worldwide horticultural problem, and approximately 30% of the world’s soil is currently Fe deficient, which may trigger chlorosis and reduce fruit yields [4].
Plants utilize two mechanisms to increase Fe uptake capacity to cope with Fe deficiency: strategy I (used by dicots and nongraminaceous monocot plants) and strategy II (used by graminaceous monocots) [5, 6]. Pear, as a strategy I plant, reduces Fe3+ to Fe2+ by ferric chelate reductase (FCR) in the root surface, and Fe2+ then enters the cytoplasm in the root [7]. Before entering the xylem, Fe2+ in the cytoplasm is reoxidized to Fe3+ and then combined with citric acid and transported aboveground in the xylem, and finally, Fe3+ is reduced to Fe2+ for assimilation and utilization [8]. Thus, FCR is one of the key enzymes for iron absorption and recycling in plants [9].
Studies have shown that iron deficiency induces various reactions at the root level to increase the uptake of available iron in the rhizosphere. Plants can alleviate iron deficiency stress by increasing iron storage, mainly through three ways: (I) improving the iron reduction ability of root tissue, (II) acidifying the rhizosphere to increase iron solubility, and (III) improving the absorption capacity of root cells to cope with iron deficiency in soil. Ammonium nitrate significantly affected the pH of plant rhizosphere, and then affected the absorption and utilization of iron. Under iron deficiency, the addition of NH4+ increased the reuse of iron in primary leaves. NH4+ treatment affected the transfer of iron from primary leaves and stems to young leaves, and significantly increased the iron level in young leaves of maize [10]. In addition, the iron content of spinach shoots significantly increased under the culture of nutrient solution containing NH4+ [11], further confirming that different forms of nitrogen affect Fe uptake. Fe is also a metal cofactor of enzymes in the nitrogen reductive assimilatory pathway, and iron absorption affects nitrogen assimilation [12]. Nitrate reductase (NR), nitrite reductase (NiR), and glutamate synthase (GOGAT) all require Fe as the Fe-heme group or Fe-S cluster [13].
Nitric oxide (NO) is a signaling molecule that is involved in a variety of physiological processes in response to Fe deficiency [14,15,16,17,18,19]. Sodium nitroprusside (SNP; NO donor) decreased the pH of soil and increased the available Fe in the soil [20]. In addition, the application of exogenous SNP significantly increased the total iron concentration in leaves by increasing the H+-ATPase and Fe3+ reductase activities [21]. SNP can also prevent iron deficiency induced oxidative stress, promote iron activation, and regulate the balance of mineral elements [22, 23].
Higher plants produce NO from various enzymatic and non-enzymatic sources [24,25,26,27]. Although much progress has been made in the production of NO, there are still many problems to be solved [28]. As a key enzyme in the nitrogen cycle, NR also participates in the biosynthesis of NO [29]. At present, studies have shown that ammonium regulates Fe deficiency responses by enhancing NO signaling in Arabidopsis thaliana [30]. However, how ammonium nitrate and NO alleviate the symptoms in pear caused by iron deficiency has not yet been reported.
In this work, we aimed to analyze the role of ammonium nitrate and NO in alleviating iron deficiency in pear. Through the exogenous application of ammonium nitrate and SNP, combined with physiological and biochemical data, the possible regulatory relationship between them was explained. The regulatory mechanisms behind this relationship were also investigated, which elucidated the interaction between ammonium nitrate, NO, and iron deficiency in pear.
Methods
Plant material and growth conditions
Pear variety ‘Qingzhen D1’ (P. communis L. × P. bretschneideri Rehd.) was independently bred at the college of horticulture, Qingdao agricultural university. In vitro shoots of ‘Qingzhen D1’ pear were proliferated on rooting medium that consisted of 1/2 Murashige and Skoog (MS) medium supplemented with 0.2 mg L− 1 naphthalene acetic acid (NAA), 1.5 mg L− 1 3-indolebutyric acid (IBA), 3% (w/v) sucrose, and 0.7% (w/v) agar. After 20 days, seedlings with the same growth and development were selected and subjected to the following treatments for 60 days: +Fe (100 μmol L− 1 Fe(III)-EDTA), −Fe (1 μmol L− 1 Fe(III)-EDTA), −Fe + AN (1 μmol L− 1 Fe(III)-EDTA+ 6.85 mmol L− 1 NH4NO3; the application amount of NH4NO3 was 0.5 times the amount of total nitrogen in NN69 medium), and -Fe + SNP (1 μmol L− 1 Fe(III)-EDTA+ 100 μmol L− 1 SNP). The NO-elimination experimental treatments were applied for 20 days and consisted of: -Fe (1 μmol L− 1 Fe(III)-EDTA), −Fe + cPTIO (1 μmol L− 1 Fe(III)-EDTA+ 100 μmol L− 1 2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide (cPTIO; NO scavenger), −Fe + AN (1 μmol L− 1 Fe(III)-EDTA + 6.85 mmol L− 1 NH4NO3), and -Fe + AN+cPTIO (1 μmol L− 1 Fe(III)-EDTA + 6.85 mmol L− 1 NH4NO3 + 100 μmol L− 1 cPTIO). NN69 medium supplemented with 0.3% (w/v) agar [31], pH 5.8, was used for the cultures, which were grown at 22–27 °C with a 14 h photoperiod, relative humidity (RH) of 65–85%, and light intensity ranges from 6000 to 8000 lx. The samples were immediately frozen in liquid nitrogen and stored at − 80 °C for later use.
Determination of relative chlorophyll content and photosynthetic characteristics
A portable chlorophyll meter (Konica Minolta SPAD-502, Japan) was used to determine the chlorophyll content, which is expressed as the soil plant analysis development (SPAD) value. The net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of the fully expanded leaves, which were the third leaves from the top, were measured using a portable photosynthesis system (PP Systems CIRAS-3, USA). Leaf temperatures were maintained at 25 ± 1 °C. The RH in the assimilation chamber was maintained at 70%, the external CO2 concentration remained at 380 ± 10 μmol mol− 1, the light intensity was consistent at 200 μmol photons m− 2 s− 1, the gas flow rate was 300 mL min− 1, and the air humidity was 65–85%.
Analysis of root morphology
Root tip number, root length, root surface area, and average root diameter were scanned (Epson Perfection V800 photo scanner, China), and the relevant data were analyzed with WinRHIZO 2007 software.
Determination of soluble iron content
A fresh sample was dried and ground into powder, and 1.00 g was placed in a corkscrew test tube. Then, 10 mL of 0.1 mol L− 1 diluted hydrochloric acid was added, and the tube was continuously shaken for 12 h for extraction. The liquid was then filtered, and the concentration of iron in the supernatant was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES; PE Optima 8000DV, USA) [32].
Determination of total nitrogen
The samples were heated at 105 °C for 30 min, and then dried to constant weight at 80 °C. After being crushed through a 0.25 mm sieve, total nitrogen was determined using a Kjeltec apparatus (FOSS Kjeltec™ 8000, Denmark) [33].
Determination of FCR activity
FCR activity was quantified by the dipyridine method. First, 0.5 g of sample was soaked in 0.5 mmol L− 1 CaSO4 for 5 minutes, washed with deionized water, and then soaked for 2 hours in 50 mL determination solution (pH 5.8) containing 0.4 mmol L− 1 dipyridine and 0.1 mmol L− 1 FeNa-EDTA. The absorbance of the solution was determined by spectrophotometer (Shimadzu UV 1800, Japan) at 523.3 nm [34].
Determination of NO content
Nitric oxide content was determined according to the method of nitrate reductase, which is used to specifically reduce NO3− to NO2−. Through color development, colorimetry was performed at the wavelength of 550 nm and light diameter of 0.5 cm. A NO determination kit was used to measure the amount of NO (JianCheng A0112–1, Nanjing, China).
Determination of nitrogen assimilation-related enzymatic activities
Nitrate reductase (NR), nitrite reductase (NiR), glutamine synthetase (GS), glutamate synthetase (GOGAT), and glutamate dehydrogenase (GDH) were determined using activity detection kits (SolarBio, Beijing, China). NR activity was determined by soaking in inducer for 2 h, drying by filter paper, and freezing at − 20 °C for 30 min. After the filter paper was aspirated to dryness, 0.1 g sample was weighed, and 1 mL extract was added. The sample was ground in an ice bath, centrifuged at 4000 rpm and 4 °C for 10 min, and the supernatant was placed on ice for testing. NiR and GOGAT activity was determined by weighing 0.1 g sample, adding 1 mL extract, grinding in an ice bath, centrifuging at 10,000 rpm and 4 °C for 10 min, and then, the supernatant was removed and placed on ice for testing.
For determination of the GS and GDH activity, 0.1 g sample was weighed, and 1 mL extract was added. The sample was ground in ice bath, centrifuged at 8000 rpm and 4 °C for 10 min, and the supernatant was then removed and placed on ice for testing. Deionized water was used as the reference solution. The colorimetric wavelengths of NR, GDH, and GOGAT are 340 nm, while those of NiR and GS are 540 nm.
Real-time quantitative PCR (RT-qPCR) analysis
The homologous sequence of pear was found in the National Center for Biotechnology Information (NCBI) database, and the full length of the pear genome was obtained by comparison. RT-qPCR primers were designed (Table 1), and the primers were synthesized by Beijing Tsingke Biotechnology Co., Ltd. The total RNA of pear leaves that underwent different treatments was extracted using an EASY Spin Plant RNA Kit (Tiangen Biotech Co., China). cDNA was synthesized using a reverse transcription kit (Vazyme Biotech Co., China), and a Real-time quantitative PCR System (Lightcycler® 480II System, Roche) and ChamQ SYBR Color qPCR Master Kit (Vazyme Biotech Co., China) were used to analyze the relative gene expression levels under different treatments. The reaction system (20 μL total volume) consisted of template cDNA 2 μL, forward primer and reverse primer 1 μL each, Supermix 10 μL, and RNA-free H2O 6 μL. The reaction process was as follows: 95 °C for 5 min, 45 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s [35]. Using PbActin1 and PbActin2 as internal reference genes, the relative expression levels for each gene were calculated by the 2−ΔΔCT method, and each sample analysis was repeated 3 times [36].
Statistical analysis
All data were processed using DPS and GraphPad Prism 6 software, and are presented as the mean ± standard error (mean ± SD) of triplicate determinations. Differences were defined as significant at P<0.05 (least significant difference (LSD) test). All experiments were conducted at least in triplicate.
Results
Effects of exogenous NH4NO3 and NO treatment on the growth and development of pear under iron deficiency
Exogenous NH4NO3 and NO effectively alleviated the chlorosis of new leaves by iron deficiency stress, with NO exerting a stronger effect (Fig. 1A). Iron deficiency significantly decreased stem diameter and fresh and dry weights, and inhibited the overall growth of plants. NH4NO3 and NO partially restored plant growth and increased stem diameter and fresh and dry weights. Under iron deficiency, the root-shoot ratio of plants increased, indicating that iron deficiency had a greater inhibitory effect on shoots than roots. Exogenous NH4NO3 reduced the root-shoot ratio, but exogenous NO had no effect on the root-shoot ratio (Fig. 1B).
Effects of exogenous NH4NO3 and NO application on the growth and development of pear with iron deficiency. A The growth phenotypes. B Growth index, including stem diameter, fresh weight, dry weight, and root shoot ratio. The data are expressed as the mean ± SD (n = 6). Different letters indicate significant differences at P < 0.05 (LSD test)
Effects of exogenous NH4NO3 and NO on the relative chlorophyll content and photosynthetic capacity of pear leaves under iron deficiency
Iron deficiency decreased the SPAD value and the photosynthetic capacity of pears. Exogenous NH4NO3 and NO significantly increased the SPAD value and the net photosynthesis rate of pear leaves under iron deficiency. In addition, Fe deficiency significantly decreased the stomatal conductance and transpiration rate, and increased the intercellular CO2 concentration. Under Fe deficiency, exogenous NH4NO3 and NO significantly increased the stomatal conductance and transpiration rate, and decreased the intercellular CO2 concentration of mesophyll cells (Fig. 2).
Effects of exogenous NH4NO3 and NO on the root growth of pear under iron deficiency
As the most important organ for plant nutrient absorption, roots play an important role in the absorption and utilization of plant shoots. Iron deficiency promotes root growth (Fig. 3A). Iron deficiency increased the total root length, root surface area, and total root tip number in pears. However, iron deficiency results in a decrease in the mean root diameter. Under iron deficiency, exogenous NH4NO3 and NO significantly decreased the total root length and total root surface area of plants, but significantly increased the average root diameter. The addition of NH4NO3 significantly decreased the number of root tips, while the addition of NO significantly increased the number of root tips (Fig. 3B).
Effects of exogenous NH4NO3 and NO on the root phenotype of pear under iron deficiency stress. A Root phenotype of pear treated with NH4NO3 and NO. B Effects of exogenous NH4NO3 and NO on root length, root surface area, root average diameter, and total root tip number under iron deficiency. The data are expressed as the mean ± SD (n = 3). Different letters indicate significant differences at P < 0.05 (LSD test)
Effects of exogenous NH4NO3 and NO on the iron absorption capacity of pear under iron deficiency
To prove whether NH4NO3 and NO increased the soluble iron content in plants to alleviate iron deficiency stress, we measured the active iron content in new leaves and roots. Under iron deficiency, NH4NO3 and NO significantly increased the FCR activity (Fig. 4A) and the soluble iron content in new leaves (Fig. 4B). However, NH4NO3 did not significantly increase the soluble iron content in roots. In addition, NH4NO3 and NO increased the expression of iron absorption genes PbFRO2 and PbFRO4, as well as the expression of iron transport-related genes PbIRT1 (Fig. 4C). NO also significantly increased the expression of PbIRT1-like.
Effects of NH4NO3 and NO on iron absorption capacity and soluble iron content of pear under iron deficiency stress. A Effects of NH4NO3 and NO on FCR activity in pear leaves under iron deficiency. B Soluble Fe content of newly expanded leaves and roots. C The relative expression levels of iron absorption genes (PbFRO2, PbFRO4) and iron transport genes (PbIRT1, PbIRT1-like). The data are expressed as the mean ± SD (n = 3). Different letters indicate significant differences at P < 0.05 (LSD test)
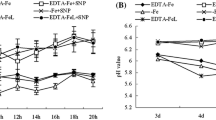
Effects of exogenous NH4NO3 and NO on the total nitrogen content and NO content in pear under iron deficiency
Iron deficiency significantly decreased the total nitrogen content in roots. Under iron deficiency, exogenous NH4NO3 significantly increased the total nitrogen content in young leaves and roots. Exogenous NO also significantly increased the total nitrogen content in young leaves, but had NO significant effect on the total nitrogen content in roots. Iron deficiency significantly increased the NO in young leaves, and exogenous NH4NO3 and NO increased the NO content in young leaves under iron deficiency (Fig. 5).
NH4NO3 alleviates leaf chlorosis through NO
The chlorosis of leaves were accelerated by cPTIO treatment under iron deficiency. Ammonium nitrate treatment alleviated leaf chlorosis, and the SPAD value was significantly higher than that under iron deficiency. However, the chlorosis of leaves were not alleviated after treatment with ammonium nitrate and cPTIO (Fig. 6). cPTIO treatment significantly decreased the activity of Fe3+ reductase. Ammonium nitrate treatment increased the activity of Fe3+ reductase, but when the treatment with ammonium nitrate and cPTIO, the activity of Fe3+ reductase did not increase and was significantly lower than that under iron deficiency (Fig. 6C).
Exogenous NH4NO3 and NO increased the activities of nitrogen assimilation-related enzymes in pear leaves under iron deficiency
Iron deficiency decreased the activities of NR, NiR, GS, GOGAT, and GDH, while exogenous NH4NO3 and NO significantly increased these enzymatic activities under iron deficiency. After treatment with NH4NO3, the activities of NR and NiR were significantly increased, but did not reach normal levels. Exogenous NH4NO3 and NO significantly increased the activity of GS to levels even higher than those of the normal iron treatment group. Compared with NO treatment, GOGAT activity and NiR activity were significantly increased after ammonium nitrate treatment (Fig. 7). NH4NO3 and NO significantly increased the relative expression levels of nitrogen assimilation-related genes PbNR, PbNiR, PbGS, PbGOGAT, and PbGDH (Fig. 8).
Discussion
Fruit trees are very sensitive to iron deficiency. Pear is one of the species of iron deficiency-sensitive fruit trees. Under iron deficiency stress, the young leaves at the tip of the new shoot lose their green color first. With the aggravation of stress, the mesophyll gradually loses its green color and turns yellow. In severe cases, the green color of the leaf veins fades, the entire leaf turns yellowish white, and dry patches occur at the edge of the leaves. Finally, the leaves dry and fall off, which eventually leads to a decrease in tree vigor and fruit quality, and even death, causing great economic losses to growers [37, 38].
In the current study, under iron deficiency, the chlorophyll content of pear variety ‘Qingzhen D1’ decreased. The leaves from young to old gradually turned yellow, resulting in the yellowing phenotype of the plant, which inhibited photosynthesis and finally led to thinning of the stem and poor growth. After NO treatment, we found that the phenomenon of plant yellowing was alleviated, and plant growth did not cause significant changes at 60 days. Previous studies have reported the role of NO in plants under iron deficiency stress. It is considered that the accumulation of NO caused by iron deficiency is a factor in the response to iron deficiency stress. NO alleviates iron deficiency stress in plants through increasing FCR activity and improving iron ion transport [39, 40]. This is consistent with our research results. As an important nitrogen source for plants, we used ammonium nitrate to treat plants under iron deficiency stress. It was found that its effect of alleviating iron deficiency stress was weaker than that of NO, but it also significantly increased plant growth and alleviated plant yellowing compared with the iron deficiency treatment group.
Through observation of root growth, we found that iron deficiency resulted in the growth of roots. In contrast, the treatment of ammonium nitrate and NO weakened this phenotype, and root growth was inhibited, but the root length and root surface area were significantly higher than those of plants that received a normal iron treatment. It is worth noting that the number of root tips in the no-treatment group significantly increased. In previous studies, NO promoted root tip growth, and it may act in different pathways with root growth caused by iron deficiency [41, 42].
To further understand the effects of ammonium nitrate and NO on plant iron absorption, we measured the soluble iron content and FCR activity. Under iron deficiency, ammonium nitrate and NO treatment significantly increased the activity of FCR. The soluble iron content significantly increased in the underground and aboveground parts of the plant. In pear (a strategy I plant), free iron is reduced to Fe2+ by FCR on the plasma membrane, and it is then transported to the cell through IRT1. qPCR analysis showed that ammonium nitrate and NO promoted the high expression of PbIRT1 and PbFRO1. For the Arabidopsis homologous PbIRT1 gene [43], NO significantly increased its expression, while ammonium nitrate did not result in a high expression trend. This shows that both ammonium nitrate and NO increase the absorption of irons, and there are more transporter proteins for iron regulated by NO than those ammonium nitrate, which may be the reason for the difference between them in alleviating plant iron deficiency.
Iron deficiency stress decreased nitrogen accumulation, especially aboveground nitrogen accumulation. Ammonium nitrate as a nitrogen source induced nitrogen accumulation. Interestingly, NO treatment also increased nitrogen accumulation. Meanwhile, we found NO increased after ammonium nitrate treatment, which may indicate that ammonium nitrate could indirectly affect the response to iron deficiency through NO. Ammonium nitrate could alleviate the chlorosis caused by iron deficiency, but the alleviating effect was weakened after adding cPTIO. Additionally, leaf chlorosis was more severe than iron deficiency, which indicated that NO is important for facilitating the action of ammonium nitrate in alleviating leaf chlorosis under iron deficiency. The nitrogen cycle also plays an important role in alleviating iron deficiency. Iron deficiency significantly decreased the nitrogen metabolism level in Areca catechu L., and similar conclusions were also reached in cucumber and Arabidopsis [12, 44, 45], which is also consistent with our study.
Through the determination of key enzymatic activities and the identification of related gene expression, it was found that the activities of NR, NiR, GS, GOGAT, and GDH were significantly increased after treatment, and the activity of GS after treatment was higher than that in the normal Fe application group. This may be due to the increase in NH4+ caused by exogenous ammonium nitrate and NO treatment in plants. GS has high affinity for NH4+ [46], which can ensure that the plant maintains a low level of NH4+ to protect its tissue from damage.
Conclusions
We used ammonium nitrate and NO treatment to explore the effects of the two substances on iron deficiency stress. It was found that NO promoted plant nitrogen accumulation and alleviated the yellowing symptoms caused by iron deficiency. Ammonium nitrate can indirectly alleviate the symptoms of plant yellowing by promoting the increase of NO, but it is not the only method. NO increases the response of iron transporters. Both substances increase nitrogen accumulation in plants by promoting the activity of key enzymes of nitrogen assimilation. The increase in GS activity alleviated the adverse effects of excessive NH4+ to a certain extent (Fig. 9). This study provides a theoretical basis for further examination of iron stress alleviation in plants.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Guerinot ML. Improving rice yields—ironing out the details. Nat Biotechnol. 2001;19:417–8.
Curie C, Mari S. New routes for plant iron mining. New Phytol. 2017;214:521–5.
Parveen S, Ranjan RK, Anand A, Singh B. Combined deficiency of nitrogen and iron increases senescence induced remobilization of plant immobile iron in wheat. Acta Physiol Plant. 2018;40:211.
Álvarez-Fernández A, Paniagua P, Abadía J, Abadía A. Effects of Fe deficiency chlorosis on yield and fruit quality in peach (Prunus persica L. Batsch). J Agric Food Chem. 2003;51:5738–44.
Brumbarova T, Bauer P, Ivanov R. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. 2015;20:124–33.
Connorton JM, Balk J, Rodríguez-Celma J. Iron homeostasis in plants – a brief overview. Metallomics. 2017;9:813–23.
Römheld V, Kramer D. Relationship between proton efflux and rhizodermal transfer cells induced by iron deficiency. Zeitschrift Pflanzenphysiologie. 1983;113:73–83.
Schmidke I, Krüger C, Frömmichen R, Scholz G, Stephan UW. Phloem loading and transport characteristics of iron in interaction with plant-endogenous ligands in castor bean seedlings. Physiol Plant. 1999;106:82–9.
Ding H, Duan L, Wu H, Yang R, Ling H, Li WX, et al. Regulation of AhFRO1, an Fe(III)-chelate reductase of peanut, during iron deficiency stress and intercropping with maize. Physiol Plant. 2009;136:274–83.
Zou C, Shen J, Zhang F, Guo S, Rengel Z, Tang C. Impact of nitrogen form on iron uptake and distribution in maize seedlings in solution culture. Plant Soil. 2001;235:143–9.
Assimakopoulou A. Effect of iron supply and nitrogen form on growth, nutritional status and ferric reducing activity of spinach in nutrient solution culture. Sci Hortic. 2006;110:21–9.
Borlotti A, Vigani G, Zocchi G. Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biol. 2012;12:189.
Martin MH, Marschner H. Mineral nutrition of higher plants. J Ecol. 1988;76:1250.
Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 2007;52:949–60.
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, et al. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010;154:810–9.
García MJ, Lucena C, Romera FJ, Alcántara J, Alcántara E, Pérez-Vicente R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot. 2010;61:3885–99.
Meiser J, Lingam S, Bauer P. Posttranslational regulation of the iron deficiency basic helix-loop-helix transcription factor FIT is affected by iron and nitric oxide. Plant Physiol. 2011;157:2154–66.
Yang J, Chen W, Chen L, Qin C, Jin C, Shi Y, et al. The 14-3-3 protein general regulatory factor11 (FRF11) acts downstream of nitric oxide to regulate iron acquisition in Arabidopsis thaliana. New Phytol. 2013;197:815–24.
Sun H, Tao J, Zhao Q, Xu G, Zhang Y. Multiple roles of nitric oxide in root development and nitrogen uptake. Plant Signal Behav. 2017;12:e1274480–3.
Zhang XV, Dong YJ, Kong J, Wang QH. Exogenous nitric oxide effects on physiological characteristics of a peanut cultivar growing on calcareous soil. Commun Soil Sci Plant Anal. 2014;45:1011–24.
Kong J, Dong Y, Xu L, Liu S, Bai X. Effects of foliar application of salicylic acid and nitric oxide in alleviating iron deficiency induced chlorosis of Arachis hypogaea L. Bot Stud. 2014;55:9.
Zhang X, Dong Y, Kong J, Liu Z, Wang Q. Effects of nitric oxide on iron-deficiency stress alleviation of peanut. J Plant Nutr. 2014;37:2108–27.
Kong J, Dong Y, Xu L, Liu S, Bai X. Role of exogenous nitric oxide in alleviating iron deficiency-induced peanut chlorosis on calcareous soil. J Plant Interact. 2014;9:450–9.
Moreau M, Lindermayr C, Durner J, Klessig DF. NO synthesis and signaling in plants - where do we stand? Physiol Plant. 2010;138:372–83.
Fröhlich A, Durner J. The hunt for plant nitric oxide synthase (NOS): is one really needed? Plant Sci. 2011;181:401–4.
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT. On the origins of nitric oxide. Trends Plant Sci. 2011;16:160–8.
Astier J, Gross I, Durner J. Nitric oxide production in plants: an update. J Exp Bot. 2018;69:3401–11.
Corpas FJ, Barroso JB. Nitric oxide synthase-like activity in higher plants. Nitric Oxide. 2017;68:5–6.
Chamizo-Ampudia A, Sanz-Luque E, Llamas Á, Galván A, Fernández E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017;22:163–74.
Zhu XF, Dong XY, Wu Q, Shen RF. Ammonium regulates Fe deficiency responses by enhancing nitric oxide signaling in Arabidopsis thaliana. Planta. 2019;250:1089–102.
Nitsch JP, Nitsch C. Haploid plants from pollen grains. Science. 1969;163:85–7.
Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ. 2014;37:852–63.
Magomya AM, Kubmarawa D, Ndahi JA, Yebpella GG. Determination of plant proteins via the Kjeldahl method and amino acid analysis: a comparative study. Int J Sci Technol Res. 2014;3:68–72.
Gao L, Shi Y. Genetic differences in resistance to iron deficiency chlorosis in peanut. J Plant Nutr. 2007;30:37–52.
Liu JL, Zhou FL, Cui SQ, Yang YJ, Sun QR, Guan QZ, et al. Effects of ploidy variation on DNA methylation and gene expression in pear (Pyrus communis L.). Sci Hortic. 2021;293:110713.
Livak KJ. Schmittgen T D (2001) analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8.
Lucena JJ, Hernandez-Apaolaza L. Iron nutrition in plants: an overview. Plant Soil. 2017;418:1–4.
Yadavalli V, Neelam S, Rao A, Reddy AR, Subramanyam R. Differential degradation of photosystem I subunits under iron deficiency in rice. J Plant Physiol. 2012;169:753–9.
Zhai L, Xiao D, Sun C, Wu T, Han Z, Zhang X, et al. Nitric oxide signaling is involved in the response to iron deficiency in the woody plant Malus xiaojinensis. Plant Physiol Biochem. 2016;109:515–24.
Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol. 2009;150:272–80.
Sun H, Feng F, Liu J, Liu J, Zhao Q. The interaction between auxin and nitric oxide regulates root growth in response to iron deficiency in rice. Front Plant Sci. 2017a;8:2169.
Bai S, Yao T, Li M, Guo X, Zhang Y, Zhu S, et al. PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol Plant. 2013;7:616–25.
Quintana J, Bernal M, Scholle M, Hollander-Czytko H, Nguyen NT, Piotrowski M, et al. Root-to-shoot iron partitioning in Arabidopsis requires iron–regulated transporter1 (IRT1) protein but not its iron(ii) transport function. Plant J. 2021;109:992–1013.
Li J, Cao X, Jia X, Liu L, Cao H, Qin W, et al. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front Plant Sci. 2021;12:710093.
Coleto I, Bejarano I, Marín-Peña AJ, Medina J, Rioja C, Burow M, et al. Arabidopsis thaliana transcription factors MYB28 and MYB29 shape ammonium stress responses by regulating Fe homeostasis. New Phytol. 2021;229(2):1021–35.
Mondal R, Kumar A, Chattopadhyay SK. Structural property, molecular regulation, and functional diversity of glutamine synthetase in higher plants: a data-mining bioinformatics approach. Plant J. 2021;108:1565–84.
Acknowledgements
We thank the Engineering Laboratory of Genetic Improvement of Horticultural Crops of Shandong Province, Qingdao Agricultural University for providing the test platform.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA (CARS-28-07), the Agricultural Variety Improvement Project of Shandong Province (2019LZGC008), and the Natural Science Foundation of Shandong Province (ZR2020QC150).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.W., Z.W., C.L., D.L, R.W. and M.L. methodology, J.W., Z.W., formal analysis, J.W., Z.W., writing—original draft preparation; J.L., writing—review and editing, J.L., supervision, R.W. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The pear material (Qingzhen D1) used in this study was cultivated and authorized by Professor Wang Ran of Qingdao Agricultural University. Pear is a plant commonly used in molecular biology, and this study complied with the laws and regulations of the People’s Republic of China.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Wang, J., Wang, Z. et al. Alleviation of iron deficiency in pear by ammonium nitrate and nitric oxide. BMC Plant Biol 22, 434 (2022). https://doi.org/10.1186/s12870-022-03826-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03826-z