Abstract
Background
In light of existing regulations regarding the use of nematicides coupled with the global loss of agricultural outputs due to nematodes, new strategies are needed to ensure soil ecosystem health while promoting crop production without the use of potentially dangerous chemicals. Proof of concept methodologies can be used by soil/agricultural scientists interested in identifying potential shortcomings of a given strategy and to identify additional parameters for future work. Using this limited approach allows for the dissemination of information in a stepwise fashion so that changes in research strategies can be initiated prior to final 'definitive’ results. This work tests the viability of using coal fly ash, stabilized biosolids, or a mixture of the two to manage plant-parasitic nematode populations and increase carrot yield.
Results
The fly ash and biosolids chosen for this work did not alter soil pH or metal content enough to impact significantly on nematode populations. Data on all parameters were combined to see overall trends. The soil and amendments are basic in nature with pH values close to 8 and only fluctuating between 0.2 (season 1) and 0.4 (season 2) pH units from baseline to harvest. Fly ash played a minor role in B and Fe increases, and biosolids contained slightly more Ca, Cu, K, Mg, P, and Zn than the soils, but none of these elements were present in concentrations that affected nematode ontogeny. Fly ash was more important in altering electrical conductivity than biosolids and had the greatest impact on nematode population changes. Biosolids were most important for increasing carrot yields either alone or in mixtures.
Conclusions
Not all fly ash or biosolids are equal. The choice of these materials as soil amendments, or natural nematicides, should be based on pre-examination of the soils and the raw materials. Subsequently, ratios and application rates should be chosen so that the physicochemical and microbiological conditions favor nematode management. Biosolids and biosolids mixed with fly ash are capable of enhancing carrot yield significantly at the ratios and application rates tested in this study but had little effect on nematode populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This study was designed as a first approximation of the ability of fly ash and/or biosolids to manage plant-parasitic nematodes through either direct control (via changes in pH, electrical conductivity (EC), metals) or by promoting bacterial, fungal, and non-plant-parasitic nematode activities (indirect control), which act negatively upon plant-parasitic nematode populations.
The use of chemical compounds to manage populations of plant-parasitic nematodes has been the preferred method used by agriculturalists the world over (Evenson and Gollin 2003). During the last 50 years, the use of such compounds has altered the natural balance of the soil ecosystem, affecting soil microbial biodiversity and creating soils that are dependent on synthetic compounds (Edwards 1993) and less capable of self-managing plant pathogens (Westphal 2005). The necessity for alternative nematode management has led soil and plant specialists to apply a wide range of byproducts as soil amendments (Akhtar and Alam 1993; Stirling et al. 2005; Walker 2007). The ways in which these amendments affect plant-parasitic nematode populations are quite diverse and can be direct, indirect, or a combination of both. Direct effects rely on chemical and physical changes in the soil matrix which in turn make the medium inhospitable or noxious to plant-parasitic nematodes (Haydock et al. 2006; Guerena 2006). Indirect effects can be due to increased populations of biocontrol agents (bacteria and fungi) (Rodriguez-Kabana et al. 1987; Akhtar and Malik 2000; Stirling 1991, Siddiqui and Mahmood 1999) or increased nematode biodiversity, which does not favor plant-parasitic nematodes (Qi and Hu 2007). Additionally, the application of some soil amendments provides cropped plants with macro and micronutrients not found in traditional fertilizers and promotes healthy plant growth (Davies 1997; Kabirinejad and Hoodaji 2012). The combination of direct and indirect effects plus healthy plant growth work to manage plant-parasitic nematode populations (Magdoff 2001).
There has been some research on the application of stabilized biosolids and coal combustion fly ash as soil amendments mainly from the standpoints of yield or introduction of potentially toxic organic and inorganic pollutants. The application of these amendments can improve crop yield as well as soil health (Parkpian et al. 2002; Punshon et al. 2002). Some commercial products made from biosolids and fly ash have been tested under laboratory conditions and in microplot experiments and are shown to affect plant-parasitic nematodes by increasing soil pH and favoring the release of ammonia, both detrimental to nematode propagation (Zasada and Tenuta 2004; Zasada 2005; Zasada et al. 2007). The objective of this study was to determine the influence of mixtures of stabilized biosolids and coal combustion fly ash on different nematode feeding groups under field conditions. Specific objectives were to (a) determine if the physical and chemical changes in the amended soils produce nematicidal effects, (b) determine the effects of the amendments on fungal and bacterial activity, and (c) determine the effect of the amendments on carrot yield. Here, we report the findings of two consecutive growing seasons where soil amendments were applied in field microplot experiments that resulted in improved carrot yield and slight changes in nematode populations and biodiversity.
Methods
Experimental design
In order to evaluate the effect of either fly ash, biosolids or mixtures of the two, treatments were combined into those with only soil plus soil with fertilizer (C), fly ash only (AH), biosolids only (BS), or mixtures of fly ash and biosolids (M) at the ratios given in Table 1. Using this methodology, the average data for each of the four groups of treatments could be assessed relative to their effect on the physicochemical and microbial characteristics of the soil and carrot yield.
The impact of the amendments was tested for two consecutive growing seasons at the University of Western Ontario, Environmental Sciences Western, Field Station, Ilderton, Ontario, Canada. The local soil is a Bryanston silt loam so the soils were first amended with brick sand to approximate the texture of a sandy loam (46% sand, 46% silt, and 8% clay), which is more conducive to nematode proliferation. Stabilized biosolids were collected from the municipal sewage settling ponds at the town of Glencoe, Ontario, Canada, and the fly ash came from the Lambton coal-fuelled generating station, St. Clair River, St. Clair Township, Ontario, Canada. For each season, triplicates of each treatment (Table 1) were placed in microplots (12-L plastic pots) and then randomly buried in the field with a distance of 0.75 m between pots and 1.5 m between rows. Treatment ratios and application rates were prepared based on previous studies (Christie et al. 2001; Canadian International Development Agency 2002). Collection and preparation of samples for analyses is given below. Treatment details are given in Table 1, which gives the percentages of each amendment (fertilizer, ash, biosolids, or a combination of both) added to the soil of each 12-L microplot.
Carrot sowing
Treatments were sown with 2 to 3 carrot (Daucus carota L.) seeds each in six holes equidistant from the center of the pot. Soil amendments were prepared and sampled for baseline analyses. Carrot seeds were sown immediately after amendments were prepared. Sprouts were thinned to three plants per pot 2 weeks after emergence based simply on the size of the sprouts.
Treatment sampling
The samples for analyses were prepared by combining samples from each of the triplicates of each treatment into a composite sample. The composite samples included a 2.5 cm × 15 cm core from each of the microplots. Core samples were placed into plastic buckets and disaggregated/mixed until homogeneous. Replicates for different analyses were taken by either cone and quarter or riffle splitter method. In this manner, each of the samples (n) from Table 1 actually represents the average of three replicates.
Electrical conductivity (EC) and pH
From each sample, 20 g was transferred to a 100-mL beaker; 40 mL of deionized water was added followed by rotary shaking for 30 min and settling for 30 min. The pH was measured using an Accumet Model 10 pH meter (Thermo-Fisher Scientific, Waltham, MA, USA) with an Orion 9172 BN probe (Thermo-Fisher Scientific, Waltham, MA, USA) calibrated at room temperature with standard buffers of pH 4, 7, and 10. Subsequently, EC was determined on a 15-mL aliquot of the suspension using a HI 8033 handheld EC/TDS meter (Hanna Instruments, Smithfield, RI, USA) for the range 0 to 1,999 μS.
Nematode extraction and counting
A 100-mL aliquot of the composite sample from each treatment was placed in 2 L of water and stirred using a metal spatula for 2 min to disaggregate the sample. The suspension was allowed to settle for 30 s and then passed through a series of 60 and 325 mesh sieves (openings of 250 and 45 μm, respectively). The 325 mesh fraction was transferred onto a Baermann tray (Whitehead and Hemming 1965) and incubated at room temperature for 24 h. Nematodes were counted using a dissecting microscope and categorized into four feeding groups (bacterial feeders, fungal feeders, plant feeders, and predators; Yeates et al. 1993).
Bacterial and fungal colony-forming units
An LB broth with nystatin (0.5 g L-1) was used to culture bacterial colony-forming units (CFU), and a PDA agar with streptomycin (0.1 g L-1) and tetracycline (0.01 g L-1) was used for fungal CFU (Riegel et al. 1996). A subsample of each treatment (0.5 g) was mixed with 4.5 mL of autoclaved deionized water and vortexed for 2 min. One hundred microliters of diluted suspension for each treatment triplicate was plated for either bacteria or fungi counts under a laminar flow hood and incubated at 25°C in the dark. Bacterial CFU were counted 3 days after plating and fungal CFU were counted 5 days after plating.
Statistical analyses
The data for each set of treatments were combined in order to determine the impact of fly ash, biosolids, and mixtures of both (Table 1). Data were then subjected to (one-way) ANOVA followed by Tukey's range test (SPSS Statistics 17.0, Chicago, IL, USA).
Elemental analyses
The concentration of plant-available elements was determined after Mehlich III extraction (Mehlich 1984) followed by ICP-AES analysis on baseline samples collected for season 1. Because the same soils and the same raw materials were used to prepare the amendments, the data collected for the first set of samples were used as a reference for the entire study.
Results
For both growing seasons, data (average of 3%, 6%, 9%, 12%, and 15% (w/w) addition of amendment types given) on nematodes, pH, and EC for the average of triplicate microplots for each treatment were used when evaluating the impact of the treatments on soils and nematodes (Table 2). Samples were grouped so that differences due to the amendments could be determined. Soil and soil with fertilizers (C) represent the control and give the average data for those samples that received no ash or biosolids, while all samples receiving AH or BS or a mixture of the two (M) represent amended soils. It should be noted that the average data for the triplicate microplots of each of the 3%, 6%, 9%, 12%, and 15% (w/w) application rates for the amendments were extremely variable. It was therefore necessary to compare the averages in order to discern the slight trends observed between treatments and over time.
Nematodes (bacterial feeders, fungal feeders, and plant feeders)
For seasons 1 and 2, average counts for all nematode types from each replicate of a given treatment were extremely variable. As shown in Table 2, even the average numbers vary significantly and are not consistent going from baseline to harvest in most cases. For season 1 across all treatment types, plant feeder (PF) numbers were highest followed by bacterial feeders (BF) and fungal feeders (FF). Numbers for all nematode types were higher in season 2 than in season 1. For the different treatments, the ratio of BF and FF at baseline relative to harvest increased with the exception of BF with M in season 2 and FF with BS in season 1. The opposite was true for PF which decreased in numbers (or remained similar) going from baseline to harvest. In general, addition of AH resulted in decreased numbers relative to C, and numbers were highest in either the BS or M treatments.
pH
The pH of the soils, ash, and biosolids are very similar, varying by only 0.2 to 0.4 pH units within treatment types going from baseline to harvest and only varying by about 0.1 to 0.2 units between treatment types at any given time. Overall, the pH data across treatments and times was slightly higher in season 2.
Electrical conductivity (EC)
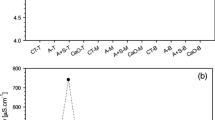
Overall, EC was significantly higher in AH treatments, with the exception of season 2 and baseline, and was lowest in the C group. For the mixtrures (M), those containing the higher percentage of ash had higher EC, at baseline and harvest. Electrical conductivity decreased from baseline to harvest in both seasons.
Bacterial and fungal colony-forming units (season 2 only)
Bacterial and fungal CFU data for season 2 are given in Table 3. Bacterial units are greater than fungal units by over ten times for all treatment types. Within treatment types, there is no trend from baseline to harvest for bacterial colonies, but there is a distinct decrease in fungal colonies. As shown, numbers do not correlate between sampling periods when only AH is added but increase significantly for either BS or M treatments.
Concentrations of elements in amendments (ash and biosolids)
The average concentrations of 14 elements in each of the treatment groups are given in Table 4; Ontario guidelines for some of the elements are included (OMAFRA 1996). Most of the differences between treatment groups are negligible with marginal increases due to either the ash (As, B, Cr, and Fe) or biosolids (Ca, Cu, K, Mg, P, and Pb). All of the elements of potential concern are well below international standards for use on agricultural fields except B, which would not cause a problem at the ratios and application rates chosen for this study. Therefore, no further testing (e.g., at harvest) was done.
Carrot (Daucus carota) yield
Significant differences in carrot yield were found among treatment groups for both seasons (Table 5). Higher yields during season 1 may have been due to climate/soil variations; determining the reasons for these differences was not part of this study. For both seasons, addition of AH caused significant decreases in yields relative to C while the highest yields were observed for either BS or M treatments.
Discussion
Bacterial-feeding nematodes (BF)
Bacterial-feeding nematodes increased marginally between baseline and harvest for all treatment groups with the exception of season 2, M treatments. There were no significant correlations between BF and the other parameters when all data for all treatments were considered together. However, when averaged data for each treatment type is considered, BF activity is inversely related to both PF and pH for seasons 1 and 2 and strongly correlated with carrot yields for season 1, but less so for season 2. Other parameters showed negligible associations. Addition of ash only resulted in decreasing BF numbers while BS and/or M caused significant increases.
These results are consistent with numerous other studies using ash or biosolids. Decreases in BF populations towards the end of the seasons are consistent with the findings of Mitchell et al. (1978), who found higher BF populations at the beginning of the test, declining toward the end. These results support the idea that addition of either amendment (ash or biosolids) or a mixture can have a positive effect on BF nematode populations. It is likely that ash alone, which did provide a better media for nematode propagation, was not as effective as when biosolids were added.
Even though the fertilizer also supplied additional nutrients, the biosolids appear to be more significant. These findings are consistent with Weiss and Larink (1991) who suggested that the increase in BF populations found in their sewage sludge-amended treatments was due to an increased nutrient content that led to an increase in microbial biomass, which is also consistent with the results obtained by Dmowska and Kozlowska (1988). In an experiment involving sludge application to a gravelly loam at pH of 7.2 to 7.6, Mannion et al. (1994) reported very little variation in BF populations, suggesting that at mildly basic pH levels, the influence of sludge on BF is insignificant. These finding are consistent with the present study, where pH ranges were small and close to 8.0.
Elements that can affect nematode populations include As, Cd, Cr, Cu, Ni, Pb, and Zn. Georgieva et al. (2002) found that soils that received heavy metals go through changes that might alter the microbial community, favoring a few species and reducing the positive interactions among them. Higher nematode populations were associated with anaerobically digested sludge with heavy metals in the range of 250 to 2,600 mg kg-1 Zn, 150 to 500 mg kg-1 Cu, 3 to 43 mg kg-1 Cd, 60 to 500 mg kg-1 Cr, 200 to 1,400 mg kg-1 Pb, and 28 to 201 mg kg-1 Ni. These data are much higher than for this study (Table 4) and explain why metal effects were not noted.
Bardgett et al. (1994) found that microbial respiration decreased and bacterial-feeding nematodes increased as Cu, Cr, and As increased for a stony silt loam at pH 5.7. For a loamy, moderately fine sandy soil, Korthals et al. (1996a) found that at pH of 4–4.7 and 125 mg kg-1 Cu, total nematode populations decreased; additionally, lower pH and higher levels of Cu caused BF populations to decrease. Korthals et al. (1996b) found that concentrations of 1600 mg kg-1 Cu applied to a sandy soil resulted in increases of BF relative to other species. The difference between the last two experiments is that the first looked at the long-term effects and the latter at short-term effects, suggesting that exposure time to the contaminant caused BF to react differently. Weiss and Larink (1991) reported that BF populations increased dramatically with the addition of sewage sludge and/or sewage sludge with metals added for a loamy sandy soil at pH 6.4; where total nematode populations and bacterial-feeding nematodes increased from 1729 to 7216/100 g and 318 to 2950/100 g of soil, respectively. These data are much higher than the present study (Table 4). In another experiment (Georgieva et al., 2002) that used sludge with metals added (Ni, Cu, and Zn), total BF populations were 16% and 21% higher in treatments to which Ni and Zn had been added, respectively. In the latter study, it was suggested that the increase in BF populations might be due to the wide range of life strategies exhibited by these nematodes, allowing them to adapt to polluted environments. Several studies have found that different concentrations of Cd, Cr, Se, and Zn reduce BF populations because the bacteria they feed on adsorbed enough metals during their life cycle to make them undesirable as food (Bisessar 1981; Doelman et al. 1984; Bouwman et al. 2005; Smit et al. 2002; Korthals et al. 1998; Bakonyi et al. 2003).
In a lab assay, Doelman et al. (1984) showed that the addition of 2–20 mg kg-1 Cd and 20–1000 mg kg-1 Pb to growth media lead to the adsorption of the metals onto bacteria, which resulted in a reduction of BF. As far as metal concentrations are concerned, it bears mentioning again, that in all of these studies the concentrations of the selected metals were much higher than for this work, which might explain why differences in BF were not noted here. In addition, the pH values of the treatments in the present study were high, and limited metal mobility, also decreasing the likelihood of interfering with nematode proliferation.
Fungal-feeding nematodes (FF)
Fungal-feeding nematodes also seemed to have responded well to BS and M treatments and in one case to AH (season 2, baseline). They also showed a mild positive correlation with PF and pH in season 1, but this trend was negative for season 2. These nematodes showed a moderate to strong association with carrot yields for the 2 seasons. The C for season 1 produced the highest numbers while season 2 showed a preference for both BS and M. Fungal-feeding nematodes at different sampling times were, on average, lower in numbers relative to the BF. Reduced numbers for BS and M at harvest for each season may be the result of decaying organic matter with time, which may influence the mobility and the availability of elements (McBride et al. 1997). The changes in the stored biosolids might have caused changes in nutrient availability, which in turn caused microbial populations to differ from year to year, ultimately affecting nematode populations.
Similarly to BF populations, FF populations are controlled by direct and indirect effects. In the experiment by Korthals et al. (1996a) higher FF populations were found under the same soil conditions described previously. In another experiment using the same soil, Korthals et al. (1998) found that at 400 mg kg-1 each of Cu and Zn, there was an increase in FF. The authors suggest that higher concentrations of Cu and Zn and lower pH cause bacterial biomass to decrease, favoring fungal growth. This shift in microbial communities related to pH change was shown in the experiment conducted by Rousk et al. (2009). Additionally, there is reduced food competition and predation for FF, causing an increase in their populations. Smit et al. (2002) and Nagy (1999) found that at 1,800 mg kg-1 Zn and 270 mg kg-1 Cu, few species of FF increased. Contrasting with the results from Nagy (1999), a long-term study by Bakonyi et al. (2003) showed a reduction in FF at a total of 270 mg kg-1 Cd, Cr, and Zn in different proportions. Georgieva et al. (2002) showed that low levels of Ni (19 mg kg-1), Zn + Ni (97 and 16 mg kg-1, respectively), and Zn + Cu (109 and 68 mg kg-1, respectively) resulted in an increase in FF.
In a short-term study, the results reported by Korthals et al. (1996b) showed a reduction in FF relative to other species. Bisessar (1981) reported that concentrations of Pb, As, Cd, and Cu of 3,564, 163, 26, and 333 mg kg-1, respectively, decreased FF populations. In the lab assay by Doelman et al. (1984) described previously, levels of Cd and Pb ranged from 1 to 25 mg kg-1 and 10 to 250 mg kg-1, respectively. The latter authors have suggested that the reduction in FF is due to the absorption of heavy metals by fungal hyphae, which makes this food source toxic, causing the nematodes to stop feeding. Furthermore, high heavy metal concentration in the soil reduces fungal biomass, reducing food availability. These studies were carried out in the pH range between 4.5 and 6.4, suggesting that extreme pH levels enhance heavy metal effects, which was not the case for this study, where pH was near 8.0 in all cases.
Plant-feeding nematodes
Overall, counts for PF nematodes were greater than either BF or FF. Plant feeders (as well as FF season 1) also showed a higher preference for C relative to the other treatments at harvest times, which is opposite for both FF and BF. This difference may be due to their reliance on carrot biomass/hosting in order to support their ontogeny, which is not the case for the other nematode types. Plant feeders also showed a strong positive association with pH and an inverse association with EC. In addition, the PF did not correlate well with carrot yield for either season which may indicate that the total carrot biomass produced is not as important as the simple presence of the carrots as a host organism. The moderate to strong negative correlation between PF and BF and FF can be explained by the fact that PF nematodes are more affected by indirect rather than the direct effects, i.e., microbiology of the soil over physicochemical changes due to the ash or biosolids.
Weiss and Larink (1991) reported higher PF populations in plots that had higher plant biomass when sludge and heavy metals were added to a loamy sandy soil at pH 6.4. Similar results were found by Bouwman et al. (2005) and Georgieva et al. (2002), where higher PF populations were associated with higher levels of Cd and Zn. Bouwman et al. (2005) suggested that PF populations kept feeding on the roots of plants with high levels of heavy metals because metal content was lower in the root than the shoot. Additionally, higher Zn contents, which reduced plant biomass, might have also reduced PF antagonists including root nodule bacteria, mycorrhizal fungi, and predatory nematodes. Bakonyi et al. (2003) found higher PF populations associated with improved plant growth in plots that received 270 mg kg-1 Zn. It has also been shown that intermediate levels of Zn (50 to 200 mg kg-1) applied to a sandy loam at pH of 4.1 caused PF populations to increase, suggesting that higher root leakage diminished the plant's defense mechanism (Korthals et al. 1998). In a calcareous loamy chernozem with a pH of 7.4, Nagy (1999) found that plots that received a total of 270 mg kg-1 Ni and Zn at different ratios had higher wheat yield and higher PF populations. In the same study, 228 mg kg-1 Cd and 10 mg kg-1 Cr proved to be phytotoxic, reducing the availability of wheat as a food source for PF. These results suggest that PF populations are more closely related to food supply and not directly to the concentrations of heavy metals. Under experimental conditions described previously, Mannion et al. (1994) found that low applications of sludge (8% to 24% w/w) did not have an effect on PF populations. Once again, these levels of metals, pH, and sludge application are far different from the present study and may account for why the same impact on nematodes was not noted.
Electrical conductivity
The most pronounced changes in soil physicochemical status for this study was caused by the addition of ash and the attendant increase in EC (approximately 1.5 to 3 times increase over C only at harvest) either by itself or when mixed with biosolids. Changes in EC are negatively correlated with all other parameters except pH. Soil electrical conductivity can be an important predictor of soil biological activity, affecting important soil processes related to physical and chemical interactions. Such interactions can influence nematode activity in soils in relationship with plant development and microbial communities. In a report by Vellidis et al. (2006), soil electrical conductivity was used in combination with nematode sampling to correlate areas of specific EC with root-knot nematode abundance. The authors reported that lower populations of nematodes (4 to 115 nematodes/unit of soil) were found in areas with EC in the range of 27 to 100 μS cm-1. These numbers are low compared to the present study where EC ranged (average range for all treatments) from 168 to 640 and 111 to 732 μS cm-1 for season 1 and 2, respectively (Table 2), suggesting that EC might be responsible for low nematode counts. Nkem et al. (2006) reported no nematode survival in soil samples with EC >4,100 μS cm-1, while at 1,945 μS cm-1 they reported 80% to 97% survival rates. These researchers suggest that different species of nematodes can tolerate different levels of salinity by entering into a state of osmobiosis. Electrical conductivity levels from the present study did not reach these levels during either season.
pH
The average pH within/between all treatment groups and at different times from this study is very close, at 8.0 ± 0.2 (±0.1 in most cases). At these pH ranges, there is little difference in neither metal/nutrient mobility nor availability nor an appreciable effect on nematode ontogeny. Any trends/correlation between pH and the other parameters was probably due more to EC, which also increased pH slightly.
Several studies (Korthals et al. 1996a, b, 1998, 2000; Bardgett et al. 1994; Bouwman et al. 2005; Burns 1970) have demonstrated that pH values in the range of 4.1 to 6.0 might enhance the direct and indirect effects that influence changes in nematode community structure. The combination of direct and indirect effects might favor one or more nematode feeding groups based on increased availability of food or reduced predation from omnivorous or predatory nematodes, which appear to be highly sensitive to heavy metals. Other effects from heavy metals, enhanced by extreme pH levels, might be the higher bioavailability of metals that cause a reduction in plant, bacterial, or fungal biomass, influencing the shifts in nematode populations from different feeding groups. In the previously discussed experiment by Weiss and Larink (1991), the authors found increased nematode populations from all feeding groups at a pH of 6.4. Populations of bacterial, fungal, and plant-feeding nematodes remained stable throughout the experiment by Mannion et al. (1994), which is described in the discussion on BF.
Soil bacteria and fungi
Bacterial and fungal CFU were cultured in season 2 only and both (Table 3) increased with addition of ash and biosolids. Changes in bacterial CFU were less pronounced than for fungal CFU. The addition of AH did not have much effect on bacterial CFU, and only minor impact was noted for BS and M treatments. Fungal CFU responded dramatically to the application of BS and M to a lesser degree; the response to AH was less pronounced and mixed.
These results are in agreement with the literature where addition of organic matter to soils has been shown to benefit bacterial and fungal populations by improving nutrient cycling and enhancing soil health (Riegel and Noe 2000; Litterick et al. 2004). Conversely, addition of fly ash to soils at greater than 10% (w/w) has been shown to slow microbial respiration.
Levels of heavy metals in the present study were adequate for maintaining/improving microbial communities. Bacterial and fungal colony-forming units are affected by the same parameters that affect nematodes, favoring or inhibiting their growth and their role as decomposers. In a soil medium that received anaerobically digested sewage sludge with different concentrations of Cd (0.1 to 111 mg kg-1), Cu (11 to 556 mg kg-1), and Cr (1 to 556 mg kg-1), Zibilske and Wagner (1982) reported an initial positive response in bacterial and fungal activity that declined over time and even exhibited inhibition at the end of the incubation period. Microbial populations exhibited these changes at different incubation times depending on the metal added (2 weeks for Cd and Cr, and 1 week for Cu). Additionally, the authors suggest that the higher tested levels of Cd and Cr affect fungal sporulation favoring some species and inhibiting others. The levels of the selected heavy metals from that study are similar to those found in some sludges, presenting a realistic approach on reduction in microbial activity fundamental to sludge decomposition. A study by Schutter and Fuhrmann (2001) showed that application of 25% of fly ash to soils might benefit fungi and some bacteria. They determined this by measuring the fatty acid content in whole soil, which indicated that these populations were enhanced in a soil system that had improved plant growth and nutrient content as a result of fly ash application. The heavy metal levels in the soil amended with fly ash used by the latter authors were 10 mg kg-1 As, 5 mg kg-1 B, 0.03 mg kg-1 Cd, 1 mg kg-1 Cr, 1 mg kg-1 Cu, 0.70 mg kg-1 Ni, and 0.50 mg kg-1 Pb, which in some cases are higher and in some cases lower than those used in the present study (Table 4).
Carrot yield
Carrot yield data are given in Table 5 for both seasons. The application of ash only resulted in a substantial decrease in yield for both seasons, which is not consistent with the literature and may be a result of the large increase in EC that resulted from ash application. A twofold increase in yield was noted for both seasons with the addition of either BS or M. Biosolids had the largest effect in season 1 while the mixtures were more prevalent in season 2. Carrot yield correlated positively with BF activity in season 1 and was inversely related to PF, FF, pH, and EC. During season 2, yield was positively related to BF and FF and negatively related to the other parameters. Recommendations by Fritz et al. (2006) for carrot cultivation include the selection of well-drained soils with a sandy loam texture in the pH range 5.5 to 7.0. Soil texture for the present study resembles a sandy loam in which drainage and organic matter were improved with increased biosolid application. Sterrett et al. (1982) reported phytotoxicity to several cropped plants at >500 mg kg-1 Zn, >500 mg kg-1 Mn, >25 mg kg-1 Cu, and >50 mg kg-1 Ni. Levels of these heavy metals in the present study were 3.3 mg kg-1 Zn, 72 mg kg-1 Mn, 2.4 kg-1 Cu, and 0.5 mg kg-1 Ni, being much lower than those reported by the previous authors.
Conclusions
Foremost, it must be noted that this study attempts to delineate the benefits of coal fly ash and/or stabilized biosolids as soil amendments in the roll of suppressing harmful nematode damage. The scope of the study did not allow for optimization trials; rather, its goal was to provide proof of concept trials which can be expanded on during future research. It should also be noted that the vast majority of the research quoted here has been done in a greenhouse environment, where most external variables can be controlled. This work was done in the field, and shortcomings regarding the results may very well be related to ambient climatic/soil changes that were outside the purview of this work. We would suggest that until trials are conducted under field conditions, the results are not necessarily applicable to real agricultural scenarios. The data for this study were extremely variable, which enforces the fact that statistically there is a need to include as many replications as possible when doing nematode studies; this work was not allowed that benefit.
Positive results from this work indicate that at the pH and metal ranges for the raw materials used, little impact on nematode activity was noticed. Future studies would be well advised to analyze raw materials before running trials so that the materials can be preselected for the most potential impact on nematode management. In the case of this study, our goal was to use locally available materials that would be financially viable 'if’ they produced the desired outcomes.
Electrical conductivity - as provided by the fly ash - had the greatest impact on nematodes and yield, which is sometimes positive and sometimes negative. Biosolids had, for the most part, the largest positive influence on nematode numbers, which is not necessarily good, but also provided the best increases in yield. Mixtures of ash and biosolids showed similar results.
Author’s information
PJ-L is a postdoctoral fellow at the Centro de Investigaciones en Geografía Ambiental, Universidad Nacional Autónoma de México, Campus Morelia and is currently carrying out research using biosolids and fly ash in reforestation practices. PJ-L has worked with organic wastes for more than 10 years and has been involved in research activities since 2003. PJ-L carried out the research for this study and drafted the manuscript. MP is an adjunct professor at the University of Alberta and has more than 30 years of research experience using waste materials in soil remineralization studies in India, Canada, China, Ecuador, and Colombia. MP participated in the design and coordination of the study and helped to draft the manuscript. Both authors read and approved the final manuscript.
References
Akhtar M, Alam MM: Utilization of waste materials in nematode control: a review. Bioresour Technol 1993, 45: 1–7. 10.1016/0960-8524(93)90134-W
Akhtar M, Malik A: Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: a review. Bioresour Technol 2000, 74: 35–47. 10.1016/S0960-8524(99)00154-6
Bakonyi G, Nagy P, Kádár I: Long-term effects of heavy metals and microelements on nematode assemblage. Toxicol Lett 2003, 141: 391–401.
Bardgett RD, Speir TW, Ross DJ, Yeates GW, Kettles HA: Impact of pasture contamination by copper, chromium, and arsenic timber preservative on soil microbial properties and nematodes. Biol Fert Soils 1994, 18: 71–79. 10.1007/BF00336448
Bisessar S: Effect of heavy metals on microorganisms in soils near a secondary lead smelter. Water Air Soil Pollut 1981, 17: 305–308.
Bouwman LA, Bloem J, Römkens PFAM, Japenga J: EDGA amendment of slightly heavy metal loaded soil affects heavy metal solubility, crop growth and microbivorous nematodes but not bacteria and herbivorous nematodes. Soil Biol Biochem 2005, 37: 271–278. 10.1016/j.soilbio.2004.07.039
Burns NC: Soil pH effects on nematode populations associated with soybeans. J Nematol 1970, 3: 238–245.
Canadian International Development Agency: Land restoration through waste management & fly ash management in India. Kharagpur, West Bengal, India: IIT-Kharagpur; 2002.
Christie P, Easson DL, Picton JR, Love SCP: Agronomic value of alkaline-stabilized sewage biosolids for spring barley. Agron J 2001, 93: 144–155. 10.2134/agronj2001.931144x
Davies BE: Deficiencies and toxicities of trace elements and micronutrients in tropical soils: limitations of knowledge and future research needs. Environ Toxicol Chem 1997, 16: 75–83. 10.1002/etc.5620160108
Dmowska E, Kozlowska J: Communities of nematodes in soil treated with semi-liquid manure. Pedobiologia 1988, 32: 323–330.
Doelman P, Nieboer G, Schrooten J, Visser M: Antagonistic and synergistic toxic effects of Pb and Cd in a simple foodchain: nematodes feeding on bacteria or fungi. Bull Environ Contam Toxicol 1984, 32: 717–723. 10.1007/BF01607562
Edwards CA: The impact of pesticides on the environment. In The pesticide question: environment, economics, and ethics. Edited by: Pimentel D, Lehman H. New York: Chapman Hall; 1993.
Evenson RE, Gollin D: Assessing the impact of the green revolution, 1960 to 2000. Science 2003, 300: 758–762. 10.1126/science.1078710
Fritz V, Tong C, Rosen C, Wright J: Carrots (Daucus carota). University of Minnesota Extension Service; vegetable crop management; 2006. http://www.extension.umn.edu/distribution/horticulture/dg7196.html
Georgieva SS, McGrath SP, Hopper DJ, Chambers BS: Nematode communities under stress: the long-term effects of heavy metals in soil treated with sewage sludge. Appl Soil Ecol 2002, 20: 27–42. 10.1016/S0929-1393(02)00005-7
Guerena M: Nematode: alternative controls. Fayetteville, AR: ATTRA Publication IP287. National Sustainable Agriculture Information Service; 2006.
Haydock PPJ, Woods SR, Grove IG, Hare MC: Chemical control of nematodes. In Plant nematology. Edited by: Perry RN, Moens M. Wallingford: CABI; 2006.
Kabirinejad S, Hoodaji M: The effects of biosolid application on soil chemical properties and Zea mays nutrition. Int J Recycling Org Waste Agric 2012, 1: 4. 10.1186/2251-7715-1-4
Korthals GW, Alexiev AD, Lexmond TM, Kammenga JE, Bongers T: Long-term effects of copper and pH and the nematode community in an agroecosystem. Environ Toxicol Chem 1996, 15: 979–985. 10.1002/etc.5620150621
Korthals GW, Van de Enge A, Van Megen H, Lexmond TM, Kammenga JE, Bongers T: Short-term effects of cadmium, copper, nickel and zinc on soil nematodes from different feeding and life-history strategy groups. Appl Soil Ecol 1996, 4: 107–117. 10.1016/0929-1393(96)00113-8
Korthals GW, Popovici I, Iliev I, Lexmond TM: Influence of perennial ryegrass on a copper and zinc affected terrestrial nematode community. Appl Soil Ecol 1998, 10: 73–85. 10.1016/S0929-1393(98)00039-0
Korthals GW, Bongers M, Fokkema A, Dueck TA, Lexmond TM: Joint toxicity of copper and zinc to a terrestrial nematode community in an acid sandy soil. Ecotoxicology 2000, 9: 219–228. 10.1023/A:1008950905983
Litterick AM, Harrier L, Wallace P, Watson CA, Wood M: The role of uncomposted materials, composts, manures, and compost extracts in reducing pest and disease incidence and severity in sustainable temperate agricultural and horticultural crop protection - a review. Crit Rev Plant Sci 2004, 23: 435–479.
Magdoff F: Concept, components, and strategies of soil health in agroecosystems. J Nematol 2001, 33: 169–172.
Mannion CM, Schaffer B, Ozores-Hampton M, Bryan HH, McSorley R: Nematode population dynamics in municipal solid waste-amended soil during tomato and squash cultivation. Nematropica 1994, 24: 17–24.
McBride MB, Richards BK, Steenhuis T, Russo JJ, Sauvé S: Mobility and solubility of toxic metals and nutrients in soil fifteen years after sludge application. Soil Sci 1997, 162: 487–500. 10.1097/00010694-199707000-00004
Mehlich A: Mehlich III soil test extractant: a modification of the MII extractant. Comm Soil Sci Plant Anal 1984, 15: 1409–1416. 10.1080/00103628409367568
Mitchell MJ, Hartenstein R, Swift BL, Neuhauser EF, Abrams BI, Mulligan RM, Brown BA, Craig D, Kaplan D: Effects of different sewage sludges on some chemical and biological characteristics of soil. J Environ Qual 1978, 7: 551–559.
Nagy P: Effect of an artificial metal pollution on nematode assemblage of a calcareous loamy chernozem soil. Plant Soil 1999, 212: 35–43. 10.1023/A:1004657924496
Nkem JN, Virginia RA, Barrett JE, Wall DH, Li G: Salt tolerance and survival thresholds for two species of Antarctic soil nematodes. Polar Biol 2006, 29: 643–651. 10.1007/s00300-005-0101-6
OMAFRA: Guidelines for the utilization of biosolids and other wastes on agricultural land. Ontario, Canada: Ministry of environment and energy; 1996.
Parkpian P, Leong ST, Laortanakul P, Juntaramitree J: An environmentally sound method for disposal of both ash and sludge wastes by mixing with soil: a case study of Bangkok Plain. Environ Monit Assess 2002, 74: 27–43. 10.1023/A:1013850620819
Punshon T, Adriano DC, Weber JT: Restoration of drastically eroded land using coal fly ash and poultry biosolid. Sci Total Environ 2002, 296: 209–225. 10.1016/S0048-9697(02)00128-6
Qi Y, Hu C: Soil nematode abundance in relation to diversity in different farming management system. World J Agric Sci 2007, 3: 587–592.
Riegel C, Noe JP: Chicken litter soil amendment effects on soilborne microbes and Meloidogyne incognita on cotton. Plant Dis 2000, 84: 1275–1281. 10.1094/PDIS.2000.84.12.1275
Riegel C, Fernandez FA, Noe JP: Meloidogyne incognita infested soil amended with chicken litter. J Nematol 1996, 28: 369–378.
Rodriguez-Kabana R, Morgan-Jones G, Chet I: Biological control of nematodes: soil amendments and microbial antagonists. Plant Soil 1987, 100: 237–247. 10.1007/BF02370944
Rousk J, Brookes PC, Bååth E: Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 2009, 75: 1589–1596. 10.1128/AEM.02775-08
Schutter ME, Fuhrmann JJ: Soil microbial community responses to fly ash amendment as revealed by analyses of whole soils and bacterial isolates. Soil Biol Biochem 2001, 33: 1947–1958. 10.1016/S0038-0717(01)00123-7
Siddiqui ZA, Mahmood I: Role of bacteria in the management of plant parasitic nematodes: a review. Bioresour Technol 1999, 69: 167–179. 10.1016/S0960-8524(98)00122-9
Smit CE, Schouten AJ, Van den Brink PJ, Van Esbroek MLP, Posthuma L: Effects of zinc contamination on a natural nematode community in outdoor soil mesocosms. Arch Environ Con Tox 2002, 42: 205–216. 10.1007/s00244-001-0029-y
Sterrett SB, Chaney RL, Reynolds CW, Schales FD, Douglas LW: Transplant quality and metal concentrations in vegetable transplants grown in media containing sewage sludge compost. HortSci 1982, 17: 920–922.
Stirling GR: Biological control of plant parasitic nematodes. Wallingford, UK: CAB International; 1991.
Stirling GR, Wilson EJ, Stirling AM, Pankhurst CE, Moody PW, Bell MJ, Halpin N: Amendments of sugarcane trash induce suppressiveness to plant-parasitic nematodes in a sugarcane soil. Australas Plant Pathol 2005, 34: 203–211. 10.1071/AP05022
Vellidis G, Perry C, Rucker K, Kemerait B: Using soil electrical conductivity and pH to identify nematode-prone areas. Final report submitted to Georgia agricultural commodity commission for peanuts. Tifton, GA: NESPAL, University of Georgia; 2006.
Walker GE: Effects of organic amendments, fertilizers and fenamiphos on parasitic and free-living nematodes, tomato growth and yield. Nematol Medit 2007, 35: 131–136.
Weiss B, Larink O: Influence of sewage sludge and heavy metals on nematodes in an arable soil. Biol Fert Soils 1991, 12: 5–9. 10.1007/BF00369381
Westphal A: Detection of soils with specific nematode suppressiveness. J Nematol 2005, 37: 121–130.
Whitehead AG, Hemming JR: A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 1965, 55: 25–38. 10.1111/j.1744-7348.1965.tb07864.x
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS: Feeding habits in soil nematode families and genera - an outline for soil ecologists. J Nematol 1993, 25: 315–331.
Zasada IA: Factors affecting the suppression of Heterodera glycines by N-Viro soil. J Nematol 2005, 37: 220–225.
Zasada IA, Tenuta M: Chemical-mediated toxicity of N-Viro soil to Heterodera glycines and Meloidogyne incognita. J Nematol 2004, 36: 297–302.
Zasada I, Rogers S, Sardanelli S: Application of alkaline-stabilized biosolids for Meloidogyne incognita suppression in microplots. Nematology 2007, 9: 123–129. 10.1163/156854107779969682
Zibilske LM, Wagner GH: Bacterial growth and fungal genera distribution in soil amended with sewage sludge containing cadmium, chromium, and copper. Soil Sci 1982, 134: 364–370. 10.1097/00010694-198212000-00004
Acknowledgements
The authors would like to thank Mr. Peter Duenk and Ms. Caroline Rasenberg (Environmental Sciences Western Field Station, University of Western Ontario, London, Canada) for help with all phases of the field experiment. Funding for this research was provided by Dr. Don Hayden from the Department of Biology at the University of Western Ontario. Many thanks go to Dr. Gary Lawrence from Mississippi State University for help with data analysis and interpretation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they do not have any competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jaramillo-López, P.F., Powell, M.A. Application of stabilized biosolids and fly ash mixtures as soil amendments and their impact on free living nematodes and carrot (Daucus carota) yield. Int J Recycl Org Waste Agricult 2, 22 (2013). https://doi.org/10.1186/2251-7715-2-22
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7715-2-22