Abstract
Background
Cold is a major abiotic stress and Huanglongbing and citrus canker disease are two devastating bacterial diseases for citrus. The Ca2+-CBL-CIPK network is known to regulate different types of stress signalling in plants. How do CBL–CIPK signalling networks function in response to cold and infection by CLas or Xcc in citrus?
Results
Eight calcineurin B-like proteins (CBLs) and seventeen CBL-interacting protein kinases (CIPKs) were identified from the cold-tolerant satsuma mandarin ‘Guijing2501’ (Citrus. unshiu) and CLas/Xcc-sensitive sweet orange (C. sinensis). Phylogenetic analysis revealed that both CBL and CIPK family members in citrus were classified into an ancient and a recent clade according to their conserved domain characteristics and/or intron/exon structures. Genome duplication analysis suggested that both tandem and segmental duplications contributed to the amplification of the CBL and CIPK gene families in citrus under intense purifying selection, and the duplication events only existed in the recent clades. Expression comparison of the duplicated gene pairs indicated that the duplicated CBL and CIPK genes underwent functional differentiation. Further expression analysis identified that CBL1, 5, 6, and 8 and CIPK2, 8, 12, 15, 16, and 17 were significantly regulated by multiple stresses, including cold, Xcc infection and/or CLas infection, in citrus, whereas CBL2/7 and CIPK1/4/5/11/13/14 were independently highly regulated by cold and CIPK3 was uniquely responsive to Xcc infection. The combination analyses of targeted Y2H assay and expression analysis revealed that CBL6-CIPK8 was the common signalling network in response to cold and Xcc infection, while CBL6/CBL8-CIPK14 was uniquely responsive to cold in citrus. Further stable transformation and cold tolerance assay indicated that overexpression of CuCIPK16 enhanced the cold tolerance of transgenic Arabidopsis with higher POD activity and lower MDA content.
Conclusions
In this study, evolution, gene expression and protein‒protein interaction analyses of citrus CBLs and CIPKs were comprehensively conducted over a genome-wide range. The results will facilitate future functional characterization of individual citrus CBLs and CIPKs under specific stresses and provide clues for the clarification of cold tolerance and disease susceptibility mechanisms in corresponding citrus cultivars.
Similar content being viewed by others
Background
Specific mechanisms have evolved in plants to detect and respond to various abiotic and biotic stresses. Calcium plays an essential role in sensing and signalling these stresses in plants. Under specific stress conditions, plants can release stress-induced Ca2+ signals, which are subsequently decoded by various Ca2+-sensors [1]. Calmodulin (CaM), calmodulin-like protein (CML), calcineurin B-like protein (CBL) and calcium-dependent protein kinase (CDPK) are four sensors of Ca2+ [2]. CDPK, which has a kinase domain, can function independently of protein kinases [3]. However, CaM, CML and CBL harbour no kinase domain and require interactions with specific protein kinases to activate phosphorylation cascades and regulate downstream gene expression [4, 5].
Complexes of CBL and CBL-interacting protein kinase (CIPK) have been identified as calcium-decoding signalling networks that decode intracellular calcium signals and mediate the response of downstream genes to various stresses [1]. With a highly conserved core region that is composed of four elongation factor hand (EF-hand) domains, CBLs are responsible for Ca2+ binding and interaction with CIPKs [1, 5, 6]. Furthermore, the PFPF motif located at the C-terminus is critical for CBL phosphorylation by CIPKs [1]. CIPK proteins are classified into the sucrose nonfermenting 1-related serine/threonine kinases 3 (SnRK3) subfamily [7] and consist of an N-terminal catalytic kinase domain and a self-inhibitory C-terminal regulatory domain [8]. The N-terminal catalytic kinase domain contains an ATP binding site and an activation loop, and the C-terminal regulatory domain harbours a unique NAF/FISL motif and a protein-phosphatase interaction (PPI) motif [1]. Ca2+-bound CBLs can interact with the conserved NAF/FISL motif and activate the catalytic activity of target CIPKs [9]. Some CIPKs can also target specific members of the protein phosphatase 2C (PP2C) by the PPI motif [10].
In previous studies, the CBL–CIPK signalling networks have been extensively investigated in response to nutrient deficiency and salt stress in plants. For instance, AtCBL1/9-AtCIPK23 and AtCBL4-AtCIPK6 complexes can phosphorylate AKT1 and AKT2, respectively, and then modulate their activities to maintain K+ homeostasis under low-K+ stress in Arabidopsis [11,12,13]. In addition, AtCIPK24, a well-known gene identified in the salt overly sensitive (SOS) pathway of Arabidopsis, can interact with AtCBL4 and AtCBL10 [14, 15]. The AtCBL4-AtCIPK24 complex is responsive to salt stress and controls long-distance Na+ transport from the root to the shoot, while the AtCBL10-AtCIPK24 complex functions mainly in the response of shoots to salt toxicity [14, 15]. However, there have been few studies of the CBL–CIPK network in response to cold stress [1]. To the best of our knowledge, the AtCBL1-AtCIPK7 complex may play a role in the cold response of Arabidopsis [16]. In addition, the CBL–CIPK network also plays important functions in response to pathogen elicitors [1]. For example, the OsCBL2-CIPK31-AKT1L, TaCBL4-TaCIPK5 and MaCBL1/9-MaCIPK23 complexes can positively regulate disease resistance in rice, wheat and cassava, respectively [17,18,19]. Overall, these findings suggest that CBL–CIPK signalling networks may have important functions in response to abiotic and biotic stresses in plants.
Citrus is one of the most widely cultivated fruit crops in the world. Cold is a major limiting factor affecting citrus production and survival in citrus northern fringe growing areas [20,21,22]. ‘Guijing2501’ satsuma mandarin (Citrus. unshiu) is a novel citrus cultivar with cold tolerance selected from Hubei Province in China, which is a northern fringe region for citrus growing [23]. Citrus Huanglongbing (HLB) disease, mainly caused by Candidatus Liberibacter asiaticus (CLas), and citrus canker disease, caused by Xanthomonas citri subsp. citri (Xcc), are two devastating bacterial diseases that affect the global citrus industry [24,25,26,27]. Sweet orange (C. sinensis) is a citrus cultivar sensitive to CLas and Xcc infection. CBL-CIPK interactions are known to regulate different types of stress signalling in plants. Although genome-wide analysis has identified CBL and CIPK family genes as involved in various stresses in many fruit crops, including grape [28], apple [29], pineapple [30] and pear [31], and coexpression of VvCBL and VvCIPK combined with Arabidopsis CBL-CIPK interaction networks retrieved from STRING was employed to predict VvCBL-VvCIPK interactions in grape [28], few genome-wide studies in fruit crops have verified the CBL–CIPK interaction networks by protein‒protein interaction experiments. To the best of our knowledge, MdCIPK24-LIKE1 can physically interact with MdCBL1/4/10 to increase salt tolerance in apple [32]. In a previous study, citrus CBL and CIPK genes were identified in response to drought and arbuscular mycorrhizal fungi colonization according to expression data [33]. How do CBL–CIPK signalling networks function in response to cold and infection by CLas or Xcc in citrus? In this study, evolution, gene expression and protein‒protein interaction analyses were comprehensively conducted over a genome-wide range to identify candidate CBL-CIPK networks implicated in regulating responses to cold in ‘Guijing2501’ and CLas and Xcc infection in sweet orange. The findings may facilitate future functional characterization of individual citrus CBLs and CIPKs under specific stresses and provide clues for the clarification of cold tolerance and disease susceptibility mechanisms in corresponding citrus cultivars.
Results
Identification and cloning of CBLs and CIPKs in citrus
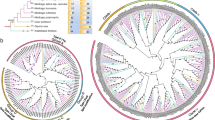
A total of eight C. sinensis CBL (CsCBL) candidate genes and 17 CsCIPK candidate genes were identified from the sweet orange genome, and they were named CsCBL1–8 and CsCIPK1–17 based on their positions in the chromosomes. These CsCBL and CsCIPK genes exhibited uneven distributions on chromosomes (Fig. 1A and 1C). We further analysed the intron/exon structures of these genes using the gene sequences (Table S1). As shown in Figure S1, all CsCBL genes were intron rich, whereas the CsCIPK genes could be divided into intron-rich and intron-less clades.
Chromosomal locations of CsCBLs (A) and CsCIPKs (C) and the phylogenetic trees based on the predicted CuCBL and CuCIPK protein sequences along with their sequence alignment in the conserved domains (B and D). Note that only chromosomes with CsCBL and CsCIPK genes are shown in the drawing. The phylogenetic trees for CuCBLs and CuCIPKs of ‘Guijing2501’ satsuma mandarin were generated using the maximum likelihood method. Four EF-hand domains, PFPF motifs and the lengths of the linker amino acid sequences for CuCBL proteins are shown in B. The ATP-binding site and activation loop in the N-terminal catalytic kinase domain and the NAF/FISL and PPI motifs in the C-terminal regulatory domain for CuCIPK proteins are shown in D
In addition, eight C. unshiu CBL (CuCBL) and 17 CuCIPK genes with full-length CDSs were cloned from the cold-tolerant ‘Guijing2501’ satsuma mandarin. Multiple sequence alignment analysis revealed that the deduced proteins of all the CuCBL genes from ‘Guijing2501’ contain four EF-hand domains and a PFPF motif. The EF1 and EF2, EF2 and EF3, and EF3 and EF4 domains were separated by 23, 25 and 32 amino acids, respectively, for all CuCBL proteins (Fig. 1B, File S1). All CuCIPK proteins except CuCIPK15 contain an ATP-binding site and an activation loop in the N-terminal catalytic kinase domain and an NAF/FISL motif and a PPI motif in the C-terminal regulatory domain (Fig. 1D, File S2).
Phylogenetic analysis of CBL and CIPK family genes
To better understand the evolutionary relationship of the CBL and CIPK members, a maximum likelihood phylogenetic tree was generated based on the full deduced amino acid sequences of the CBL and CIPK family proteins from sweet orange, ‘Guijing2501’ satsuma mandarin, Arabidopsis and two ancient species (Physcomitrella patens and Selaginella moellendorffii) (Fig. 2, Tables S2-S5). Based on the phylogenetic tree, both the predicted citrus CBL and CIPK proteins could be divided into an ancient clade and a recent clade (Fig. 2). Cs(u)CBL2, 3 in Group A and Cs(u)CBL7 in Group B might be ancient genes because they were clustered with the CBLs from ancient species. However, Cs(u)CBL1, 4 in Group C and Cs(u)CBL5, 6, 8 in Group D might have evolved recently, as both groups included no members from the ancient species. Similarly, Cs(u)CIPKs in Group I and Group II might have evolved long ago; in contrast, the remaining Cs(u)CIPKs in Group III might have evolved recently. More interestingly, CIPKs in the ancient clade are intron rich, whereas CIPKs in the recent clade are intron less (Figure S1).
Phylogenetic tree of CBL (A) and CIPK (B) proteins. Eight Cs (u) CBLs and 17 Cs (u)CIPKs from sweet orange and ‘Guijing2501’ satsuma mandarin, 10 AtCBLs and 26 AtCIPKs from Arabidopsis, five CBLs and seven CIPKs from Physcomitrella patens, and three CBLs and five CIPKs from Selaginella moellendorffii were used to generate the maximum likelihood tree by using MEGA 7.0 with 1000 bootstraps. Cs (u) CBLs and Cs (u) CIPKs from citrus are highlighted in red. Branch lines of CBLs and CIPKs from eudicots are marked with rose red, and those of CBLs and CIPKs from ancient bryophyta and pteridophyta are marked with light green
Expression analysis of CuCBL and CuCIPK genes in cold-tolerant ‘Guijing2501’ satsuma mandarin under cold stress
To test whether the CBL and CIPK family genes are responsive to cold stress in citrus,‘Gujing2501’ trees were subjected to low temperature (4 °C). Leaf samples were collected from the tree under test at 0, 3, 6, and 12 h and 0, 1, 4, and 16 days after the cold treatment. The gene expression levels of all the CuCBL and CuCIPK genes were determined by qRT‒PCR (Fig. 3, Table S6). The results showed that 75% of CuCBL genes (except CuCBL3, 4) exhibited significant transcriptional changes upon cold treatment. CuCBL1, 2, 5, and 8 genes were upregulated 2.4 ~ 4.2-fold at the early time points (3 ~ 12 h). Almost all CuCBL genes were downregulated at the later time points (4 ~ 16 days), except that CuCBL7 was upregulated 3.1-fold at 4 days. In particular, the recently evolved CuCBL5, 6, and 8 genes were downregulated by 12.5 ~ 50.0-fold at 16 days after cold stress. In the case of CuCIPK genes, almost all CuCIPK genes were significantly upregulated upon cold treatment, except that CuCIPK3, 6, 7, 9, and 10 remained at a relatively stable expression level. Among the upregulated CuCIPK genes, only CuCIPK8 and CuCIPK1 were upregulated at the later time points (4 ~ 16 days), and reached the peak expression level at 16 days. The remaining CuCIPK genes were upregulated from early time points. CuCIPK17 reached the peak of expression level at 3 h, CuCIPK13 and 5 at 12 h, CuCIPK16 and 2 at 1 day, and CuCIPK14, 12, 11, 4, and 15 at 4 days. Thus, these genes exhibited significant cold-induced transcriptional changes, implying that they may be functionally involved in mounting the physiological response of ‘Guijing2501’ satsuma mandarin to cold stress.
Expression patterns of eight CuCBLs and 17 CuCIPKs in cold-tolerant ‘Guijing2501’ satsuma mandarin under cold stress as determined by qRT‒PCR. Two-year-old healthy and uniform bud-grafting ‘Gujing2501’ satsuma mandarin trees growing in a greenhouse were subjected to low-temperature (4 °C) treatment as described in the Methods section. Three biological replicates were used for measuring gene expression, and the experiment was repeated once with similar results. The relative expression levels of candidate genes were calculated using the 2−∆∆CT method. The CuEF1α gene was used as an internal reference gene for normalization of the transcript levels of the tested genes. Error bars indicate inferential statistics according to the SE. *indicates statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; Student’s t test) when compared to the expression level at 0 h/d
Expression analysis of CsCBL and CsCIPK genes during CLas or Xcc infection in sweet orange
To determine whether the CBL and CIPK family genes are responsive to CLas and Xcc infection in citrus, the expression patterns of all CsCBL and CsCIPK genes from CLas-infected orange leaves (verified by HLB typical symptoms and CLas titre quantification; Figure S2A and Figure S2B) and Xcc-inoculated orange leaves (Figure S2C) were evaluated by qRT‒PCR analysis (Fig. 4–5; Table S7 and Table S8). The results showed that only a few CsCBL and CsCIPK genes exhibited significant transcriptional changes during CLas and Xcc infection. For example, the expression of CsCBL8 showed a significant increase in CLas-infected trees (11.2 x) and in trees after 12 days of Xcc inoculation (2.7 x). In contrast, the expression of CsCBL1 and CsCBL5 was reduced by 14.3 × and 33.3 x, respectively, in CLas-infected trees, and the expression of CsCBL6 was reduced by 33.3 × in trees after 12 days of Xcc inoculation. These expression patterns suggest that the recently evolved CsCBL1, 5, 6 and 8 genes might also play important roles in the response to bacterial infection. Among the CsCIPK genes, the expression of CsCIPK2, 8, 16 and 17 was decreased 5.6–25.0-fold in the CLas-infected trees; that of CsCIPK2, 12 and 16 was reduced 5.6–7.7-fold in trees after 12 days of Xcc inoculation, while that of CsCIPK3 and 8 was increased 5.2- and 6.5-fold in trees after 12 days of Xcc inoculation, respectively. Only one gene, CsCIPK15, was upregulated (4.0-fold) at the early time points (12 h) after Xcc inoculation. The above expression data suggest that these seven genes (CsCIPK2, 3, 8, 12, 15, 16, 17) might be implicated in the responses of sweet orange to bacterial infection.
Expression patterns of eight CsCBLs and 17 CsCIPKs in leaves of healthy sweet orange trees and in leaves of those infected with CLas as determined by qRT‒PCR. Healthy and CLas-infected leaves from 15-year-old ‘Yuanfeng’ navel orange trees were collected in the same orchard in Wugang County of Hunan Province. Three biological replicates were used for measuring gene expression, and the experiment was repeated once with similar results. The relative expression levels of candidate genes were calculated using the 2−∆∆CT method. The CsEF1α gene was used as an internal reference for the nominalization of the transcript levels of the genes tested. Error bars indicate inferential statistics according to the SE. *indicates statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; Student’s t test) when compared to the expression level in leaves of healthy sweet orange trees
Expression patterns of eight CsCBLs and 17 CsCIPKs in leaves of sweet orange trees infiltrated by water and in leaves of those infiltrated by Xcc bacteria as determined by qRT‒PCR. Two-year-old healthy and uniform bud-grafting ‘Taoye’ sweet orange trees growing in a greenhouse were subjected to the Xcc inoculation assay as described in the Methods section. Three biological replicates were used for measuring gene expression, and the experiment was repeated once with similar results. The relative expression levels of candidate genes were calculated using the 2−∆∆CT method. The CsEF1α gene was used as an internal reference for the nominalization of the transcript levels of the genes tested. Error bars indicate inferential statistics according to the SE. *indicates statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; Student’s t test) when compared to the expression level in leaves of sweet orange trees infiltrated by water
Synteny analysis of CsCBLs or CsCIPKs within the sweet orange genome
Duplication and diversification play essential roles in the evolution and expansion of gene families. One Step MCScanX was conducted within the sweet orange genome by TBtools software to identify the duplication events for the CsCBL and CsCIPK gene families. The results revealed that CsCBL5/CsCBL6 and CsCIPK1/CsCIPK2 were tandemly duplicated gene pairs, while CsCBL1/CsCBL4, CsCIPK4/CsCIPK10 and CsCIPK10/CsCIPK13 were segmentally duplicated gene pairs (Fig. 6A, Table S9). Interestingly, all the above duplicated genes were classified into recently evolved clades. In addition, the Ka/Ks ratios for all the duplicated gene pairs were lower than 0.5, suggesting that the CsCBL and CsCIPK gene families have evolved under intense purifying selection (Table S9).
Segmental duplication gene pairs of CsCBLs and CsCIPKs within the sweet orange genome (A) and divergent expression patterns of the duplication gene pairs in tissues and under different abiotic and biotic stresses (B). A. Grey lines in the background show the collinear blocks within the sweet orange genome, and the segmental duplication gene pairs of CsCBLs and CsCIPKs are highlighted by light green lines. B. The expression patterns in different tissues were analysed based on the RPKM values of RNA-seq data from the sweet orange genome database [34]
In addition, subfunctionalization and/or neofunctionalization are major evolutionary fates of duplicated genes, in which a large proportion of the duplicated genes undergo functional differentiation through gene expression divergence [35]. In this study, except for CsCBL5/CsCBL6, the other four duplicated genes shared the same patterns: one was responsible for normal tissue development, and the other was in response to abiotic and/or biotic stress (Fig. 6B). For example, CsCBL4, CsCIPK1 and CsCIPK10 were highly expressed in tissues including callus, flower, leaf and fruit, while the corresponding duplicated genes CsCBL1, CsCIPK2 and CsCIPK4/13 were significantly upregulated by cold stress and downregulated by CLas infection and/or Xcc infection. Although both CsCBL5 and CsCBL6 were expressed at low levels in tissues, CsCBL5 was significantly upregulated by cold stress and downregulated by CLas infection, whereas CsCBL6 was significantly downregulated by cold stress and Xcc infection. These results suggest that duplication might play an important role in the expansion of the CsCBL and CsCIPK gene family, which might also contribute to the adaptive feature of citrus CBL and CIPK for cold and bacterial infection.
Interactions and expression patterns of CBLs and CIPKs in the stress response to cold and bacterial infection in citrus
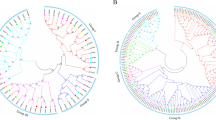
To investigate how CBLs interact with CIPKs in citrus, we examined the interaction specificity of CBL and CIPK proteins by using yeast two-hybrid assay. Theoretically, eight CBLs and 17 CIPKs in citrus could form 136 possible interaction pairs. However, we found that only 15 CBL–CIPK pairs exhibited interactive relationships (Fig. 7A, Figure S3). Interestingly, all recently evolved CBLs (except CBL4) had interacting CIPKs (accounting for 86.7% of the total interactive pairs) and were involved in multiple stresses (Fig. 8A). However, the majority of the candidate key stress-inducible CIPKs had no interacting CBLs (Fig. 8B). Among the positive interactive genes, CBL1, 5, 6, and 8 and CIPK8 were substantially regulated by multiple stresses, including cold, Xcc infection and/or CLas infection, whereas CBL2 and CIPK1 and 14 were independently highly regulated by cold stress (Fig. 8A). Among the negative interactive genes, CIPK2, 12, 15, 16, and 17 were involved in multiple stresses, including cold, Xcc infection and/or CLas infection, whereas CBL7 and CIPK4, 5, 11 and 13 were independently regulated by cold stress, and CIPK3 was uniquely responsive to Xcc infection (Fig. 8B). More importantly, CBL6 can interact with CIPK8 and respond to cold stress and Xcc infection in an antagonistic way, as both CBL6 and CIPK8 were highly regulated by the two stresses but showed opposite expression patterns (Fig. 7B and Fig. 8A). However, the CBL6/CBL8-CIPK14 interaction was independently involved in cold stress. Therefore, it can be inferred that CBL6-CIPK8 might be the common signalling network in response to cold and Xcc infection, while CBL6/CBL8-CIPK14 might be uniquely responsive to cold in citrus.
Interactive CBL-CIPK pairs in citrus verified by yeast two-hybrid assay (A) and comparison of their expression patterns under abiotic and biotic stresses (B). A. DDO (SD/–Leu/–Trp) agar plates were used to select the positive mating clones, and QDO/X/A (SD/-Trp/-Leu/-His/-Ade/X-α-Gal/AbA) agar plates were used to select the positive interaction proteins. (pGADT7-T + pGBKT7-Lam) was used as the negative control, and (pGADT7-T + pGBKT7-53) was used as the positive control. B. The expression patterns are presented by line chart according to each CIPK and its interactive CBLs, and the same genes in different line charts are highlighted with the same colour
Potential CBL-CIPK signalling networks in citrus in response to abiotic and biotic stresses. A. Interaction relationships between CBL and CIPK proteins indicated by yeast two-hybrid assay and the observed gene expression responses to stresses according to qRT‒PCR assay. The predicted hub networks are highlighted by red arrows. B. The candidate key genes responsive to stresses according to expression analysis and the potential network need to be further investigated
Overexpression of CuCIPK16 enhanced the cold tolerance of transgenic Arabidopsis
According to the expression profiles, 75% of CuCBL genes and 71% of CuCIPK genes exhibited significant transcriptional changes upon cold stress, implying their important function in the cold response of ‘Guijing2501’ satsuma mandarin. Among the cold-responsive genes, the expression level of CuCIPK16 was the highest under cold stress. Therefore, we cloned CuCIPK16 and overexpressed it in Arabidopsis for functional analysis. Three transgenic Arabidopsis lines at the T2 generation were selected for the cold tolerance assay. One-month-old Arabidopsis plants were subjected to 4 ℃ for seven days, followed by 0 ℃ treatment for one day and growth recovery at 21 ℃ for one day. As a result, the abaxial leaf surface of the wild type (WT) turned deep purple on a large scale and exhibited severe freezing damage, whereas the three transgenic lines remained green or turned only light purple over a small range and exhibited slight freezing damage (Fig. 9A). Malondialdehyde (MDA) content and peroxidase (POD) activity were further measured. The results revealed that the transgenic plants had significantly improved POD activity but decreased MDA content relative to the WT (Fig. 9B-9C). These results suggest that overexpression of CuCIPK16 enhanced the cold tolerance of transgenic Arabidopsis with higher POD activity and lower MDA content.
Comparison of phenotype (A), MDA content (B), and POD activity (C) between transgenic Arabidopsis lines and WT after cold treatment. For cold treatment, one-month-old Arabidopsis plants were subjected to 4 ℃ for seven days, followed by 0 ℃ treatment for one day and growth recovery at 21 ℃ for one day. Error bars in B and C indicate inferential statistics according to the SE. *indicates statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; Student’s t test) when compared to WT
Discussion
The evolution of CBLs and CIPKs in citrus is closely associated with their adaptive feature for cold and bacterial infection
Conserved domain characteristics, intron/exon structures and duplications in gene families are often taken as imprints of gene evolution. In this study, the CBL and CIPK gene families in citrus can be divided into an ancient clade and a recent clade according to their conserved domain characteristics, which is in accordance with a previous analysis [35]. Moreover, CIPKs in the ancient clade are intron rich, whereas CIPKs in the recent clade are intron less. These characteristics of CIPK genes were also found in Arabidopsis [35], grape, apple, pineapple, and pear [28,29,30,31]. These results suggest that intron gain and loss are essential to the evolution of the CIPK family and that intron number has decreased during evolution. More importantly, CuCBL2 and CuCBL3, which are in the ancient clade, share the same amino acid sites in the conserved domains. However, the CBL proteins in the recent clade harbour more polymorphic amino acid sites. For instance, CuCBL6, which is tandemly duplicated from CuCBL5, harbours a shortened PFPF motif that is critical for CBL phosphorylation by CIPKs [1]. Additionally, CuCBL6 and CuCBL8 harbour the N-myristoylation site, which is critical for protein membrane binding [1]. Interestingly, these two CBL genes were further identified as signalling network hubs in response to cold stress and/or Xcc infection in citrus. Meanwhile, CBLs in the recent clade frequently interact with CIPKs and function in multiple stresses in citrus. In addition, the duplication events of citrus CBLs and CIPKs only exist in the recent clades, and the duplication might also contribute to the adaptive feature of citrus CBLs and CIPKs for cold and bacterial infection. Taken together, the evolution of CBLs and CIPKs in citrus might be closely associated with their adaptive feature for cold and bacterial infection.
Specific expression patterns and stable transformation indicate the important roles of CBLs and CIPKs in the response to cold and bacterial infection in citrus
As the ubiquitous second messenger in plants, the increase in Ca2+ concentration is an early event during the perception of different types of stress signalling in plants [16, 36,37,38,39]. Ca2+ influx into the cytosol is also an early event during cold acclimatization, which can help plants increase freezing tolerance [36]. CBLs, one of the Ca2+ sensors, bind Ca2+ and interact with CIPKs to activate downstream targets that regulate specific biochemical processes and protect plants from stresses [16, 37, 38]. In tomato, the expression of SlCIPK genes was significantly upregulated in the early stages after 4 ℃ treatment [40]. In Arabidopsis, the expression of AtCBL1 and AtCIPK3, 7 was induced by cold, and disruption of AtCIPK3 altered the expression patterns of many stress marker genes under cold stress [16, 41]. In addition, overexpression of the apple MdCIPK6L gene remarkably enhanced the tolerance of transgenic Arabidopsis and apple to cold stress [42]. In this study, 75% of CuCBL and 71% of CuCIPK genes in ‘Guijing2501’ satsuma mandarin were significantly regulated by cold acclimatization (4 ℃ treatment). Among these cold-responsive genes, 67% of CuCBLs and 83% of CuCIPKs were significantly upregulated starting in the early stages of cold treatment. Interestingly, the expression level of all four upregulated CuCBLs at early stages peaked at 12 h and then decreased. However, the majority of cold-responsive CuCIPKs were upregulated starting from early stages until later stages. The expression profiles of CuCBLs and CuCIPKs imply that CuCBLs, as Ca2+ sensors and CuCIPK activators, might function earlier than CuCIPKs, while CuCIPKs, as downstream target activators, might function until later stages. CuCIPK16 was upregulated from early stages until later stages, and the peak expression level was the highest among the cold-responsive genes. Indeed, overexpression of CuCIPK16 enhanced the cold tolerance of transgenic Arabidopsis with higher POD activity and lower MDA content. Taken together, these cold-responsive CuCBL and CuCIPK genes may be functionally involved in the cold response of ‘Guijing2501’ satsuma mandarin, and their potential function in citrus needs to be further studied.
The Ca2+ signalling pathway is also involved in the early steps of plant‒pathogen interactions [39]. CBLs and CIPKs can usually positively regulate disease resistance. In rice, OsCIPK14/15 is critical for the microbe-associated molecular pattern-induced defence signalling pathway [43], and CBL2-CIPK31-AKT1L was confirmed as a new signalling pathway to promote resistance to blast disease by increasing K+ uptake [17]. In wheat, the TaCBL4-TaCIPK5 complex positively modulates resistance to Puccinia striiformis f. sp. tritici depending on ROS [18]. In pepper, CaCIPK1 was found to mediate defence against Phytophthora capsici [44]. In cassava, MaCBL1/9-MaCIPK23 was revealed to enhance the defence response to Xanthomonas axonopodis pv. Manihotis [19]. However, AtCIPK6 negatively regulates effector- and PAMP-triggered immunity responses to Pseudomonas syringae in Arabidopsis [45]. In this study, except CsCIPK15, which was substantially upregulated at 12 h after Xcc inoculation, almost all the CsCBL and CsCIPK genes in sweet orange remained at a stable expression level at the early stages after Xcc inoculation. However, most of the Xcc infection-responsive genes (including CsCIPK2, 3, 8, 12, and 16 and CsCBL6 and 8) were regulated at the later stage (12 d after Xcc inoculation). The possible reason might be the low Xcc inoculation concentration (104 cfu/ml), leading to a relatively long time for Xcc proliferation in the leaf cells; thus, the typical infection symptoms appeared until 12 d after Xcc inoculation. In addition, the majority of the CLas infection-responsive genes (including CsCIPK2, 8, 16, and 17 and CsCBL1 and 5) were significantly downregulated, while only the CsCBL8 gene was drastically upregulated. Whether these bacterial infection-responsive CsCBL and CsCIPK genes negatively or positively regulate the defence response of sweet orange still needs to be further studied.
Citrus potential CBL-CIPK signalling networks involved in response to cold and bacterial infection in citrus
CBL-CIPK signalling networks have been found to decode intracellular calcium signals and regulate responses to various abiotic and biotic stresses in plants [1, 35]. In our study, CBL6-CIPK8 was identified as the common signalling network in response to cold and Xcc infection, while CBL6/CBL8-CIPK14 was uniquely responsive to cold in citrus. Compared with Arabidopsis, CBL-CIPK interactive networks might be different in citrus. For instance, in Arabidopsis, approximately 43% of CBL-CIPK showed interactive relationships, as indicated by a previous study [35], whereas only 11% of CBL-CIPK showed interactions in citrus (Fig. 10). Meanwhile, ancient anc-AtCBL1/9 and anc-AtCBL2/3 could interact with 85% of AtCIPKs in Arabidopsis; however, their orthologous proteins in citrus (CuCBL7 and CuCBL3) had no interactive CuCIPKs (Fig. 10). In contrast, the recently evolved CuCBL1, 5, 6, and 8 could form 13 interactive pairs (accounting for 86.7% of the total) with CuCIPKs (Fig. 10). Furthermore, Cs(u)CIPK8 may interact with Cs(u)CBL6 and is involved in the response to cold stress, Xcc infection (this study) and AMF colonization [33] in citrus. However, its orthologues in Arabidopsis (AtCIPK6) cannot interact with AtCBL5 (orthologue of Cs(u)CBL6) but interact with AtCBL4 (orthologue of Cs(u)CBL8) and are involved in the response to low K+, salt stress and bacterial infection [13, 45, 46]. Moreover, CuCBL6 can interact with CuCIPK14 and play a role in response to cold stress in citrus; however, their Arabidopsis orthologues cannot interact with each other [35]. The interaction between CuCBL8 and CuCIPK14 is conserved since their Arabidopsis orthologues (AtCBL4 and AtCIPK1) can also interact with each other. However, CuCBL8-CuCIPK14 is involved in the response to cold stress in citrus, while the AtCBL4 gene is highly inducible by low K+ and salt stress, the expression of the AtCIPK1 gene was not affected by these stress signals in Arabidopsis [13, 14, 47]. Therefore, CBL-CIPK signalling networks in response to abiotic and biotic stresses are extremely complicated, and the potential mechanism in citrus needs to be further studied.
Comparison of the CBL-CIPK interaction patterns between citrus (A) and Arabidopsis (B). The red square indicates interaction, and the blue square indicates no interaction for the test CBL-CIPK pairs. The white square indicates that there are no citrus orthologous genes corresponding to Arabidopsis, and the conserved CBL-CIPK pairs shared by citrus and Arabidopsis are marked with “Y” in the squares. The genes in the brackets are the corresponding orthologous genes. A. The CBL-CIPK interaction patterns for citrus were constructed according to the yeast two-hybrid assay in this study. B. The ancestral interaction states of CBLs and CIPKs before the α duplication event in Arabidopsis were reconstructed according to a previous study [35]. anc- indicates ancestral gene, and the gene pairs duplicated from the recent α event in Arabidopsis are considered as one ancestral gene
Conclusions
Cold is a major abiotic stress for citrus in regions prone to unpredictable winter weather, while Huanglongbing (HLB) and citrus canker disease are two devastating bacterial diseases that affect global citrus production. The Ca2+-CBL-CIPK signalling network plays important roles in the response to abiotic and biotic stresses in plants. How does this signalling network function in response to cold and infection by CLas or Xcc in citrus? Here, genome-wide range analyses based on evolution, gene expression and protein‒protein interactions were conducted to identify the key CBLs, CIPKs and CBL-CIPK interaction pairs implicated in responses to the above three stresses in citrus. As a result, CBL1, 5, 6, and 8 and CIPK2, 8, 12, 15, 16, and 17 in citrus were identified as common key genes responsive to multiple stresses, including cold, Xcc infection and/or CLas infection, whereas CBL2/7/CIPK1/4/5/11/13/14 and CIPK3 were unique key genes responsive to cold and Xcc infection, respectively. Meanwhile, CBL6-CIPK8 was the common signalling network in response to cold and Xcc infection, while CBL6/CBL8-CIPK14 was uniquely responsive to cold in citrus. In addition, stable transformation of CuCIPK16 in Arabidopsis preliminarily verified its function in the cold response. The results from this comprehensive analysis may facilitate future functional characterization of individual citrus CBLs and CIPKs under specific stresses.
Materials and methods
Plant materials and stress treatment
The cold-tolerant satsuma mandarin (C. unshiu) variety ‘Guijing 2501’ was used as the material for cold treatment, and the Xcc-sensitive sweet orange (C. sinensis) variety ‘Taoye’ was used for Xcc inoculation. All plant materials employed for cold treatment and Xcc inoculation were kept at the Institute of Fruit and Tea, Hubei Academy of Agricultural Sciences, Wuhan, China. Healthy and uniform bud-grafting plants (2 years old) were grown in a greenhouse with a 16 h light/8 h dark photoperiod at 25 °C and routinely pruned to stimulate the growth of new leaves. For the cold acclimation assay, the ‘Guijing 2501’ satsuma mandarin plants were kept in a low-temperature (4 °C) growth chamber with a 16 h light/8 h dark photoperiod, and the leaves were sampled at certain time points (0 h/d, 3 h, 6 h, 12 h, 1 d, 4 d and 16 d). For the Xcc inoculation assay, fully expanded young leaves of ‘Taoye’ sweet orange were infiltrated with 104 cfu/ml Xcc bacterial suspension, and sterile distilled water infiltration inoculation was performed as the control assay. The inoculated plants were kept in a high-humidity growth chamber with a 16 h light/8 h dark photoperiod and 85% humidity at 28 °C, and the leaves were sampled at certain time points (3 h, 6 h, 12 h and 12 d after Xcc inoculation). The symptoms were observed after 12 days of inoculation. The CLas-infected leaves with typical HLB symptoms from the 15-year-old ‘Yuanfeng’ navel orange trees were collected from Wugang County of Hunan Province, and healthy leaves in the same orchard were collected as the control samples. The main vein and petiole of the leaves were harvested for further CLas titre identification and expression analysis. All the samples were immediately frozen in liquid nitrogen and stored at –80 °C until further qRT‒PCR.
Genome-wide identification and construction of phylogenetic trees for CBL and CIPK family genes in citrus
The reported CBL and CIPK protein sequences of Arabidopsis were downloaded from TAIR (http://www.Arabidopsis.org/) and then taken as queries to blast against Citrus sinensis V1.0 protein with identity > 90% and E-value < 1e–5 as cut-offs by the BLAST Tool in the citrus pangenome to breeding database (CPBD) (http://citrus.hzau.edu.cn/blast/query.php). The BLAST hits were further investigated and filtered manually based on the specific gene family motif features. EF-hand domains and PFPF motifs were used to validate the CsCBLs, and NAF/FISL motifs were used to validate the CsCIPKs. The genomic sequences of CsCBLs and CsCIPKs were employed to analyse the intron/exon structure by GSDS 2.0 [48]. The chromosomal locations of the CsCBLs and CsCIPKs were determined using TBtools software [49]. The CuCBL and CuCIPK genes with full-length CDSs were cloned by using cDNA from ‘Guijing 2501’ satsuma mandarin as the template. The protein sequences of the CuCBLs and CuCIPKs were deduced from the corresponding cloned nucleotide coding sequences, and the conserved domains/motifs were predicted by Pfam. The CBL and CIPK protein sequences of sweet orange, Physcomitrella patens and Selaginella moellendorffii were downloaded from CPBD (http://citrus.hzau.edu.cn) and PHYTOZOME v.11 (http://www.phytozome.net/), respectively. Multiple protein sequence alignments were then generated and trimmed using MEGA 7.0. A maximum likelihood phylogenetic tree was constructed based on the multiple sequence alignments, and 1000 bootstrap replicates were used to estimate the reliability of the tree topology by MEGA 7.0.
Expression pattern analysis of CBL and CIPK genes in different sweet orange tissues and under different stresses
The RPKM values of CsCBL and CsCIPK genes in the sweet orange callus, leaf, flower, and fruit were retrieved from the RNA-seq data in sweet orange genome database (http://citrus.hzau.edu.cn/orange/). Samples collected from the above stress treatments were subjected to qRT‒PCR analysis. Total RNA was extracted using an RNAprep Pure Plant Kit (Tiangen, China) following the manufacturer’s instructions. RNA integrity was tested on 1.0% agarose gels stained with ethidium bromide, and the RNA concentration was calculated by a NanoPhotometer-NP80 (Implen, Germany). First strand cDNA was synthesized using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, USA). qRT‒PCR was conducted using Applied Biosystems 7500 with PowerUP™ SYBR™ Green Master Mix (Thermo Scientific, USA). Specific primers for qRT‒PCR were designed by Primer Premier 5.0 software (Table S10). The specificity of the primers was further confirmed with a melting curve analysis after amplification of each tested gene. Each PCR pattern was verified using three biological replicates, and the experiment was repeated once. The Cs(u)EF1α gene was used as an internal reference gene to normalize the expression level. Samples before 4 °C treatment, infiltrated samples inoculated with sterile distilled water and samples confirmed without CLas infection were used as the internal controls for cold treatment, Xcc infection and CLas infection, respectively. The relative expression values were calculated by using the 2−∆∆CT method. Standard error (SE) showing inferential statistics was calculated by the formula “STDEV.S/SQRT (number)” by using Excel software. Statistical analysis was performed using Student’s t test. A bar graph overlaying all the individual data points was employed to show the relative expression level. Error bars are indicated in the bar graph according to the SE. The asterisk indicates statistical significance according to Student’s t test (*p < 0.05; **p < 0.01; ***p < 0.001).
Synteny analysis of the CBL and CIPK gene families
One Step MCScanX was conducted within the sweet orange genome using TBtools software [49]. E-values of 1e-10 and 5 of BlastHits were employed to filter the potential false gene duplication events. Advanced Circos in TBtools software was employed to display the synteny relationship of the orthologous CBL and CIPK genes within the sweet orange genome. The nucleotide coding sequences of CsCBL and CsCIPK genes were aligned using ClustalW 2.0, and then nonsynonymous substitution (Ka), synonymous substitution (Ks), and the Ka/Ks ratio were calculated using DNA Sequence Polymorphism v6.12.03 software.
Yeast two-hybrid assay
The Matchmaker gold yeast two-hybrid System (Clontech, USA) was used to identify protein interactions. The eight CuCBL genes and 17 CuCIPK genes with full-length CDSs were cloned by specific primers (Table S11) and inserted into the pGADT7 vector and pGBKT7 vector, respectively. Then, the CuCBL-AD and CuCIPK-BD plasmids were transformed into the yeast strains Y187 and Y2HGold, respectively. After one-to-one yeast mating and spreading on DDO (SD/–Leu/–Trp) agar plates, the positive clones were examined by PCR. Subsequently, the positive clones were incubated in YPDA medium at 30 °C for 1 day. Ten microlitres of 1/1 (OD600 = 0.6), 1/10, and 1/100 dilutions were dripped on DDO (SD/-Trp/-Leu) agar plates and selective medium QDO/X/A (SD/-Trp/-Leu/-His/-Ade/X-α-Gal/AbA, supplemented with 8 mg/mL X-α-Gal and 125 ng/mL aureobasidin A) for 3 days to test protein interactions.
Stable transformation of CuCIPK16 in Arabidopsis
The full-length CDS of CuCIPK16 was cloned using the primer CuCIPK16-OE-F/R and adapter primer attB1/attB2 (Table S11), inserted into the pDONR201 vector by Gateway BP Clonase II Enzyme Mix (Invitrogen, USA), and then ligated into the pK7YWG2 vector by Gateway LR Clonase II Enzyme Mix (Invitrogen, USA) following the manufacturer’s instructions. The constructs were verified by PCR and sequencing and then transformed into Agrobacterium tumefaciens strain GV3101. The floral dip method was employed for Arabidopsis transformation according to a previous study [50]. MS medium containing 50 μg ml–1 kanamycin was used to select the positive transformants, followed by genomic PCR and qRT‒PCR confirmation. Seeds of the transgenic lines were harvested until the T2 generation.
Cold tolerance verification and measurement of MDA content and POD activity
Three transgenic Arabidopsis lines were selected for the cold tolerance assay. Seeds of three transgenic lines at the T2 generation and the wild type (WT) were germinated on soil pots and kept in a growth chamber with a 16 h light/8 h dark photoperiod at 21 °C. Two weeks later, three seedlings of each transgenic line and WT were independently transferred to each soil pot. For cold treatment, one-month-old Arabidopsis plants were subjected to 4 ℃ for seven days, followed by 0 ℃ treatment for one day and growth recovery at 21 ℃ for one day. The leaves were collected after cold treatment for MDA content and POD activity measurement. The MDA content and POD activity were determined using the relevant detection kits (A003-1–2 for MDA, A084-3–1 for POD, Nanjing Jiancheng Bioengineering Institute, China) following the manufacturer’s instructions.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- CaM:
-
Calmodulin
- CML:
-
Calmodulin-like protein
- CBL:
-
Calcineurin B-like protein
- CDPK:
-
Calcium-dependent protein kinase
- CIPK:
-
CBL-interacting protein kinase
- PP2C:
-
Protein phosphatase 2C
- CLas:
-
Candidatus liberibacter asiaticus
- Xcc :
-
Xanthomonas citri subsp. citri
- HLB:
-
Huanglongbing
- Ka:
-
Non-synonymous substitution
- Ks:
-
Synonymous substitution
- Ka/Ks:
-
Non-synonymous/synonymous substitution ratio
- RPKM:
-
Reads per kilobase per million mapped reads
- WT:
-
Wild type
- OE:
-
Over-expression
- MDA:
-
Malondialdehyde
- POD:
-
Peroxidase
References
Ma X, Li QH, Yu YN, Qiao YM, ul Haq S, Gong ZH. The CBL-CIPK pathway in plant response to stress signals. Int J Mol Sci. 2020;21(16):5668.
Ranty B, Aldon D, Cotelle V, Galaud JP, Thuleau P, Mazars C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front Plant Sci. 2016;7:327.
Harmon AC, Gribskov M, Harper JF. CDPKs–a kinase for every Ca2+ signal? Trends Plant Sci. 2000;5(4):154–9.
Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22(3):541–63.
Kolukisaoglu U, Weinl S, Blazevic D, Batistic O, Kudla J. Calcium sensors and their interacting protein kinases: Genomics of the arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004;134(1):43–58.
Batistiˇc O, Kudla J. Plant calcineurin B-like proteins and their interacting protein kinases. BBA-Mol Cell Res. 2009;1793:985–92.
Hrabak EM, Dickmann LJ, Satterlee JS, Sussman MR. Characterization of eight new members of the calmodulin-like domain protein kinase gene family from Arabidopsis thaliana. Plant Mol Biol. 1996;31(2):405–12.
Guo Y, Halfter U, Ishitani M, Zhu JK. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell. 2001;13(6):1383–400.
Sánchez-Barrena MJ, Martínez-Ripoll M, Albert A. Structural biology of a major signaling network that regulates plant abiotic stress: The CBL-CIPK mediated pathway. Int J Mol Sci. 2013;14(3):5734–49.
Ohta M, Guo Y, Halfter U, Zhu JK. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Natl Acad Sci. 2003;100(20):11771–6.
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in arabidopsis. Cell. 2006;125(7):1347–60.
Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG, Lee SC, Kudla J, Luan S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in arabidopsis. Plant J. 2007;52(2):223–39.
Held K, Pascaud F, Eckert C, Gajdanowicz P, Hashimoto K, Corratgé-Faillie C, Offenborn JN, Lacombe B, Dreyer I, Thibaud JB. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011;21(7):1116–30.
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci. 2002;99(12):8436–41.
Quan R, Lin H, Mendoza I, Zhang Y, Cao W, Yang Y, Shang M, Chen S, Pardo JM, Guo Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect arabidopsis shoots from salt stress. Plant Cell. 2007;19(4):1415–31.
Huang C, Ding S, Zhang H, Du H, An L. CIPK7 is involved in cold response by interacting with CBL1 in arabidopsis thaliana. Plant Sci. 2011;181(1):57–64.
Lin QJ, Kumar V, Chu J, Li ZM, Wu XX, Dong H, Sun Q, Xuan YH. CBL-interacting protein kinase 31 regulates rice resistance to blast disease by modulating cellular potassium levels. Biochem Biophys Res Commun. 2021;563:23–30.
Liu P, Duan YH, Liu C, Xue QH, Guo J, Qi T, Kang ZS, Guo J. The calcium sensor TaCBL4 and its interacting protein TaCIPK5 are required for wheat resistance to stripe rust fungus. J Exp Bot. 2018;69(18):4443–57.
Yan Y, He XY, Hu W, Liu GY, Wang P, He CZ, Shi HT. Functional analysis of MeCIPK23 and MeCBL1/9 in cassava defense response against xanthomonas axonopodis pv. Manihotis. Plant Cell Rep. 2018;37(6):887–900.
Rieger M, Krewer G, Lewis P, Linton M, McClendon T. Field evaluation of cold hardy citrus in coastal Georgia. HortTechnol. 2003;13(3):540–4.
Ebel RC, Nesbitt M, Dozier WA, Dane F. Freeze risk and protection measures of satsuma mandarins grown in the southeastern United States. HortSci. 2008;43(2):287–9.
Inch S, Stover E, Driggers R, Lee RF. Freeze response of citrus and citrus-related genotypes in a Florida field planting. HortSci. 2014;49(8):1010–6.
He LG, Wang ZJ, Song F, Wu LM, Jiang YC, Peng JQ, Huang YM, Liu T. A novel citrus cultivar ‘Guijing 2501’ with cold tolerance. Acta Horticulturae Sin. 2021;48(S2):1–2.
Zheng Z, Chen JC, Deng XL. Historical perspectives, management, and current research of citrus HLB in Guangdong Province of China, Where the disease has been endemic for over a hundred years. Phytopathol. 2018;108(11):1224–36.
Zhou CY. The status of citrus Huanglongbing in China. Trop Plant Pathol. 2020;45(3):279–84.
Brown K. Florida fights to stop citrus canker. Sci. 2001;292(5525):2275–6.
Singh R, Hardy TN, Spitzer WE. Citrus canker: Another hurricane for Louisiana’s citrus industry. Phytopathol. 2014;104(5):9–9.
Xi Y, Liu J, Dong C, Cheng ZMM. The CBL and CIPK gene family in grapevine (Vitis vinifera): genome-wide analysis and expression profiles in response to various abiotic stresses. Front Plant Sci. 2017;8:978.
Niu L, Dong B, Song Z, Meng D, Fu Y. Genome-wide identification and characterization of CIPK family and analysis responses to various stresses in apple (Malus domestica). Int J Mol Sci. 2018;19(7):2131.
Aslam M, Fakher B, Jakada BH, Zhao L, Cao S, Cheng Y, Qin Y. Genome-wide identification and expression profiling of CBL-CIPK gene family in pineapple (Ananas comosus) and the role of AcCBL1 in abiotic and biotic stress response. Biomolecules. 2019;9(7):293.
Tang J, Lin J, Li H, Li X, Yang Q, Cheng ZM, Chang Y. Characterization of CIPK family in Asian pear (Pyrus bretschneideri Rehd) and co-expression analysis related to salt and osmotic stress responses. Front Plant Sci. 2016;7:1361.
Hu DG, Ma QJ, Sun CH, Sun MH, You CX, Hao YJ. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol Plant. 2016;156(2):201–14.
Shu B, Cai D, Zhang F, Zhang DJ, Liu CY, Wu QS, Luo C. Identifying citrus CBL and CIPK gene families and their expressions in response to drought and arbuscular mycorrhizal fungi colonization. Biol Plant. 2020;64:773–83.
Xu Q, Chen LL, Ruan X, Chen D, Zhu A, Chen C, Bertrand D, Jiao WB, Hao BH, Lyon MP. The draft genome of sweet orange (Citrus sinensis). Nat Genet. 2013;45(1):59.
Zhang XX, Li XX, Zhao R, Zhou Y, Jiao YN. Evolutionary strategies drive a balance of the interacting gene products for the CBL and CIPK gene families. New Phytol. 2020;226(5):1506–16.
Örvar BL, Sangwan V, Omann F, Dhindsa RS. Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 2000;23(6):785–94.
Zhang Y, Lv Y, Jahan N, Chen G, Ren D, Guo L. Sensing of abiotic stress and ionic stress responses in plants. Int J Mol Sci. 2018;19(11):3298.
Luan S. The CBL–CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14(1):37–42.
Thuleau P, Aldon D, Cotelle V, Brière C, Ranty B, Galaud J-P, Mazars C. Relationships between calcium and sphingolipid-dependent signalling pathways during the early steps of plant–pathogen interactions. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2013;1833(7):1590–4.
Zhang Y, Zhou Xn, Liu S, Yu A, Yang C, Chen X, Liu J, Wang A. Identification and functional analysis of tomato CIPK gene family. Int J Mol Sci. 2019;21(1):110.
Kim KN, Cheong YH, Grant JJ, Pandey GK, Luan S. CIPK3, a calcium sensor–associated protein kinase that regulates abscisic acid and cold signal transduction in arabidopsis. Plant Cell. 2003;15(2):411–23.
Wang RK, Li LL, Cao ZH, Zhao Q, Li M, Zhang LY, Hao YJ. Molecular cloning and functional characterization of a novel apple MdCIPK6L gene reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Plant Mol Biol. 2012;79(1–2):123–35.
Kurusu T, Hamada J, Nokajima H, Kitagawa Y, Kiyoduka M, Takahashi A, Hanamata S, Ohno R, Hayashi T, Okada K. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin b-like protein-interacting protein kinases, OsCIPK14/15. Rice Cultured Cells Plant Physiol. 2010;153(2):678–92.
Ma X, Gai WX, Qiao YM, Ali M, Wei AM, Luo DX, Li QH, Gong ZH. Identification of CBL and CIPK gene families and functional characterization of CaCIPK1 under Phytophthora capsici in pepper (Capsicum annuum L.). BMC Genomics. 2019;20(1):775.
Sardar A, Nandi AK, Chattopadhyay D. CBL-interacting protein kinase 6 negatively regulates immune response to Pseudomonas syringae in arabidopsis. J Exp Bot. 2017;68(13):3573–84.
Chen L, Wang QQ, Zhou L, Ren F, Li DD, Li XB. Arabidopsis CBL-interacting protein kinase (CIPK6) is involved in plant response to salt/osmotic stress and ABA. Mol Biol Rep. 2013;40(8):4759–67.
Shi JR, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J. Novel protein kinases associated with calcineurin B-like calcium sensors in arabidopsis. Plant Cell. 1999;11:2393–405.
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Chen C, Chen H, He Y, Xia R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv. Preprint. 2018. https://doi.org/10.1101/289660.
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1(2):641–6.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFD1000302), the National Natural Science Foundation of China (31701908), the Natural Science Foundation of Hubei Province (2019CFB601) and the Natural Science Foundation of Hubei Academy of Agricultural Sciences (2020NKYJJ13). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
XC and SZH conceived and designed the research. XC, ZH and XF performed the experiments. XC, ZH and XF analysed the data, WZQ, HXJ and XYH participated in data analysis. XC wrote the paper. PZY, QWM and TZ revised the paper. All authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments did not involve endangered or protected species. The data collection of plants was carried out with permission of related institution, and complied with national or international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Intron/exon structures and phylogenetic trees of the CsCBL and CsCIPK gene families. Figure S2. HLB-typical symptoms (A) and CLas titre quantification (B) for CLas-infected orange leaves and symptoms observed after 12 days of 104 cfu/ml Xcc inoculation for Xcc-infected orange leaves (C). Figure S3. 136 possible interaction sets for CuCBL and CuCIPK verified by yeast two-hybrid assay. Table S1. The full-length gene sequences of 8 CsCBL and 17 CsCIPK genes in sweet orange. Table S2-S5. Protein sequences of CBLs and CIPKs from sweet orange, ‘Guijing2501’ satsuma mandarin, Arabidopsis, Physcomitrella patens and Selaginella moellendorffii. Table S6-S8. Expression profiles of Cs(u)CBL and Cs(u)CIPK genes under cold stress, CLas infection and Xcc infection by qRT‒PCR. Table S9. One-to-one synteny relationships of the CBL or CIPK gene family within the sweet orange genome. Table S10-S11. Primer sequences used for qRT‒PCR, yeast two-hybrid assays and stable transformation.
Additional file 2: File S1-S2.
Multiple alignment of amino acid sequences of CuCBLs and CuCIPKs from ‘Guijing2501’ satsuma mandarin.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiao, C., Zhang, H., Xie, F. et al. Evolution, gene expression, and protein‒protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biol 22, 420 (2022). https://doi.org/10.1186/s12870-022-03809-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03809-0