Abstract
Background
Toxicity of silver nanoparticles (AgNP) has been studied frequently due to a rise in application in various products. Various studies on AgNP toxicity with terrestrial and aquatic organisms confirmed their negative effects. In our previous experiments, strong variability was observed in the reproduction of Collembola (Folsomia candida) in different repeats. To investigate the effects of silver on the reproduction of Folsomia candida, they were exposed in laboratory-controlled conditions to AgNP and silver nitrate (AgNO3) at a concentration of 30 mg/kg dry soil for 28 days and compared to controlled individuals not exposed to silver. We repeated reproduction tests on the toxicity of silver to Folsomia candida four times throughout one year (April, July, October and January) in order to explore the temporal variability of their outcome.
Results
While adult survival was similar in all treatments and seasons, reproduction in the control increased from April to October. Significantly lower reproduction was found in January with only 385–424 juveniles per vessel, compared to 504–633 individuals in other months. Strong toxic effects of both silver treatments were observed in July, April and October. However, AgNP showed no toxic effects on the reproduction of F. candida in January. The relative toxicity of both substances varied between single experiments: AgNP were more toxic than AgNO3 in April and July, and less toxic in October and January.
Conclusion
These findings indicate that the reproduction of F. candida in the control had a significant effect on the results of the toxicology experiments. Moreover, we demonstrated the reproductive toxicity of AgNP in soil at a much lower concentration than reported thus far. Therefore, to guarantee reliability and reproducibility, we recommend to disregard any test results where the reproduction rate of F. candida in the control is significantly different from the average in the respective laboratory, even if the validity criteria of the test are met.
Similar content being viewed by others
Background
The application of silver nanoparticles (AgNP) is strongly increasing in several areas such as electrical, medicine, food and textile products. AgNP can be released from these products during washing [1], disposal, and via industry wastewater [2, 3] to the environment. Up to 90% of Ag remains in sewage sludge in which the estimated Ag annual increase is 1.6 µg/kg [4, 5]. There are concerns about unintended exposure of humans and the environment to AgNP [6], resulting in a large research effort into the hazards and behavior of AgNP in the environment [7]. Numerous aquatic toxicity tests with AgNP and silver ions using a wide range of species have resulted in classifying AgNP as “particularly toxic” [8,9,10]. Nano-sized particles may pass through cell membranes, and the accumulation of intracellular nanoparticles can lead to cell malfunction, and the toxicity is presumed to be size and shape dependent [11]. A combined effect of Ag+ and nano-size was illustrated for the discrepancy in toxicity pattern of AgNP compared to AgNO3 [12,13,14]. Research focusing on the terrestrial matrix has shown that soil pH, texture, organic matter, and ionic composition can affect the toxicity of AgNP to soil invertebrates [15,16,17]. Negative effects of AgNP were shown for reproduction, survival and growth of nematodes [18,19,20], earthworms [20,21,22,23,24] and enchytraeids [24]. The toxicity of AgNP for Collembola was first reported by Waalewijn-Kool et al. [25], who observed no effect on survival and reproduction for Folsomia candida (F. candida) exposed to AgNP (3–8 nm coated with paraffin) at a measured concentration of 673 mg Ag/kg dry soil. Mendes et al. [26] reported a negative effect of AgNP (NM-300 K) on the reproduction of F. candida, with EC20 and EC50 values of 173 and 540 mg Ag/kg, respectively.
F. candida Willem 1902 is a common Collembola species that is present in soils all over the world and has been used for ecotoxicological testing in numerous publications [27,28,29,30,31,32,33,34]. Series of tests are conducted to examine mortality, reproduction, bioaccumulation, and effects on the behavior of F. candida to evaluate the toxicity of organic and inorganic contaminants. Filser et al. [35] introduced a miniaturized reproduction test of F. candida according to the OECD 232 (2009) [36] standard reproduction test in which 4 adults and 10-g soil were used instead of 10 adults and 30-g soil.
Based on our years of experience with F. candida, we have found that its reproduction decreases significantly at certain times of the year, particularly in winter. Does this difference in reproduction affect the results of the toxicity test? To test the accuracy and reproducibility of the test, we evaluated the toxicity of AgNP and AgNO3 on the reproduction of F. candida throughout one year. We hypothesized that (a) the reproduction of F. candida is different between equally repeated reproduction experiments and (b) the toxic effects of AgNP and AgNO3 vary between repeats.
Materials and methods
Chemicals
AgNO3 (purity 99.0%, Sigma-Aldrich, Steinheim, Germany) was used to provide a reference for dissolved silver (Ag+) toxicity. AgNP were NM-300 K, a representative manufactured dispersion containing uncoated spherical nanoparticles (diameter: 15 nm). NM-300 K has been used in a variety of studies and projects, and was included in the OECD Working Party on Manufactured Nanomaterials sponsorship program. As a dispersion in stabilizing agents, NM-300K contains 4% w/w each of polyoxyethylene glycerol trioleate and polyoxyethylene (20) sorbitan monolaurate (Tween 20) with a silver content of 10.16% by weight [37]. It was distributed by the Fraunhofer Institute for Molecular Biology and Applied Ecology (IME) and provided by Joint Research Centre of the European Commission as a part of the UMSICHT project (BMBF 0340091A).
Test soil
RefeSol soils were selected as reference soils by the German Federal Environment Agency, and they matched the properties stated in various OECD terrestrial ecotoxicological guidelines. In this study, we used RefeSol 01-A (provided by the Fraunhofer IME, Schmallenberg, Germany), a loamy sand soil with a pH of 5.67, 0.93% organic carbon, 71% sand, 24% silt, and 5% clay.
Soil preparation and reproduction test
The reproduction test was carried out following a miniaturized version of OECD 232 [35]. F. candida was taken from our lab culture, originally obtained from the working group of Professor Achazi at Freie Universität Berlin in the early 1990s. As the initial difference in age and size may affect the observed difference in reproduction, controlling the age of the individuals is important [38, 39]. To synchronize F. candida, adults were placed in a breeding container for three days to lay eggs and then were removed. After hatching, four 9–12-day-old juveniles of similar size were placed randomly in each vessel with 10 g test soil. The vessels were incubated in a climate chamber (Sanyo MLR-350H) at 20 °C with a 12-hour light/12-h dark cycle with 80% humidity and 500 Lux illumination. During the test, 5 pieces of dried baker’s yeast (Dr. Oetker) were added to the animals twice a week, and the old food bunches were removed. The test vessels were aerated twice a week, and moisture content of the soil was kept constant at 50% of the maximum water holding capacity by replenishing the water loss once a week. After 28 days of exposure, 100-mL deionized water was added to each test container, and the soil was transferred to a plastic container. F. candida floating on the surface of the dispersion were visible after adding two drops of ink to the water. A picture was taken of each container, to count the juveniles and adults using Image J 1.46r software package.
Stock solutions of AgNO3 and AgNP were prepared by diluting both substances with deionized water. The flasks with stock dispersions of AgNP were placed in an ultrasonic bath (Bandelin sonorex RK 100H with an output of 160 W, 35 kHz) and sonicated for 20 min before use. After the dispersions and solutions were prepared, they were added to the soil to obtain a concentration of 30 mg Ag/kg dry soil. To obtain a homogeneous distribution, the test substance solution was first added to a small portion of the soil (20 g), which then was mixed to the final test soil thoroughly with a spoon. All soil samples were adjusted to 50% of the maximum water holding capacity and thoroughly mixed. Additionally, a control without any silver chemicals was included in each study. For each treatment and control, 6 replicate glass vessels (30 mL) were filled with 10 g prepared soil 1 day before the test began. For each test treatment and control, three replicate soil samples were analyzed for pH (Jürgens, WTW, Weilheim, Germany) at the end of the reproduction test. To test the accuracy and reproducibility of the experiment, the reproduction test was repeated four times: in the first week of April, the second week of July, the first week of October and the second week of January of the following year.
Statistics
Statistical analyses were performed with SPSS 23.0. Normality of the data was analyzed untransformed according to Shapiro–Wilk test (p > 0.05), albeit a minor deviation of normality in the controls in April and July (p = 0.048 and p = 0.032, respectively). Variance homogeneity was analyzed according to Levene’s test (p = 0.164). A general linear model (Two-way ANOVA) was used to analyze the main and interaction effects of treatment and month as influencing factors; pairwise comparison (Bonferroni) was used to show significant differences between single months or treatments, respectively. All tests were run with and without outliers; the outliers did not change the results of significance. Therefore, outliers were included in all final results.
Results
Reproduction in different treatments during four repeats
The mean soil \({\text{pH}}_{{\text{CaCl}}_{2}}\) was 5.59 (SD = 0.39) in the control, 5.57 (SD = 0.45) in soil spiked with AgNP and 5.44 in soil spiked with AgNO3 (SD = 0.46). No significant differences were detected during the four experiments between treatments and control, nor between the single experiments.
All reproduction tests met the validity criteria according to OECD guideline 232. The mean of adults’ mortality in control did not exceed 20%, and did not differ between the treatments (p = 0.628). The mean number of juveniles per vessel in control was not lower than 100 for each replicate control (n = 6), and the coefficient of variation of reproduction was less than 30% (Additional file 1: Table S1).
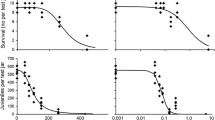
Collembola reproduction significantly differed between repeats. Figure 1 shows the number of F. candida juveniles in the AgNP treatment, AgNO3 and control in four test repeats. Only the reproduction with AgNO3 did not differ between repeats (Additional file 1: Table S2 in Appendix). The reproduction in the control was lowest in January and highest in October. In the AgNP treatment, the highest reproduction was detected in October and the lowest in July (Additional file 1: Table S1 in Appendix). The comparison between treatments in single repeats is shown in Fig. 2.
Number of F. candida juveniles in the control, AgNO3 and AgNP treatment during four test repeats. The band inside the box is the median. The top and bottom of the box are the first and third quartiles with the ends of the minimum and maximum including outliers (n = 6). Circle: outlier (> 3/2 times of the upper or lower quartile). Values followed by the same letters do not differ significantly within the same treatment (p < 0.05) by using pairwise comparisons (Bonferroni) of original data
F. candida reproduction in soil spiked with AgNP and AgNO3 during four repeats. Mean values of juveniles (a), Adults’ size and retrieved adults (b) ± SE, (n = 6) are shown. Asterisks indicate statistically significant differences of original data to controls and asterisks with line show statistically significant between AgNO3 and AgNP (*p ≤ 0.05, **p < 0.01 **p ≤ 0.001)
Toxicity of AgNP and AgNO3 during four repeats
The effect of treatment and month as impact factors is significant, and they have an interaction effect (Additional file 1: Table S4 in the Appendix). Figure 2 displays toxic effects of AgNP and AgNO3 compared to the control. Neither the retrieved adults nor the adult’s size differs significantly between treatments and repeats (p > 0.05, Additional file 1: Table sS5, S6 in Appendix). Compared to the control, the reproduction of F. candida was significantly reduced in April, July and October in the AgNP treatment (p < 0.05), but not in January (p = 0.736), and during all tests in the AgNO3 treatment (p < 0.05, Fig. 2, Additional file 1: Table S2 in the Appendix). AgNP were (1) less toxic for the reproduction of F. candida than AgNO3 in October (p < 0.05), (2) but the same as AgNO3 in toxicity in other months (p < 0.05, Fig. 2, Additional file 1: Table S3 in Appendix).
Discussion
We investigated the toxicity of AgNP and AgNO3 to F. candida in four repeats during 1 year. For the first time, strong toxic effects of AgNP at a concentration of 30 mg Ag/kg on the reproduction of F. candida were observed. This is in contrast to Waalewijn-Kool et al. [25] who found no effect on survival and reproduction for F. candida exposed to AgNP at a measured concentration of 673 mg Ag/kg dry soil, which was more than 20 times higher than the concentration in our study. Mendes et al. [26] found that NM-300K reduced F. candida reproduction by about 50%, yet at a concentration of 640 mg Ag/kg soil. Mainly three factors may explain the differences between these studies: (1) Waalewijn-Kool et al. [25] used paraffin-coated AgNP in a water-only dispersion, while NM-300 K are uncoated and dispersed in a suspension that contains three organic agents; (2) The size of AgNP used by Waalewijn-Kool et al. was 3–8 nm AgNP, whereas NM-300K has a diameter of 15 nm.; (3) Loamy sand soil (LUFA-Speyer 2.2, Sp 2121, Germany, 2009) with a \({\text{pH}}_{{\text{CaCl}}_{2}}\) of 5.5 was used by [25] and [26], whereas we used RefeSol 01A, a loamy, medium-acidic, and lightly humic sand with \({\text{pH}}_{{\text{CaCl}}_{2}}\) of 5.67.
For NM-300K, an effect of the organic dispersion can be excluded, because tests had been made in advance to ensure that the dispersion showed no toxic effect of on the reproduction of F. candida (X. Zhang, unpublished data). McKee et al. studied the dispersion of NM-300K in OECD soil pore water and found that the dispersion caused significant immobilization of F. candida at 10 mg/l, whereas no toxic effect occurred at 40 mg/l [40]. This is in line with our findings. Second (except for differences in release kinetics, see Engelke et al.), particle size can also be excluded as nanoparticle reactivity increases with decreasing size [41]. Therefore, coating and soil type might be the main reasons for the fate and toxicity of the particles found in our study. The presence of a coating is important because it can modify the particle structure, the electrostatic surface charge and, therefore, its potential toxicity over time [42]. Nguyen et al. [43], for instance, found considerable differences in toxicity between AgNP coated with citrate and polyvinylpyrrolidone and uncoated AgNP to macrophages and epithelial cells. They reported that uncoated AgNP, at a concentration of 1 µg/ml, decreased cell viability by 20–40% and that 20- and 40-nm particles were 10% more cytotoxic than the 60- and 80-nm particles. In exposures to coated AgNPs, cell viability dropped at 25 µg Ag/ml or higher concentrations. Similar coating effects were observed with ZnO-NPs and F. candida [44] and with iron oxide nanoparticles and mouse fibroblast cells [45]. There is strong support for the assumption that the different soil types were the main reason for the large difference in toxicity between our study and the one by Mendes et al. [26], as various studies in our lab with Collembola (McKee et al. [40]) and enchytraeids (Voua Otomo et al. under revision) have rendered much stronger toxic effects of AgNP in RefeSol 01A than in Lufa 2.2 and artificial OECD soil. In fact, it is challenging to evaluate the results of different studies on F. candida if different clonal lineages were used in the different studies. A large number of studies have shown that different lineages of F. candida can exhibit very different life history traits, and that they also differ genetically in their ability to cope with environmental stress [39, 46,47,48].
In the present study, the toxicity of AgNP varied significantly between repeats. Significant toxic effects of AgNP on reproduction were observed in April, July and October, but not in January, while AgNO3 caused toxic effects during all repeats. The reproduction of F. candida in the control in January was 19.5–35.9% lower than in July and October. On the other hand, in the soil spiked with AgNP, the reproduction of F. candida in January was even higher than in July. In the following, we discuss four possible explanations: fungi compromising Collembola reproduction by defensive strategies or being entomopathogenic, differences in dissolution kinetics of AgNP and AgNO3, avoidance behavior and circannual biological rhythms.
Fungi observed in our test vessels during winter might account for the reduction of the reproduction of F. candida in January. It is possible that spores from fungi emitted in autumn or winter are brought into the laboratory by the ventilation system. We speculate that these fungi might have defence properties (toxins or crystals at the hyphal surface) that inhibited reproduction of F.candida [49, 50]. An alternative explanation could be Entomopathogenic Fungi (EPF), although not all of them are toxic to Collembola [51, 52]. Outbreaks of infection with entomopathogens such as Entomophthora muscae tend to occur in spring and autumn, and sporulation usually takes place in cool, humid conditions [53]; it might explain why we observed fungi in our test vessels in January and the reproduction of F. candida was significantly decreased in control. Interestingly, there was no significant reduction in reproduction in January in the soil treated with AgNP (Fig. 1), most likely due to their continuous antimicrobial activity [54, 55]. On the one hand, AgNP are capable of inhibiting fungi that compromise the reproduction of F. candida; on the other hand, the direct negative effects of silver on the Collembola would partly be masked by the indirect positive effect through its suppressing effect on such fungi.
In January, the reproduction was significantly lower in the treatment of AgNO3, but there was no difference in the treatment of AgNP compared to control (Fig. 2). We postulate that the different performance of both Ag forms is due to their reaction kinetics. AgNO3 dissociates readily in water, but only part of the Ag+ ions are bioavailable: they will react with anions in the soil solution, forming insoluble precipitates, or complexes with organic acids. In turn, AgNP dissolve slowly, constantly releasing new Ag+ ions. Therefore, over a longer period, it is likely that more Ag+ is bioavailable from AgNP than from AgNO3.
But what are the reasons for those differences between repeats? The hypothetical model in Fig. 3 illustrates why the treatment with AgNO3 had a negative effect on F. candida in January, not the one with AgNP: The presumed contamination with fungal spores should have been present in low numbers at the beginning of January, then might increase due to favorable conditions and decrease again in spring due to increasing temperature. The release of dissolved Ag+ upon adding AgNP to moist soil provides a low, but constant supply of Ag+ ions. The low Ag+ concentration should be sufficient to control the small initial fungi population in winter and to prevent their further increase, thus reducing the negative effect of fungi on the reproduction of F. candida. With AgNO3, the sudden release of dissolved Ag+ upon adding AgNO3 to moist soil would kill most of the present fungi, but the population might quickly recover thereafter (Fig. 3).
Hypothetical model on the development of deleterious fungi in winter and spring in the different treatments as affected by the released Ag+ ions. There is a slow and continuous ion release from AgNP, whereas AgNO3 ions dissolve at test start. Numbers indicate different phases on fungi populations in the single treatments and tests: (1) Efficient control of the originally small population by continuous Ag+ ion release; (2) High mortality and exponential recovery due to high growth rate during winter
Some studies explained the difference in toxicity between AgNP and AgNO3 by a release of Ag+ from the particles and by a slower assimilation of AgNP, which leads to lower toxic effects on soil fauna compared with AgNO3 [56,57,58]. Such differences in toxicity were also reported in studies with earthworms [21, 22]. Similar results were observed in our study during autumn and winter. Stronger toxic effects were found in the treatment with AgNO3 than that with AgNP, which supports the ion release theory. However, the pattern was reversed in the repeats in April and July. We believe this is a combination of Ag+ release kinetics (see above) and avoidance behavior. Avoidance studies in our laboratory gave hints that F. candida and enchytraeids avoid high, but not low concentration of Ag. Assuming that they sense rather the ions than the undissolved metal, it is possible that they actively avoided (e.g., by staying mostly at the uncontaminated food patch on the surface) only the AgNO3 treatment but not the one with AgNP in our study. Thus, in the AgNP treatment, the animals were exposed to low concentrations of Ag+ permanently released by the AgNP, reducing their reproduction.
Circannual biological rhythms might be another explanation for the different toxicity results. Rozen collected earthworms (Dendrobaena octaedra) from the field and cultured them in the laboratory under constant conditions. The author found that reproduction was highest in spring and summer, and dropped significantly in the winter months, which indicated that internal regulation of reproduction may exist in the earthworm D. octaedra [59]. However, the mechanisms have not yet been understood. Nevertheless, we cannot fully elucidate what exactly caused the toxicity of AgNP in the present study. The hypothesis on the interaction with fungi should be tested in further investigations, to identify the fungi species present during winter.
Krogh summarized data of reproduction tests using F. candida and Folsomia fimetaria from 1994 to 1999 and found that the variability of reproduction in the control is obvious and many factors contribute to the variability [60]. To what extent should we trust these data in the face of different experimental results? By comparing the data from our four repeated experiments, we suggest that F. candida in each laboratory should have a database of average reproduction rate in the control, and this database should also contain information on metadata such as different test soils or strains. To increase the reliability of the experimental results beyond established validity criteria, we suggest that the test results should be disregarded when the reproduction rate in control is significantly different from the average reproduction rate established in the laboratory.
Conclusions
We demonstrated for the first time that AgNP in natural soil can have strong toxic effects on the reproduction of F. candida at a concentration of only 30 mg Ag/kg, which is about 20 times lower than reported earlier. These effects can mainly be attributed to soil conditions (compared to Lufa 2.2 and artificial OECD soil). This is the first article reporting significantly different results of repeated toxicological experiments using F. candida. Although no clear explanations for the different performances during four repeats were found, a data bank of average reproduction rate of F. candida and other species is recommended to give comprehensive results in further toxicological tests. To corroborate our hypothetical model on the different outcome in January and April, studies specifically addressing ion release kinetics of AgNP and fungi identification are needed. Furthermore, more studies of F. candida’s avoidance behavior should be taken into account as well.
Availability of data and materials
The datasets used during this study are available from the corresponding author, the materials (pictures, raw data) from the first author—both upon reasonable request.
Abbreviations
- AgNP:
-
Silver nanoparticles
- AgNO3 :
-
Silver nitrate
- IME:
-
Institute for Molecular Biology and Applied Ecology
- GLM:
-
General linear model
- EPF:
-
Entomopathogenic fungi
References
Benn T, Cavanagh B, Hristovski K et al (2010) The Release of Nanosilver from Consumer Products Used in the Home. J Environ Qual 39(6):1875–1882. https://doi.org/10.2134/Jeq2009.0363
Yin Y, Yu S, Yang X et al (2015) Source and pathway of silver nanoparticles to the environment. In: Liu J, Jiang G (eds) Silver nanoparticles in the environment. Springer, Berlin Heidelberg, pp 43–72. https://doi.org/10.1007/978-3-662-46070-2_3
Tortella GR, Rubilar O, Durán N et al (2020) Silver nanoparticles: toxicity in model organisms as an overview of its hazard for human health and the environment. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121974
Engelke M, Koser J, Hackmann S et al (2014) A miniaturized solid contact test with arthrobacter globiformis for the assessment of the environmental impact of silver nanoparticles. Environ Toxicol Chem 33(5):1142–1147
Gottschalk F, Sonderer T, Scholz RW et al (2009) Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ Sci Technol 43(24):9216–9222. https://doi.org/10.1021/Es9015553
Calzolai L, Gilliland D, Rossi F (2012) Measuring nanoparticles size distribution in food and consumer products: a review. Food Addit Contam Part A Chem Anal Control Expos Risk Assess 29(8):1183–1193. https://doi.org/10.1080/19440049.2012.689777
Arnaout CL, Gunsch CK (2012) Impacts of silver nanoparticle coating on the nitrification potential of Nitrosomonas europaea. Environ Sci Technol 46(10):5387–5395
Ribeiro F, Gallego-Urrea JA, Jurkschat K et al (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466–467:232–241. https://doi.org/10.1016/j.scitotenv.2013.06.101
Angel BM, Batley GE, Jarolimek CV et al (2013) The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 93(2):359–365. https://doi.org/10.1016/j.chemosphere.2013.04.096
McGillicuddy E, Murray I, Kavanagh S et al (2017) Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ 575:231–246. https://doi.org/10.1016/j.scitotenv.2016.10.041
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microb 73(6):1712–1720. https://doi.org/10.1128/Aem.02218-06
Kloepfer JA, Mielke RE, Nadeau JL (2005) Uptake of CdSe and CdSe/ZnS quantum dots into bacteria via purine-dependent mechanisms. Appl Environ Microb 71(5):2548–2557. https://doi.org/10.1128/Aem.71.5.2548-2557.2005
Loo SL, Krantz WB, Fane AG et al (2015) Effect of synthesis routes on the properties and bactericidal activity of cryogels incorporated with silver nanoparticles. Rsc Adv 5(55):44626–44635. https://doi.org/10.1039/c5ra08449k
Morones JR, Elechiguerra JL, Camacho A et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
McKee MS, Filser J (2016) Impacts of metal-based engineered nanomaterials on soil communities. Environ Sci Nano 3(3):506–533. https://doi.org/10.1039/C6EN00007J
Topuz E, van Gestel CAM (2017) The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus. Ecotoxicol Environ Saf 144:330–337. https://doi.org/10.1016/j.ecoenv.2017.06.037
McKee MS, Engelke M, Zhang X et al (2017) Collembola reproduction decreases with aging of silver nanoparticles in a sewage sludge-treated soil. 5(19). https://doi.org/10.3389/fenvs.2017.00019
Meyer JN, Lord CA, Yang XYY et al (2010) Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans. Aquat Toxicol 100(2):140–150. https://doi.org/10.1016/j.aquatox.2010.07.016
Roh JY, Sim SJ, Yi J et al (2009) Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Technol 43(10):3933–3940. https://doi.org/10.1021/Es803477u
Patricia CS, Nerea G-V, Erik U et al (2017) Responses to silver nanoparticles and silver nitrate in a battery of biomarkers measured in coelomocytes and in target tissues of Eisenia fetida earthworms. Ecotoxicol Environ Saf 141:57–63. https://doi.org/10.1016/j.ecoenv.2017.03.008
Heckmann LH, Hovgaard MB, Sutherland DS et al (2011) Limit-test toxicity screening of selected inorganic nanoparticles to the earthworm Eisenia fetida. Ecotoxicology 20(1):226–233. https://doi.org/10.1007/s10646-010-0574-0
Shoults-Wilson WA, Zhurbich OI, McNear DH et al (2011) Evidence for avoidance of Ag nanoparticles by earthworms (Eisenia fetida). Ecotoxicology 20(2):385–396. https://doi.org/10.1007/s10646-010-0590-0
Schlich K, Klawonn T, Terytze K et al (2013) Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test. Environ Toxicol Chem 32(1):181–188. https://doi.org/10.1002/Etc.2030
Bicho RC, Ribeiro T, Rodrigues NP et al (2016) Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus. J Hazard Mater 318:608–614. https://doi.org/10.1016/j.jhazmat.2016.07.040
Waalewijn-Kool P, Klein K, Forniés R et al (2014) Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida. Ecotoxicology 23(9):1629–1637. https://doi.org/10.1007/s10646-014-1302-y
Mendes LA, Maria VL, Scott-Fordsmand JJ et al (2015) Ag nanoparticles (Ag NM300K) in the terrestrial environment: effects at population and cellular level in Folsomia candida (Collembola). Int J Environ Res Public Health 12(10):12530–12542. https://doi.org/10.3390/ijerph121012530
Zortea T, Segat JC, Maccari AP et al (2017) Toxicity of four veterinary pharmaceuticals on the survival and reproduction of Folsomia candida in tropical soils. Chemosphere 173:460–465. https://doi.org/10.1016/j.chemosphere.2017.01.069
Fountain MT, Hopkin SP (2005) Folsomia candida (Collembola): a “standard” soil arthropod. Annu Rev Entomol 50:201–222. https://doi.org/10.1146/annurev.ento.50.071803.130331
Fountain MT, Hopkin SP (2004) A comparative study of the effects of metal contamination on Collembola in the field and in the laboratory. Ecotoxicology 13(6):573–587
Giordano R, Weber E, Waite J et al (2010) Effect of a high dose of three antibiotics on the reproduction of a parthenogenetic strain of Folsomia candida (Isotomidae: Collembola). Environ Entomol 39(4):1170–1177
Holmstrup M, Aubail A, Damgaard C (2008) Exposure to mercury reduces cold tolerance in the springtail Folsomia candida. Compar Biochem Physiol C Toxicol Pharmacol 148(2):172–177. https://doi.org/10.1016/j.cbpc.2008.05.003
Kaneda S, Kaneko N (2004) Growth of the collembolan Folsomia candida Willem in soil supplemented with glucose. Pedobiologia 48(2):165–170. https://doi.org/10.1016/j.pedrobi.2003.12.002
Krogh PH (1995) Does a heterogeneous distribution of food or pesticide affect the outcome of toxicity tests with Collembola? Ecotoxicol Environ Saf 30(2):158–163. https://doi.org/10.1006/eesa.1995.1020
Lock K, Janssen CR (2002) Ecotoxicity of nickel to Eisenia fetida, Enchytraeus albidus and Folsomia candida. Chemosphere 46(2):197–200. https://doi.org/10.1016/S0045-6535(01)00112-6
Filser J, Wiegmann S, Schröder B (2014) Collembola in ecotoxicology—Any news or just boring routine? Appl Soil Ecol 83:193–199. https://doi.org/10.1016/j.apsoil.2013.07.007
OECD (2009) Test No 232 Collembolan reproduction test in soil. OECD Publishing, Paris
Klein CL, Comero S, Stahlmecke B et al NM-300 silver characterisation, stability, homogeneity; Publications Office of the European Union: https://doi.org/10.2788/23079
Le Bourlot V, Tully T, Claessen D (2014) Interference versus exploitative competition in the regulation of size-structured populations. Am Nat 184(5):609–623. https://doi.org/10.1086/678083
Mallard F, Le Bourlot V, Le Coeur C et al (2020) From individuals to populations: how intraspecific competition shapes thermal reaction norms. Funct Ecol 34(3):669–683. https://doi.org/10.1111/1365-2435.13516
McKee MS, Köser J, Focke O et al (2019) A new test system for unraveling the effects of soil components on the uptake and toxicity of silver nanoparticles (NM-300K) in simulated pore water. Sci Total Environ 673:613–621. https://doi.org/10.1016/j.scitotenv.2019.03.493
Borak J (2009) Nanotoxicology: characterization, dosing, and health effects. J Occup Environ Med 51(5):620–621
Tourinho PS, van Gestel CA, Lofts S et al (2012) Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ Toxicol Chem 31(8):1679–1692. https://doi.org/10.1002/etc.1880
Nguyen KC, Seligy VL, Massarsky A et al (2013) Comparison of toxicity of uncoated and coated silver nanoparticles. J Phys Conf Ser 429:012025. https://doi.org/10.1088/1742-6596/429/1/012025
Waalewijn-Kool PL, Diez Ortiz M, van Straalen NM et al (2013) Sorption, dissolution and pH determine the long-term equilibration and toxicity of coated and uncoated ZnO nanoparticles in soil. Environ Pollut 178:59–64. https://doi.org/10.1016/j.envpol.2013.03.003
Mahmoudi M, Simchi A, Imani M (2009) Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J Phys Chem C 113(22):9573–9580. https://doi.org/10.1021/Jp9001516
Tully T, Ferrière R (2008) Reproductive flexibility: genetic variation, genetic costs and long-term evolution in a collembola. PLoS ONE 3(9):e3207–e3207. https://doi.org/10.1371/journal.pone.0003207
Mallard F, Farina M, Tully T (2015) Within-species variation in long-term trajectories of growth, fecundity and mortality in the Collembola Folsomia candida. 28(12): 2275–2284. https://doi.org/10.1111/jeb.12752
Nota B, de Korte M, Ylstra B et al (2013) Genetic variation in parthenogenetic Collembolans is associated with differences in fitness and cadmium-induced transcriptome responses. Environ Sci Technol 47(2):1155–1162. https://doi.org/10.1021/es303983z
Bollmann J, Elmer M, Wollecke J et al (2010) Defensive strategies of soil fungi to prevent grazing by Folsomia candida (Collembola). Pedobiologia 53(2):107–114. https://doi.org/10.1016/j.pedobi.2009.06.003
Staaden S, Milcu A, Rohlfs M et al (2010) Fungal toxins affect the fitness and stable isotope fractionation of Collembola. Soil Biol Biochem 42(10):1766–1773. https://doi.org/10.1016/j.soilbio.2010.06.014
Dromph KM, Vestergaard S (2002) Pathogenicity and attractiveness of entomopathogenic hyphomycete fungi to collembolans. Appl Soil Ecol 21(3):197–210. https://doi.org/10.1016/S0929-1393(02)00092-6
Broza M, Pereira RM, Stimac JL (2001) The nonsusceptibility of soil Collembola to insect pathogens and their potential as scavengers of microbial pesticides. Pedobiologia 45(6):523–534. https://doi.org/10.1078/0031-4056-00104
Watson DW, Petersen JJ (1993) Seasonal activity of entomophthora-muscae (Zygomycetes, Entomophthorales) in Musca-Domestica L. (Diptera, Muscidae) with reference to temperature and relative-humidity. Biol Control 3(3):182–190. https://doi.org/10.1006/bcon.1993.1026
Krishnaraj C, Ramachandran R, Mohan K et al (2012) Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta Part A Mol Biomol Spectrosc 93:95–99. https://doi.org/10.1016/j.saa.2012.03.002
Kim SW, Jung JH, Lamsal K et al (2012) Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 40(1):53–58. https://doi.org/10.5941/MYCO.2012.40.1.053
Hwang ET, Lee JH, Chae YJ et al (2008) Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 4(6):746–750. https://doi.org/10.1002/smll.200700954
Sotiriou GA, Pratsinis SE (2010) Antibacterial activity of nanosilver ions and particles. Environ Sci Technol 44(14):5649–5654. https://doi.org/10.1021/Es101072s
Gomes SIL, Soares AMVM, Scott-Fordsmand JJ et al (2013) Mechanisms of response to silver nanoparticles on Enchytraeus albidus (Oligochaeta): survival, reproduction and gene expression profile. J Hazard Mater 254:336–344. https://doi.org/10.1016/j.jhazmat.2013.04.005
Rozen A (2006) Internal regulation of reproduction seasonality in earthworm Dendrobaena octaedra (Savigny, 1826) (Lumbricidae, Oligochaeta). Soil Biol Biochem 38(1):180–182. https://doi.org/10.1016/j.soilbio.2005.04.023
Krogh PH Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest. Aarhus Universitet, Aarhus: 2008; pp 1–66
Acknowledgements
We would like to thank Moira McKee and Antje Mathews for linguistic corrections and helping proofread the manuscript. Special thanks go to Tong Wu for Laboratory photograph work.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the UMSICHT project (BMBF 0340091A) and the Chinese Scholarship Council (No. 2010633007).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the design of the experiments in this study. XZ performed the practical work and initiated and drafted the manuscript. JF supplemented, revised and commented the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Tables S1–S6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Filser, J. Low concentration effects and different outcome in repeated reproduction tests with silver nanoparticles, silver nitrate and Folsomia candida (Collembola). Environ Sci Eur 32, 136 (2020). https://doi.org/10.1186/s12302-020-00413-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00413-7