Abstract
Tropical reef ecosystems are generally considered to be sinks of marine zooplankton, mainly due to the predation by scleractinian corals and other planktivores. The present study aims to evaluate the zooplankton community of a coastal reef in two specific environments: the reef edge and open-water channels between patch reefs. Sampling was carried out at two patch reefs that border the Tamandaré coastal lagoon system (Pernambuco State, Brazil). Two passive stationary nets (64 μm mesh size) were used: the Reef Edge Net (REN) and the Channel Midwater Neuston Net (CMNN). Sampling was performed simultaneously at both reefs during eight nocturnal sampling campaigns, always at new moon ebb tides. Zooplankton was classified by “origin” (estuarine, reef, neritic and neritic/estuarine). During all campaigns and at both sites, a significant buildup of zooplankton at the reefs was observed. Reef edges showed significantly higher abundance (77,579 ± 73,985 ind. m−3) and biomass (48.9 ± 45.5 mg C m−3) of zooplankton compared to open-water channels (9982 ± 11,427 ind. m−3 and 11.4 ± 21.9 mg C m−3, respectively). A total of 65 taxonomic groups were identified. Copepods were the most abundant group with a contribution of 69% for total zooplankton abundance, followed by foraminiferans, gastropod veligers, appendicularians, cirripedians nauplii, and polychaete larvae. Copepods from neritic/estuarine environments dominated the reef edges in both relative abundance and relative biomass (91% and 88%, respectively). The unexpectedly high abundance of copepods and other holoplankton at the reef edges, when compared to Indo-Pacific and Caribbean reefs, is probably due to very low cover of corals and other zooplanktivorous sessile animals (< 0.2%) on these coastal reefs, which leads to a very low predation mortality for zooplankters. Also, we propose that the reduced water column above the reef top leads to a buildup of very high densities in these environments.
Similar content being viewed by others
Background
Reef-associated zooplankton plays an important role as a link in food webs between primary producers and higher trophic levels [1,2,3,4,5]. Shallow tropical reefs shelter a complex and heterogeneous zooplankton community from different sources, e.g., small-sized demersal species emerging from the reefs at night, such as many amphipod, isopod and cumacean species [6,7,8,9], holoplanktonic species of coastal and oceanic origin, such as many calanoid copepods [4, 5, 10,11,12] and meroplanktonic larvae of benthic species, such as larvae of polychaetes, crustaceans and mollusks [13,14,15].
The demersal zooplankton comprises larval and adult stages of organisms living in reef caves at daytime and migrating towards the water column after sunset to feed on detritus and plankton. This “emergent” strategy has probably evolved to avoid visual diurnal planktivorous organisms as well as nocturnal benthic predators [7, 9]. Holoplankton consists of species that spend their entire life cycle in the water column [16]. Meroplankton are larvae of benthic species that live and feed in the water column and when they reach a new stage of development, they can hover near the reef surface in search of the right place to shelter or settle [11]. Some holoplanktonic species form swarms near the reef bottom during the day and disperse at night [1, 17]. This behavior occurs to facilitate mating and for protection during the mating periods [17, 18].
Zooplankton show clear patterns of vertical distribution off coral reefs at night, with well-documented near-reef depletion, even at shallow (1–2.4 m) coral reefs [19]. Bottom avoidance by vagile swimmers (such as copepods, shrimp larvae, fish larvae etc.) and the intense predation on zooplankton caused by sessile planktivores, such as corals, are the main reasons for near-reef depletion of zooplankton in oligotrophic coral reefs, located far from coastlines and any estuarine influence [12, 20, 21].
Conversely, the reefs of northeastern Brazil are strongly influenced by estuarine plumes that transport continental inorganic nutrients, sediments, suspended particulate matter and plankton to coastal areas [22,23,24]. Although Brazilian reefs have a high level of endemism in their coral species, they present a low coral coverage and diversity compared to reefs from the Indo-Pacific and Caribbean [22, 23, 25,26,27].
Very little is known about the micro- and mesozooplankton off Brazilian reefs, which have mostly been caught with common plankton nets far off the reefs [23, 28, 29], or by vertical ascendance traps that were placed directly on the reef tops [30]. Reef edges are key habitats within coastal ecosystems. However, there is no published information available on the micro- and mesozooplankton of these habitats. The objective of the present study was to evaluate the abundance and biomass of micro- and mesozooplankton at the edges of shallow intertidal reefs, and, for comparison, in adjacent open waters (i.e., in deep channels between reefs).
Methods
Study area
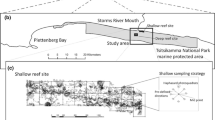
This study was carried out in a reef system located in Tamandaré, northeastern Brazil, which is part of a Marine Protected Area, the MPA “Costa dos Corais”, created in April 1999 through a federal decree. Within the reef complex of Tamandaré, a reef patch called “Ilha da Barra” was completely closed to all types of fishing, exploration, visitation and tourist activities (Fig. 1). Tamandaré Bay is a semi-open embayment, delimited by a series of loosely connected barrier reefs that form a coastal lagoon (“mar de dentro”), which is influenced by the estuaries of four small rivers, especially during the peak rainy period, from April to August.
The study area has a semidiurnal tide, i.e., the time difference between maximum high tide and maximum low tide is approximately 6 h. Tidal ranges during the sampling campaigns in November 2015 and March 2016 were 1.9 m and 2.4 m, respectively. Mean rainfall in the region during November 2015 was 0.7 mm, and local rainfall was observed only during the first day of sampling. In March 2016, average rainfall during sampling days was 0.36 mm, well below the average for that month (8.8 mm). Rainfall data were obtained by the Instituto Agronômico de Pernambuco (IPA). At the Ilha da Barra tidal channel, maximum current speed varied from 3 to 5.8 cm s−1 (at 7 to 9 m depth) measured in March and October 2015 with a S4 current meter (InterOcean Systems LLC, San Diego, CA, USA) [31]. Temperature and salinity were measured on site using a YSI CastAway CTD probe (SonTek, San Diego, CA, USA) at the beginning and at the end of each sampling.
Sampling strategy
Zooplankton sampling was carried out at two reef sites called “Ilha da Barra” (IDB, closed area) and “Pirambú” (PRB, open access area for moderate fishing and tourism) (Fig. 1). Both reef patches are characterized by steep vertical or overhanging edges and a flat reef top that completely emerges during spring low tides (at new moon and at full moon), and stays a few cm below the water line at neap low tides. Both intertidal reef tops (IDB and PRB) are almost perfectly flat, but have a slight slope towards the coast. During ebb tides, this slope creates an onshore ebb flow, that transports water, suspended particles and organisms from the reef tops towards the nearshore edges (Fig. 1).
Two stationary net systems (64 μm mesh size) were used to sample zooplankton: The Reef Edge Net (REN) and Channel Midwater Neuston Net (CMNN), which collected samples in different environments, at fixed stations near the reefs. The REN sampled organisms at the reef edges (submerged with the mouth exactly aligned with the upper reef edge, approximately 5 m away from the reef patch), which allowed sampling organisms present on the reef top, i.e., the zooplankton washed from the emerging reef top towards the edge by the ebb tide flow. The CMNN collected samples in open waters of channels between the reefs, where zooplankton is transported from reef systems towards the adjacent continental shelf. Both gears are very similar (same mesh size, same mouth opening geometry, etc.), allowing for direct comparisons [31]. Basically, the CMNN is a catamaran with two large floaters and is designed for deep, open waters, while the REN is a simpler version of the CMNN, designed for easy deployment in shallow waters close to the reefs. The orientation of the REN is fixed towards the reef, as to maximize the sampling of materials washed from the reef top. The CMNN, by contrast, is held by only one line, the mooring line holding the frame while the remainder of the net is allowed to act as a vane, orienting the net into all possible current directions, in deep, open waters. The CMNN allows for sampling simultaneously at different depths (e.g., surface, subsurface, 1 m depth). In this study, only the deepest CMNN samples (1 m depth) were used, since they are best suited for comparison with REN, that was generally sampling far below the surface.
The exact locations for the deployment of each REN were chosen to be where there was a maximum flow of water washed from the reef tops during spring ebb tides (Fig. 1). This was verified by snorkeling around these shallow reefs during spring ebb tides, previous to the sampling. The positions of the CMNNs were chosen to be in the center of the deep channels (> 5 m depth at high tide), where there is a maximum offshore flow during ebb tide (Fig. 1). The main advantage of stationary nets over towed nets is their easy handling, and that they allow for a safe navigation and deployment near the reefs, especially at night, and simultaneous, integrated sampling at several sampling spots.
REN and CMNN were deployed at high tide before dusk (i.e., between 03:03 pm and 05:10 pm) and then simultaneously sampled passively in the ebb flow currents. In the study area, sunset occurs at approximately 06:00 pm during austral summer. Finally, all nets were recovered during nocturnal low tide, after passive sampling for 3:40 to 5:03 h (mean duration: 4:21 h, std. dev.: 22 min.), depending on the weather and sea conditions. One main advantage of this timing is optimized navigation safety, since it avoids navigating close to the reefs at nocturnal high tides, when wave action is strong and the reefs are submerged, and thus less discernible, especially at new moon nights. Nocturnal navigation in this area with small boats is safest at low tide, when nearshore waters are very calm and emerging reefs are well visible with hand-held flashlights.
Filtered volume was estimated from flowmeter readings at the beginning and at the end of each sampling night. A calibrated flowmeter (Hydro-Bios, Altenholz, Germany) was attached to each net (i.e., inside a large 300 μm mesh net, that was attached to the 64 μm mesh net, Santos et al. [31]). Flowmeter rotations were checked at the end of the sampling (i.e., initial and final readings were compared), to verify whether the flowmeters had been functioning properly during peak ebb tide flow. For more details about the sampling strategy and passive net systems design, see Santos et al. [31].
Samplings were carried out during the dry period in two intensive campaigns: from November 10 to 12, 2015 (three consecutive sampling nights) and from March 7 to 11, 2016 (five consecutive sampling nights), during new moon ebb tides.
Each fixed station, Ilha da Barra (IDB) and Pirambú (PRB), was sampled using a REN and a CMNN system. The nets were fixed on the bottom (during high tide, i.e., 3:00 p.m. and 4:30 p.m.) using anchors, always placed against the ebb tide flow. All nets were recovered at low tide (they remained submerged for approximately 4.5 h). Samples were obtained at two fixed stations simultaneously, at every night of fieldwork. Average local depths in channel and reef edges stations were 9.7 and 4.6 m, respectively. All organisms were conditioned in plastic jars and the plankton was fixed with 4% formalin (final concentration in seawater), buffered with sodium tetraborate (0.5 g * L−1).
Laboratory analysis
Zooplankton was analyzed in a Sedgwick-Rafter chamber under a binocular microscope. Three 1 ml subsamples (containing from 200 to 700 individuals) were analyzed for each sample. Organisms were identified at highest taxonomic level possible [32,33,34].
Copepods (adults and copepodites) were measured (total length) in each sample (10 organisms for each species) as well as appendicularians and copepod nauplii (30 individuals per sample). Other less frequent groups belonging to phyla Ciliophora, Foraminifera, Cnidaria, Nematoda, Annelida, Mollusca, Crustacea and Chordata were also measured (approximately 100 organisms from each group in all samples analyzed).
Copepods were classified regarding their “origin” (estuarine, reef, neritic and neritic/estuarine). This species classification was based on previous literature, regarding their main habitats [11, 32, 35, 36]. Species classified as “neritic/estuarine” are commonly found in both neritic and estuarine environments. Species that are common on reefs, such as most benthic harpacticoids, that are generally associated to the reef substratum or to macroalgae, were considered “reef copepods”. Not all harpacticoids were included into the “reef” category. For example, Euterpina acutifrons, a common pelagic harpacticoid species in the tropical coastal and estuarine zooplankton, was not included in the “reef” category, but considered as “neritic/estuarine”. Some copepod taxa were withdrawn from this classification due to their unknown origin.
To estimate zooplankton carbon mass (μg C), the following regressions were used: ln(copepod biomass) = 1.82*ln(S) +1.28 for copepods (adults, copepodites, and nauplii) and ln(non-copepod Biomass) = 1.46 * ln(S) + 1.03 for other taxa, where S is total body size in mm [11, 21]. Average taxon-specific carbon values (μg C ind.−1) of all taxa were multiplied by their abundance (ind. m−3) in each sample, to calculate total zooplankton biomass (μg C m−3). A total of 32 samples were analyzed (8 nights × 2 nets × 2 areas).
Data analysis
Total abundance data (ind. m−3), relative abundance (%) and carbon mass (mg C m−3) were log(x + 1) transformed and tested for normality using Shapiro–Wilk tests [37]. Non-parametric Mann–Whitney tests were used for data without normal distribution, to test whether there were statistically significant differences regarding zooplankton abundance and carbon mass, between sampling sites (IDB vs PRB), sampling months (November vs March) and environments (reef edges vs channels). A Bonferroni correction was applied to the test results, considering three multiple comparisons (pcrit = 0.05/3 = 0.0167). All data are available at https://figshare.com/articles/Zooplankton_Abundance_Brito_Lolaia_et_al_csv/11996799.
Results
Environmental conditions
Average chlorophyll a concentration around the Tamandaré reefs was 0.12 mg m−3, in March 2016. Based on these values, this environment can be characterized as oligotrophic, with extremely low chlorophyll values (below 1 mg m−3), during the sampling period (“blue water” conditions). Water temperature was lower in November than in March, ranging from 28.36 to 28.71 °C and from 30.20 to 30.67 °C, respectively. Salinity ranged from 35.35 to 36.24 in November and from 35.83 to 36.69 in March, indicating little to none direct estuarine influence at the sampling sites.
Composition of the zooplankton
Reef edges showed a highly significant (p = 0.0005) and more than eightfold higher abundance of zooplankton (mean: 77,579 ind. m−3, st. dev.: 73,985 ind. m−3) compared to channels (9982 ± 11,427 ind. m−3) (Fig. 2).
Overall, 65 zooplankton taxa were identified (Tables 1 and 2). Copepods were the most abundant group, with an average relative abundance of 69% (adults, juveniles and nauplii), followed by foraminiferans (13%), gastropod veligers (8%), the appendicularian Oikopleura spp. (3%), cirripedian nauplii (1%), polychaete larvae (1%) and others (5%), comprising the phyla Ciliophora, Cnidaria, Nematoda, Mollusca, Crustacea, Chaetognatha and Chordata.
The planktonic copepods Parvocalanus crassirostris (adults and juveniles), Dioithona oculata, Oithona (juveniles), Oithona hebes, and Euterpina acutifrons (adults and juveniles) were the most abundant taxa, with a mean of 80% of the total copepod community. Benthic harpacticoids contributed with 9% to copepods, with a greater abundance of Harpacticidae, Longipediidae and Tegastidae.
All abundant taxa, i.e., copepod nauplii, foraminiferans, gastropods veligers, the copepods P. crassirostris, D. oculata and O. hebes (adults), presented significantly higher abundances at reef edges compared to channels (Tables 1 and 2, Figs. 3 and 4). Many other groups, such as Favella ehrenbergii, ostracods, decapod larvae and fish larvae also showed significantly higher abundance at reef edges compared to channels.
Total zooplankton carbon mass was higher at reef edges in relation to channels, with mean values of 48.0 ± 44.9 and 11.4 ± 21.9 mg C m−3, respectively (Mann–Whitney test, p = 0.001). With 52% of the zooplankton carbon mass, copepods (adults and juveniles) dominated the biomass at the Tamandaré reefs. Although nauplii contributed 37% to total abundance, the contribution of these small larvae (mostly copepod nauplii) in terms of biomass was only 12%, due to their small size. Their overall mean carbon mass was 30.2 ± 39.7 mg C m−3. There were no differences between sampling sites and months, except for the total zooplankton caught at the reef edge, with higher abundance in March (p = 0.0019) and for total zooplankton caught at the channels, with higher abundance in the IDB area (p = 0.0009).
Origin of copepods
Copepods of neritic/estuarine origin (Table 2) were the overall dominant group in this study. At the reef edges, they comprise an average 91% of copepod abundance and 88% of copepod carbon mass at reef edges. In the channels, copepods of neritic/estuarine origin comprised 83% of copepod abundance and 88% of carbon mass. Copepods of reef origin showed higher contributions in channels (13% of abundance and 3% of carbon mass) compared to reef edges (6% and 3% for abundance and carbon mass, respectively). Copepods from estuarine origin showed low abundance (2% and 3% for reef edges and channels, respectively) in relation to carbon mass values (7% and 8% for reef edges and channels, respectively). Copepods of neritic origin showed low abundance and carbon mass in both environments (1% of contribution at reef edges and channels). When comparing environments, the reef edges harboured higher abundance and carbon mass than channels for neritic/estuarine, reef, estuarine and neritic copepods (except for neritic copepod carbon mass) (Fig. 5).
Discussion
The present study is the first to reveal the existence of specific micro- and mesozooplankton communities exported from the reef tops of a shallow tropical reef ecosystem and the importance of neritic-estuarine copepod species for this environment.
The use of passive net systems adapted for zooplankton sampling at specific points in the reef environment, with a 64 μm mesh size, permitted the sampling design of the present study, that allowed the collection of reef-associated zooplankton and organisms transported in channels between reefs, as well as the evaluation of the carbon mass of zooplankton available as food source for higher trophic levels. The significant buildup of zooplankton at both reefs sites and all sampling nights indicates the existence of a hitherto unknown mechanism that enriches the zooplankton above emerging intertidal reef tops.
Zooplankton buildup at reefs—a hitherto neglected mechanism
The unexpectedly high abundances found at the reef edges point at the existence of a hitherto unknown mechanism. One part of this mechanism is the accumulation of zooplankton at the offshore side of reefs, which has been described by Genin et al. [38]. They described a situation where downwelling and upwelling driven by the interaction of currents and coastal topography generate zooplankton accumulations. In such downwelling and accumulation zones at the offshore reef edges, active zooplankters such as copepods actively maintain their vertical position in the water column, swimming upwards against the vertical flow. This may lead to huge accumulation rates at coastal frontal zones close to the offshore reef edges, constituting a well-documented aggregation and accumulation mechanism [38,39,40,41,42]. In our study area, high densities can be expected to occur at the windward offshore edges of the reefs, where there is consistent downwelling in coastal convergence fronts, mostly due to onshore winds.
In the present study, sampling could not be conducted at the windward (offshore) edge of the Tamandaré reefs, considering the year-round predominance of strong onshore trade winds in this area that lead to big waves and unsecure navigation conditions at the offshore edges of the reefs. Rather, sampling sites for the REN were chosen to receive the water washed from the emerging reef tops during ebb flow. High densities found at these sites are indicative of a hitherto unknown accumulation mechanism that acts according to the tidal cycle at new moon nights, from dusk to dawn. It consists in the following three steps (1) low tide: accumulation of zooplankton at the windward side of emerged reef tops due to the above mentioned processes, (2) flooding tide: coastal waters with high densities of zooplankton are sucked towards the submerging reefs at nocturnal low tides, (3) vertical habitat compression (i.e., decrease of available water column) above the emerging reef tops during ebb tide, (4) discharge of high-density zooplankton from emerging reef tops during ebb tides, towards the onshore reef edges.
Another important factor is that predation due to scleractinian corals is negligible in the study area, since corals are very rare and occur at a negligibly low cover on reef tops in the Tamandaré area (< 0.2%, [43]). Also, the Tamandaré reefs show very low densities of planktivores [44]. This overall low predation on zooplankton also contributes to the observed high abundances.
Conversely, many other studies showed a significant depletion of zooplankton at oligotrophic coral reef ecosystems, mainly due to intensive predation by benthic sessile planktivores (e.g., scleractinian corals). The high abundance at reef edges compared to the samples collected in open water channels thus represents an opposite pattern to the one found in many previous studies on micro- and mesozooplankton (mesh aperture between 40 and 125 μm) on coral reefs, where the lowest abundances of zooplankton were always found near the reefs [12, 19,20,21].
In contrast to the present study, Santos et al. [31] found no significant differences between CMNN and REN samples taken in the study area (regarding total wet biomass, total abundance, and length-frequency distributions), based on three sampling nights in November 2015. The apparent discrepancy between the Santos et al. [31] results and the present study can be well explained by differences in objectives, gear used, laboratory methods, and the considerably larger data set used in the present study. Santos et al. [31] presented and described the two new passive gears (REN and CMNN) in detail, and compared them with standard plankton net tows, based on a very small dataset (n = 3 sampling nights). While they used different mesh sizes, we used only 64 micron mesh nets to compare habitats, and unveiled a new community of micro- and mesozooplankton at the reef edges. Furthermore, the present study shows new results with a much larger dataset (n = 8 sampling nights, two gears, two areas, 8 * 2 * 2 = 32 samples). The Santos et al. [31] study used rapid semi-automatic laboratory methods (size distribution of large groups, no species), based on only one subsample per sample. We used detailed taxonomic identifications and determination of carbon mass, based on three subsamples per sample. The much larger dataset allowed us to detect striking, significant differences between reef edge and channel habitats, and to describe unexpectedly high abundances at the reef edges, especially in March 2016.
In most oligotrophic coral reefs, scleractinian cnidarians are considered voracious nocturnal predators of zooplankton [3, 45,46,47]. Due to this high predatory pressure near the bottom, organisms living on reefs with high coral cover show a marked vertical migration at night, i.e., where there is an evident zooplankton enrichment on the surface. The high cover of planktivorous sessile organisms, such as scleractinian corals, explained lower abundances near the reef than in adjacent open waters, in previous studies in such coral reefs [12, 19,20,21].
Yet, the coastal reefs of northeastern Brazil exhibit a very low coral cover [43, 48,49,50], probably due to the regular estuarine influx of freshwater, pollutants and sediments at peak rainy season (April to August) and during extreme flooding events [23, 25, 26]. The paucity of scleractinian corals leads to a low predatory pressure on zooplankton at the Tamandaré reef tops. Conversely, there is a rich algal cover, mostly dominated by the rhodophyte Palisada perforata [43]. Our results indicate that these reef tops covered with zoanthids and macroalgae are likely sources of zooplankton, and not sinks, as in many Indo-Pacific coral reefs. Two likely factors are probably boosting the zooplankton abundance at the Tamandaré reef tops: (1) highly productive benthic micro- and macroalgae used as food sources, and (2) vertical habitat compression on the reef tops during the tidal cycle (see above).
In theory, the observed differences could be due to some particular difference in sampling gear, or to some local aggregation of zooplankton at the studied reef edges. Aggregations in downwelling convergence zones could, in theory, be expected at the offshore side of the reef, as in Genin et al. [38]. However, we worked at the onshore side of the reefs, that receives a constant flow of water washed directly from the reef tops at ebb tide. No convergence zones (e.g. accumulation of foam or detritus at a specific spot at the surface) or aggregations (swarms) were observed at these studied edges. Instead, there was a constant horizontal flow of particles and plankton, coming from the slowly emerging reef top. Also, differences in gear used to moor the nets are most likely not the main factor explaining our data, since both gears used are very similar, use the same nets (mesh size, mouth opening, etc.) and sample the same size distributions (as shown by Santos et al. [31]). This supports the idea that the observed differences are not due to method artifacts. Furthermore, the observed differences can be well explained by the characteristics of these highly productive ecosystems, that are covered mostly by macroalgae [43], and occasionally (during the rainy season) receive important nutrient inputs from adjacent rivers. All this evidence supports the idea that these coastal reefs are sources of zooplankton, that is washed from the slightly sloped reef tops towards the nearshore edge at low tide, not sinks.
Origin and composition of coastal reef zooplankton
Compared to the study on macrozooplankton collected with a 300-micron mesh by Santos et al. [31], the present study showed a surprisingly low abundance of meroplankton (i.e., brachyuran crab zoeae). Santos et al. [31] demonstrated that these reefs are the sites of production of large-sized larvae of decapod crustaceans and fish. In the present study, copepods were dominant, and even more surprising, copepods that are not typical for oligotrophic waters, nor typical for tropical coral reefs, but species that are typical for coastal-estuarine waters, such as Parvocalanus crassirostris, although there was no measurable estuarine influence (high salinities and Secchi depths). The predominance of holoplankton indicates that there must be a large abundance of food in the water column. Holoplankton generally feeds in the water column, while early-stage meroplankton contains mostly energy and matter derived from adult benthic populations. These reefs are sites of high density and productivity of organic matter, mainly due to primary producers, such as pelagic and benthic microalgae, detritus from macroalgae and mucus [51,52,53], produced by abundant zoanthids [43]. Zoanthids are also important primary producers, due to their symbiotic zooxanthellae [54].
In spite of strong primary production with zooxanthellae, heterotrophic nutrition in zoanthids does occur [55], where a flat mucous layer that covers the whole organism can be used to trap and absorb sinking particles, such as detritus, invertebrate eggs, and diatoms [56]. This flat mucous layer on the reef surface represents a very different feeding strategy, as compared to most common scleractinian corals, which usually possess a myriad of tentacles that effectively capture zooplankton from the water column. Also, additionally to exporting plankton, the highly productive reefs off Tamandaré have been shown to produce very high densities of several types of biogenic particles [57], that may serve as food for various plankton organisms.
The high contribution of neritic/estuarine and some estuarine species to the zooplankton community at the Tamandaré reefs may indicate that the estuaries formed by the rivers that flow into Tamandaré bay and nearby coastal areas may have an important influence on these coastal reefs, even during the dry season, the time of sampling, when salinities were high and no freshwater outflow to the bay was detectable. One possible explanation is that nutrients (in the form of suspended organic matter) from these estuaries are deposited in surrounding coastal sediments during strong rainfall events, and are then slowly released to the water column in the following dry months.
Among copepods, there are several typical tropical coastal-estuarine species that are commonly found in high densities in estuaries, coastal lagoons and in regions with estuarine influence along the Brazilian coast [23, 28, 58,59,60]: Parvocalanus crassirostris, Dioithona oculata, Oithona hebes, Oithona nana, Pseudodiaptomus acutus, Euterpina acutifrons, Temora turbinata, Oithona oswaldocruzi, and Acatia lilljerborgi. These species also showed greater abundance near the coast, when compared to more distant areas, such as the Abrolhos coral reefs (located 60 km offshore), where they were not found or found only in very low numbers [29, 61, 62].
The high abundance of neritic/estuarine species collected at reef edges may be related to the retention behavior of these species [63,64,65]. In most tidal estuaries, zooplankton exhibits vertical retention strategies according to the tidal cycle, where estuarine plankters migrate towards the bottom when low-salinity waters are flushed toward the sea at ebbing tides. Outflowing freshwater remains closer to the surface. The zooplankton retention near the bottom prevents these organisms from being carried to the sea [63,64,65].
In the present study, the fact that the estuarine-coastal zooplankton was more abundant close to reefs (organisms sampled at reef edge) compared to the open water channels, may also be explained by such retention mechanisms. Samples obtained at reef edges showed a relevant contribution of neritic/estuarine species. This behavior is probably a strategy to avoid a passive drift to unfavorable offshore waters, also to remain in layers with high food concentrations and to increase the likelihood of finding partners [38, 66].
Among the reef-associated organisms are copepod swarms, which were abundantly represented by D. oculata, which is found inhabiting mangrove estuaries, reefs [1, 18, 67] and macroalgal beds [68]. The formation of swarms in this species has the characteristic of being close to structures in the background during the day and dispersion at night [1, 17]. D. oculata can remain in formation at the same site, even in persistent tidal currents, however at night, without swarm formation, copepods are unable to maintain their positions [69] and can be taken by the ebb tide currents. However, as observed in this study, only a significantly smaller portion (3 times less) of this species was transported through the channels out of the reef environment (as seen in the very low abundance at the channels), showing a retention behavior close to the reef substrate.
Another very important reef-associated group were demersal organisms, that can be included into the “reef origin” category, since they emerge in vast amounts from the Tamandaré reef tops at nocturnal high tides [30]. Alldredge and King [70] reported that demersal organisms migrate vertically at short distances. They observed that 80% of the total demersal fauna, especially those of smaller size (< 2 mm), remained 30 cm above the bottom, which is probably due to another type of selective pressure more important than predation, such as water column feeding, reproduction and dispersal [70]. In relation to dispersion, migrating short distances prevents demersals from being taken to the open sea during low tide, where food is scarce and there is no shelter.
Zooplankton exhibits a distinct behavior according to its environment and classification by origin in the reef environment cannot always be easily distinguished, especially for species that inhabit both neritic and estuarine environments. Further studies on the effective contribution of organisms from estuaries to shallow coastal reefs are needed to understand how this influence occurs.
Carbon mass by groups
The highest percentage, in units of carbon mass, in Tamandaré reefs was composed of groups of neritic and estuarine (neritic/estuarine) origin, constituting an important carbon source for upper trophic levels.
The carbon mass of zooplankton at coral reefs is generally much higher at night [12]. This is due to the behavior of demersal organisms that are vertically migrating after dark, pelagic zooplankton entering reefs from the open sea, spawning of some groups such as corals and cessation of predation by visual planktivores such as fish [4,5,6,7,8,9,10,11,12,13,14,15]. However, on the shallow reefs of Tamandaré, the contribution of demersal organisms (i.e., Amphipoda and Cumacea) and the zooplankton of neritic environment was very low.
Total biomass values found in this study were much higher than in studies with pump samplers (mesh 40 μm) near the reefs at night in the Caribbean Sea during summer (3.4 mg C m−3) and for reefs at South Florida (11.8 mg C m−3) [11, 21]. These highly oligotrophic sites, unlike the Tamandaré reefs, have no estuarine influence.
Holoplanktonic organisms were dominant at the reefs of Tamandaré, highlighted by the great abundance of planktonic copepods, which is an important community in many coral reefs [9, 11, 21]. However, they contributed with only 52% of the total carbon mass in Tamandaré reefs, when compared to a Caribbean reef where 68% of the total carbon mass were copepods [11]. This was because the copepods that were dominant at Tamandaré, such as P. crassirostris, D. oculata and O. hebes, have very small body sizes (500 to 600 μm) when compared to species commonly found in other reefs. These animals of larger size contribute with higher carbon mass [9, 21]. Small-sized organisms (100–200 μm) did not show differences in biomass between day and night at Red Sea reefs [12].
The high values of carbon mass found at reef edges compared to channels were also explained to the great participation of groups such as Foraminifera, which are generally caught in resuspension [12] caused by tidal currents that wash the reefs. Another factor was a possible “reproductive peak” of copepods during the study period, leading to a high production rate of copepod eggs and nauplii. Although these nauplii were abundant at the sampling sites, they contributed very little to the biomass [11, 71].
Conclusions
An unexpected and hitherto unknown buildup of micro- and mesozooplankton at reef edges was observed, which is opposite to the patterns found at many oligotrophic tropical coral reefs, where a depletion of zooplankton occurs near the reef. Conversely, our results indicate that coastal tropical reef tops at Tamandaré are productive sources of zooplankton, not sinks. New passive nets allowed to observe this pattern and provided a good representation of the zooplankton groups in this reef area of huge socio-economic relevance. The finding of higher zooplankton abundances at the reef edge has strong implications for our understanding of tropical coastal reefs and for the planning of future zooplankton sampling campaigns in such systems, that sustain numerous ecosystem services, such as tourism and fishing. Our data indicate that high amounts of plankton are washed from such productive reef tops towards adjacent waters. Thus, they are important sources of food for adjacent pelagic ecosystems. This is a further argument for protecting nearshore intertidal reef tops, that face multiple, severe threats.
Availability of data and materials
All data are available at https://figshare.com/articles/Zooplankton_Abundance_Brito_Lolaia_et_al_csv/11996799.
References
Emery AR. Preliminary observations on coral reef plankton. Limnol Oceanogr. 1968;13(2):293–303.
Ikeda T. Nutritional ecology of marine zooplankton. Mem Fac Fish Hokkaido Univ. 1974;22(1):1–97.
Porter J. Zooplankton feeding by the Caribbean reef-building coral Montastrea cavernosa. In: Proc 2nd Int Symp on Coral Reefs, Great Barrier Reef Committee on board the MV Marco Polo cruising in the waters of the Great Barrier Reef Province, 1974, Australia. 1974. p. 111–25.
Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DMcB. Zooplankton, planktivorous fish, and water currents on a windward reef face—Great Barrier-Reef, Australia. Bull Mar Sci. 1988;42(3):459–79.
Hamner WM, Colin PL, Hamner PP. Export-import dynamics of zooplankton on a coral reef in Palau. Mar Ecol Prog Ser. 2007;334:83–92.
Alldredge AL, King JM. Distribution, abundance, and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Mar Biol. 1977;41(4):317–33.
Ohlhorst SL. Diel migration patterns of demersal reef zooplankton. J Exp Mar Bio Ecol. 1982;60(1):1–15.
Youngbluth MJ. Sampling demersal zooplankton: a comparison of field collections using three different emergence traps. J Exp Mar Biol Ecol. 1982;61(2):111–24.
Nakajima R, Yoshida T, Othman BHR, Toda T. Diel variation of zooplankton in the tropical coral-reef water of Tioman Island, Malaysia. Aquat Ecol. 2009;43(4):965–75.
Glynn PW. Ecology of a Caribbean coral reef. The Porites reef-flat biotope: Part II. Plankton community with evidence for depletion. Mar Biol. 1973;22(1):1–21.
Heidelberg KB, Sebens KP, Purcell JE. Composition and sources of near reef zooplankton on a Jamaican forereef along with implications for coral feeding. Coral Reefs. 2004;23(2):263–76.
Yahel R, Yahel G, Genin A. Near- bottom depletion of zooplankton over coral reefs: I: diurnal dynamics and size distribution. Coral Reefs. 2005;24(1):75–85.
Babcock RC. Reproduction and distribution of two species of Goniastrea(Scleractinia) from the Great Barrier Reef province. Coral Reefs. 1984;2(4):187–95.
Babcock RC, Mundy C, Keesing J, Oliver J. Predictable and unpredictable spawning events: in situ behavioural data from free-spawning coral reef invertebrates. Invertebr Reprod Dev. 1992;22(1–3):213–28.
Francini CLB, Castro CB, Pires DO. First record of a reef coral spawning event in the Western South Atlantic. Invertebr Reprod Dev. 2002;42(1):17–9.
Brandini FP, Lopes RM, Gutseit KS, Spach HL, Sassi R. Planctologia na Plataforma Continental do Brasil- Diagnose e Revisão bibliográfica. Ministério do Meio Ambiente e da Amazônia Legal – IBAMA, Brasília. 1997. p. 196.
Ambler JW. Zooplankton swarms: Characteristics, proximal cues and proposed advantages. In: Hydrobiologia. 2002. p. 155–64.
Ambler JW, Ferrari FD, Fornshell JA. Population structure and swarm formation of the cyclopoid copepod Dioithona oculata near mangrove cays. J Plankton Res. 1991;13(6):1257–72.
Alldredge AL, King JM. Near-surface enrichment of zooplankton over a shallow back reef: implications for coral reef food webs. Coral Reefs. 2009;28(4):895–908.
Holzman R, Reidenbach MA, Monismith SG, Koseff JR, Genin A. Near-bottom depletion of zooplankton over a coral reef II: relationships with zooplankton swimming ability. Coral Reefs. 2005;24(1):87–94.
Heidelberg KB, O’Neil KL, Bythell JC, Sebens KP. Vertical distribution and diel patterns of zooplankton abundance and biomass at Conch Reef, Florida Keys (USA). J Plankton Res. 2010;32(1):75–91.
Maida M, Ferreira BP. Coral reefs of Brazil: an overview. In: Proceedings of the 8th International Coral Reef Symposium. 1997. p. 263–74.
Mayal EM, Neumann-Leitão S, Feitosa FAN, Schwamborn R, Silva TA, Silva-Cunha MGG. Hydrology, plankton, and corals of the Maracajaú reefs (Northeastern Brazil)—an ecosystem under severe thermal stress. Brazilian Arch Biol Technol. 2009;52(3):665–78.
Machado RCA, Feitosa FAN, Koening ML, Flores Montes MJ, Bastos RB, Jales MC. Phytoplankton productivity and hydrology of Porto de Galinhas Reef Ecosystem (Pernambuco, Brazil). J Coast Res. 2014;294:371–8.
Leao ZMAN, Dominguez JML. Tropical coast of Brazil. Mar Pollut Bull. 2000;719–29.
Leão ZMAN, Kikuchi RKP. A relic coral fauna threatened by global changes and human activities, Eastern Brazil. Mar Pollut Bull. 2005;51(5–7):599–611.
Figueiredo MAO, Horta PA, Pedrini AG, Nunes JMC. Benthic marine algae of the coral reefs of Brazil: a literature review. Oecologia. 2008;12(02):258–69.
Nascimento-vieira DA, Neumann-Leitão S, Porto-Neto FF, Silva TA, Silva AP. Mesozooplâncton de área recifal do atlântico sudoeste tropical. Trop Oceanogr. 2010;38(1):47–59.
Figueirêdo LGP, Melo PAMC, Melo Júnior M, Silva TA, Moura RL, Thompson FL, Neumann-Leitão S. Summer micro- and mesozooplankton from the largest reef system of the South Atlantic Ocean (Abrolhos, Brazil): responses to coast proximity. J Sea Res. 2018;141:37–46.
Melo PAMC, Silva TA, Neumann-Leitão S, Schwamborn R, Gusmão LMO, Porto-Neto FF. Demersal zooplankton communities from tropical habitats in the southwestern Atlantic. Mar Biol Res. 2010;6:530–41.
Santos GS, Brito-Lolaia M, Schwamborn R. Two new methods for sampling zooplankton and larval assemblages in tropical reef ecosystems. J Exp Mar Bio Ecol. 2017;491:27–37.
Huys R, Gee JM, Moore C, Hamond R. Marine and brackish water Harpacticoid copepods : keys and notes for identification of the species. Part 1. Published for the Linnean Society of London and the Estuarine and Coastal Sciences Association by Field Studies Council; 1996, p. 352.
Boltovskoy D (ed). South Atlantic Zooplankton, Backhuys, Leiden. J Plankton Res. 1999;22(5):997–997.
Young CM (ed). Atlas of marine invertebrate larvae. San Diego: Elsevier; 2006, p. 640.
Bakker C, Phaff WJ. Tintinnida from coastal waters of the S.W.-Netherlands I. The genus Tintinnopsis Stein. Hydrobiologia. 1976;50(2):101–11.
Dalal SG, Goswami SC. Temporal and ephemeral variations in copepod community in the Estuaries of Mandovi and Zuari-West Coast of India. J Plankton Res. 2001;23(1):19–26.
Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika. 1965;52(3/4):591.
Genin A, Jaffe JS, Reef R, Richter C, Franks PJS. Swimming against the flow: a mechanism of zooplankton aggregation. Science. 2005;308(5723):860–2.
Schwamborn R, Neumann-Leitão S, Silva TDA, Silva AP, Ekau W, Saint-Paul U. Distribution and dispersal of decapod crustacean larvae and other zooplankton in the Itamaracá estuarine system, Brazil. Trop Oceanogr. 2001;9(1):1–18.
Ryan JP, Harvey JBJ, Zhang Y, Woodson CB. Distributions of invertebrate larvae and phytoplankton in a coastal upwelling system retention zone and peripheral front. J Exp Mar Biol Ecol. 2014;459:51–60.
Derisio C, Alemany D, Acha EM, Mianzan H. Influence of a tidal front on zooplankton abundance, assemblages and life histories in Península Valdés, Argentina. J Mar Syst. 2014;139:475–82.
Michalec FG, Fouxon I, Souissi S, Holzner M. Zooplankton can actively adjust their motility to turbulent flow. PNAS. 2017;114(52):E11199–207.
Santos GS, Burgos DC, Lira SMA, Schwamborn R. The impact of trampling on reef Macrobenthos in Northeastern Brazil: how effective are current conservation strategies? Environ Manage. 2015;56(4):847–58.
Ferreira CEL, Gonçalves JEA, Coutinho R, Peret AC. Herbivory by the dusky damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community. J Exp Mar Biol Ecol. 1998;229(2):241–64.
Odum HT, Odum EP. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecol Monogr. 1955;25(3):291–320.
Sebens KP, DeRiemer K. Diel cycles of expansion and contraction in coral reef anthozoans. Mar Biol. 1977;43(3):247–56.
Sebens KP, Grace SP, Helmuth B, Maney EJ, Miles JS. Water flow and prey capture by three scleractinian corals, Madracis mirabilis, Montastrea cavernosa and Porites porites in a field enclosure. Mar Biol. 1998;131(2):347–60.
Costa Jr OS, Attrill MJ, Pedrini AG, De-Paula JC. Benthic macroalgal distribution in coastal and offshore reefs at Porto Seguro Bay, Brazilian Discovery Coast. In: Proceedings 9th International Coral Reef Symposium. Bali, Indonesia; 2001, p. 23–7.
Ramos CAC, Amaral FD, Kikuchi RKP, Chaves EM, Melo GR. Quantification of reef benthos communities and variability inherent to the monitoring using video transect method. Environ Monit Assess. 2010;162(1–4):95–101.
Feitosa JLL, Ferreira BP. Distribution and feeding patterns of juvenile parrotfish on algal-dominated coral reefs. Mar Ecol. 2015;36(3):462–74.
Gerber RP, Marshall N. Ingestion of detritus by the lagoon pelagic community at Eniwetok Atoll. Limnol Oceanogr. 1974;19(5):815–24.
Nakajima R, Yamazaki H, Lewis LS, Khen A, Smith JE, Nakatomi N, Kurihara H. Planktonic trophic structure in a coral reef ecosystem—grazing versus microbial food webs and the production of mesozooplankton. Prog Oceanogr. 2017;156:104–20.
Roman MR, Furnas MJ, Mullin MM. Zooplankton abundance and grazing at Davies Reef, Great Barrier Reef, Australia. Mar Biol. 1990;105(1):73–82.
Santos GS, Amaral FD, Sassi CFC, Schwamborn R. Response of the zooxanthellae of Palythoa caribaeorum (Cnidaria: Zoanthidea) to different environmental conditions in coastal and oceanic ecosystems of the Tropical Atlantic. Helgol Mar Res. 2016;70(1):2.
Sebens KP. Autotrophic and heterotrophic nutrition of coral reef zoanthids. In Ginsburg RN, Taylor DL (eds) Proceedings of third international coral reef symposium, Rosenstiel School of Marine and Atmospheric Science, May 1976. Miami: University of Miami Press, 1;1977. p. 397–404.
Santana EFC, Alves AL, Santos AM, Cunha MGGS, Perez CD, Gomes PB. Trophic ecology of the zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) on tropical reefs. J Mar Biol Assoc UK. 2014;95(02):301–9.
Lins Silva N, Marcolin CR, Schwamborn R. Using image analysis to assess the contributions of plankton and particles to tropical coastal ecosystems. Estuar Coast Shelf Sci. 2019;219:252–61.
Milstein A. Vertical distribution of Paracalanus crassirostris (Copepoda, Calanoidea): analysis by the general linear model. Bol do Inst Ocean. 1979;28(2):65–78.
Silva AP, Neumann-Leitão S, Schwamborn R, Gusmão LM, De Almeida Silva ET. Mesozooplankton of an impacted bay in North Eastern Brazil. Brazilian Arch Biol Technol. 2004;47(3):485–93.
Dias CDO, Bonecker SLC. Inter-annual variability of planktonic copepods in a tropical bay in Southeastern Brazil. Brazilian Arch Biol Technol. 2008;51(4):731–42.
Valentin JL, Monteiro-Ribas WM. Zooplankton community structure on the east-southeast Brazilian continental shelf (18–23°S latitude). Cont Shelf Res. 1993;13(4):407–24.
Ekau W. Topographical and hydrographical impacts on zooplankton community structure in the Abrolhos Bank region, East Brazil. Mar Res. 1999;47(2–3):307–20.
Rogers HM. Occurrence and retention of plankton within the estuary. J Fish Res Board Canada. 1940;5a(2):164–71.
Grindley JR. Effect of low-salinity water on the vertical migration of estuarine plankton. Nature. 1964;203(4946):781–2.
Wooldridge T, Erasmus T. Utilization of tidal currents by estuarine zooplankton. Estuar Coast Mar Sci. 1980;11(1):107–14.
Cowles T, Desiderio R, Carr M-E. Small-scale planktonic structure: persistence and trophic consequences. Oceanography. 1998;11(1):4–9.
Hamner WM, Carleton JH. Copepod swarms: attributes and role in coral reef ecosystems. Limnol Oceanogr. 1979;24(1):1–14.
Ueda H, Kuwahara A, Tanaka N, Azeta M. Underwater observations on copepod swarms in temperate and subtropical waters. Mar Ecol Prog Ser. 1983;11:165–71.
Buskey EJ, Peterson JO, Ambler JW. The Swarming Behavior of the Copepod Dioithona oculata: in situ and laboratory studies. Limnol Oceanogr. 1996;41(3):513–21.
Alldredge AL, King JM. The distance demersal zooplankton migrate above the benthos: implications for predation. Mar Biol. 1985;84(3):253–60.
Frangoulis C, Grigoratou M, Zoulias T, Hannides CCS, Pantazi M, Psarra S, et al. Expanding zooplankton standing stock estimation from meso- to metazooplankton: a case study in the N. Aegean Sea (Mediterranean Sea). Cont Shelf Res. 2017;149:151–61.
Acknowledgements
The authors thank the Federal University of Pernambuco, Tamandaré Municipality and CEPENE/ICMBio research center of Tamandaré for laboratory and logistical support. Many thanks to Beatrice Padovani Ferreira, Antonia Eckerlebe and Mauro Maida for logistical support and helpful suggestions. We thank two anonymous reviewers for their suggestions, which considerably improved the original manuscript.
Funding
The authors thank the Brazilian National Research Council CNPq for the M.Sc. scholarship granted to the first author and for the productivity fellowships granted to the third and fourth authors. Thanks to CNPq (Grant No. 471038/2012-1) and INCT AmbTropic (CNPq/CAPES/FAPESB), for funding the fieldwork and the construction of the sampling systems. Thanks to the Coastal Reef Institute (IRCOS), Toyota Foundation and PELD-TAMS for supporting the fieldwork.
Author information
Authors and Affiliations
Contributions
MBL collected all samples, identified and analyzed all organisms, interpreted the results and wrote the manuscript. GSS participated in the field sampling activities and helped with the interpretation of results and manuscript writing. SNL participated in the interpretation of results. RS designed and supervised this study, participated in the field sampling activities and helped with the interpretation of results and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not involve human participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brito-Lolaia, M., Santos, G.S., Neumann-Leitão, S. et al. Micro- and mesozooplankton at the edges of coastal tropical reefs (Tamandaré, Brazil). Helgol Mar Res 74, 7 (2020). https://doi.org/10.1186/s10152-020-00539-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10152-020-00539-4