Abstract
Background
The inhibition effect of two synthesized isatin compounds, namely 1-morpholinomethyl-3(1-N-dithiooxamide)iminoisatin [MMTOI] and 1-diphenylaminomethyl-3(1-N-dithiooxamide)iminoisatin [PAMTOI], on the corrosion inhibition of N80 steel in 15% HCl solution was studied by polarization, alternating current impedance (electrochemical impedance spectroscopy), and weight loss measurements. The surface examination was carried out by scanning electron microscopy and Fourier transform infrared spectroscopy.
Results
The compounds [PAMTOI] and [MMTOI] show the maximum of 91.2% and 84.3% inhibition efficiency, respectively, at 200-ppm concentration. Polarization curves revealed that the used inhibitors represent mixed-type inhibitors. Adsorption of used inhibitors led to a reduction in the double-layer capacitance and an increase in the charge transfer resistance.
Conclusions
Results show that both inhibitors were effective inhibitors and their inhibition efficiency was significantly increased with increasing concentration. Adsorption of both compounds obeys the Langmuir adsorption isotherm.
Similar content being viewed by others
Background
N80 steel is widely used as a construction material for pipe work in the oil and gas production, such as downhole tubular, flow lines, and transmission pipelines in the petroleum industry. Mineral acids, particularly hydrochloric acid, are frequently used in industrial processes involving acid cleaning, acid pickling, acid descaling, and oil well acidizing [1–3]. In the petroleum industry, 15% HCl is commonly used for acidizing treatment because it leaves no insoluble product after the treatment and is found to be commercially available and cheap, but adversely at the same time, it severely attacks the metal casings and tubular of oil well during the acidizing process. Therefore, protective measures should be required to prevent the metal loss due to corrosion by using chemical and other means. Due to the aggressiveness of acids, inhibitors are often used to reduce the rate of dissolution of metals. Most of the well-known acid inhibitors are organic compounds containing nitrogen, oxygen, and/or sulfur atoms; heterocyclic compounds; and delocalized π-electrons [4–12]. The polar functional groups are usually regarded as the reaction center for the establishment of the adsorption process [13]. It is generally accepted that organic molecules inhibit corrosion via adsorption at the metal-solution interface [14, 15], making the adsorption layer to function as a barrier and isolating the metal from the corrosion [16]. Some Mannich bases have been reported as efficient corrosion inhibitors [17, 18], and the literature available to date about the Mannich bases used as corrosion inhibitors is limited. Keeping in view the importance of Mannich bases as potential corrosion inhibitors, we have synthesized k;1-morpholinomethyl-3(1-N-dithiooxamide)iminoisatin [MMTOI] and 1-diphenylaminomethyl-3(1-N-dithiooxamide)iminoisatin [PAMTOI] and studied their corrosion inhibition properties for N80 steel in 15% HCl solution.
Methods
Materials and surface preparation
The working electrode for electrochemical studies and specimens for weight loss experiments were prepared from oil-well N80 steel sheets (supplied by ONGC, Dehradun, India) having the following percentage by weight (wt.%) composition: C, 0.31; Mn, 0.92; Si, 0.19; P, 0.01; S, 0.008; Cr, 0.20; and Fe, remainder. The specimens were mechanically polished with different grades of silicon carbide papers, degreased in ethanol to obtain a fresh oxide-free surface, washed with double-distilled water, and dried at room temperature.
Weight loss measurements
The specimens for the weight loss measurements were of the size 3 cm × 3 cm × 0.1 cm. Both sides of the specimens were exposed for weight loss measurement. For weight loss experiments, 300 mL of 15% HCl (v/v) was taken in 500-mL glass beakers. The inhibition efficiencies (η) were evaluated after a pre-optimized time interval of 6 h using 20, 50, 100, 150, and 200 ppm of inhibitors. The specimens were removed from the electrolyte, washed thoroughly with distilled water, dried, and weighed. The inhibition efficiencies were evaluated using the following formula:
where W is the weight loss in the absence of an inhibitor and Wi is the weight loss in the presence of an inhibitor.
The corrosion rate (ρ) of the specimen can be calculated with the help of the following equation:
where W is the weight loss (mg), D is the density of the specimen (g cm−3), A is the area of the specimen (cm2), and T is the exposure time (h).
Electrochemical studies
The electrochemical experiments were carried out in a three-necked glass assembly containing 150 mL of the electrolyte with different concentrations of inhibitors (from 20 to 200 ppm by weight) dissolved in it. The potentiodynamic polarization studies were carried out with N80 steel working electrodes having an exposed area of 1 cm2. A conventional three-electrode cell consisting of N80 steel as working electrode, platinum as counter electrode, and a saturated calomel electrode as reference electrode was used. Polarization studies were carried out using a VoltaLab 10 electrochemical analyzer (Radiometer Analytical, Lyon, France), and data were analyzed using the Voltamaster 4.0 software (Radiometer Analytical). The potential sweep rate was 10 mV s−1. All experiments were performed at 298 K in an electronically controlled air thermostat. For calculating inhibition efficiency by the electrochemical polarization method, the following formula was used:
where I0 is the corrosion current density in the absence of an inhibitor and Iinh is the corrosion current density in the presence of an inhibitor.
AC impedance studies
Alternating current (AC) impedance studies were carried out using the same instrument as mentioned in polarization studies. The electrochemical impedance spectra (EIS) were acquired in the frequency range of 10 kHz to 1 mHz at the rest potential by applying 5-mV sine-wave AC voltage. The charge transfer resistance (Rct) and double-layer capacitance (Cdl) were determined from Nyquist plots. The inhibition efficiencies were calculated from Rct values using the following formula:
where Rct is the charge transfer resistance in the absence of an inhibitor and Rct(inh) is the charge transfer resistance in the presence of an inhibitor.
Synthesis of inhibitors
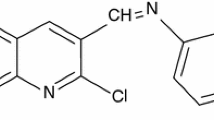
The isatin Mannich bases, namely 1-morpholinomethyl-3(1-N-dithiooxamide)iminoisatin [MMTOI] and 1-diphenylaminomethyl-3(1-N-dithiooxamide)iminoisatin [PAMTOI], were synthesized by the reported method [19]. Isatin and dithiooxamide in 1:1 molar ratio were refluxed in ethanol for 8 h and cooled, and the precipitate was filtered. This product was subsequently treated with formaldehyde and morpholine or diphenylamine to get the desired product. The compounds were characterized by Fourier transform infrared (FTIR) spectroscopy, and purity of the compounds was checked by TLC. The names and molecular structures of the studied compounds are given in Figure 1.
Scanning electron microscopy
The surface examination was carried out using a scanning electron microscope (JEOL 5400, Akishima-shi, Japan); the energy of the acceleration beam employed was 30 kV. All micrographs of the corroded specimens were carried out at a magnification of × 1,000.
FTIR study
The N80 steel specimen was immersed in 15% HCl solution containing optimum concentration of inhibitors for 6 h. The specimen was taken out and dried, and the film was scraped using a non-metallic scrapper. FTIR spectra for the pure sample and the scraped films from the inhibited specimen were recorded using a PerkinElmer FTIR (Spectrum 2000) by KBr pellet method.
Results and discussion
Weight loss study
Weight loss studies were performed in accordance with the ASTM method. Tests were conducted in 15% HCl (v/v) solution for 6 h of exposure time at different concentrations of inhibitors (20 to 200 ppm) and temperatures (298 to 333 K).
Effect of concentration
Both inhibitors were tested for 6 h of exposure period at different concentrations, and their corresponding weight loss data are presented in Table 1. The compounds [PAMTOI] and [MMTOI] have the maximum of 91.2% and 84.3% inhibition efficiency, respectively, at 200-ppm concentration. The inhibition efficiency of both inhibitors increases with increasing concentration of inhibitors (Figure 2), indicating that adsorption of inhibitors increases as concentration increases, resulting in the reduction of corrosion rate.
Effect of temperature
The effect of temperature on the corrosion inhibition efficiency of [PAMTOI] and [MMTOI] for N80 steel in 15% HCl was investigated by weight loss measurements in the temperature range of 298 to 333 K in the absence and presence of both inhibitors at optimum concentration (200 ppm) in a thermostat. Table 2 shows values of corrosion rate (ρ) and inhibition efficiency (η) at different temperatures for both inhibitors. The observation depicts that the rate of corrosion increases with increase in temperature. At this temperature, the metal organic complex layer dissociates leaving a porous diffused film, which is responsible for corrosion [20].
Adsorption isotherms
The adsorption isotherm experiments were performed to have more insights into the mechanism of corrosion inhibition since it describes the molecular interaction of the inhibitor molecule with the active sites on the N80 steel surface [21]. To determine the adsorption mode, various isotherms were tested. Langmuir adsorption isotherms were found to be the best, which give a straight line graph for the plot of log (θ/1 − θ) versus the logarithmic concentration of inhibitors (Figure 3). The inhibition of the corrosion of metals by organic inhibitors is usually attributed to either the adsorption of the inhibitor molecules or the formation of a barrier layer of insoluble metal complexes.
It is well recognized that organic inhibitor molecules set up their inhibition action via the adsorption of the inhibitor molecules onto the metal/solution interface. The adsorption process is affected by the chemical structures of the inhibitors, the nature and charged surface of the metal, and the distribution of charge over the whole inhibitor molecule. In general, owing to the complex nature of adsorption and inhibition of a given inhibitor, a single adsorption mode between inhibitor and metal surface is impossible. Organic inhibitor molecules may be adsorbed on the metal surface in one or more ways: (a) electrostatic interaction between the charged molecules and the charged metal, (b) interaction of unshared electron pairs in the molecule with the metal, (c) interaction of π-electrons with the metal, or (d) a combination of types (a) to (c) [22, 23]. In the aqueous acidic solutions, [MMTOI] and [PAMTOI] exist either as neutral molecules or as protonated molecules (cations). Generally, two modes of adsorption could be considered. In one mode, the neutral inhibitors may be adsorbed on the surface of N80 steel through the chemisorption mechanism, involving the displacement of water molecules from the N80 steel surface and the sharing of electrons between the heteroatoms and iron. The inhibitor molecules can also adsorb on the N80 steel surface on the basis of donor-acceptor interactions between π-electrons of the aromatic ring and vacant d orbital of the surface iron. In another mode, since it is well known that the steel surface bears a positive charge in acid solution [24], it is difficult for the protonated inhibitors to approach the positively charged mild steel surface (H3O+/metal interface) due to electrostatic repulsion. Since chloride ions have a smaller degree of hydration, they bring excess negative charges in the vicinity of the interface and favor more adsorption of the positively charged inhibitor molecules; the protonated inhibitors adsorb through electrostatic interactions between the positively charged inhibitor molecules and the negatively charged metal surface. Thus, there is a synergism between adsorbed Cl− ions and protonated inhibitors. Experimental data reveal that the inhibition efficiency of inhibitors follows the order [PAMTOI] > [MMTOI]. This order of performance is best explained in terms of the size of the inhibitors. Both inhibitors have the same number of active centers, but the size of [PAMTOI] is larger than that of [MMTOI], so the inhibition efficiency of [PAMTOI] is greater than that of [MMTOI].
The inhibition efficiency afforded by [MMTOI] and [PAMTOI] may be attributed to the presence of electron-rich N atom and aromatic rings. One phenylimino group and one indoline ring are common in the structure of both inhibitors. Therefore, the possible reaction centers are unshared electron pairs of sulfur of the C=S group, nitrogen of the -NH2 group and the C=N group, and π-electrons of the aromatic ring.
Kinetic and thermodynamic study
The apparent activation energy (Ea) for the dissolution of N80 steel in 15% HCl was calculated from the slope of plots using the Arrhenius equation:
where k is the rate of corrosion, Ea is the apparent activation energy, R is the universal gas constant, T is the absolute temperature, and A is the Arrhenius pre-exponential factor. By plotting log k against 1/T, the values of Ea have been calculated (Ea = −(Slope) × 2.303 × R; Figure 4). The activation energy for the reaction of N80 steel in 15% HCl increases in the presence of inhibitors. The Ea values for [PAMTOI] and [MMTOI] were found to be higher than the Ea value for the blank solution (Table 3). The values of Ea for [PAMTOI] and [MMTOI] were found to be 61.46 and 50.03 kJ mol−1, respectively.
The values of the entropy of activation (ΔS*) and enthalpy of activation (ΔH*) were calculated using the following formula:
where k is the rate of corrosion, h is Planck’s constant, N is Avogadro’s number, ΔS* is the entropy of activation, and ΔH* is the enthalpy of activation. A plot of log (k/T) versus 1/T (Figure 5) gives a straight line, with a slope of (−ΔH*/2.303R) and an intercept of [log (R/N h) + ΔS*/2.303R], from which the values of ΔS* and ΔH* were calculated (Table 3).The ΔH* values for [PAMTOI] and [MMTOI] were found to be −58.74 and −51.64 kJ mol−1, respectively, which revealed the exothermic nature of the corrosion reaction. The ΔS* values were found to be −49.42 and −68.62 J mol−1 K−1 for [PAMTOI] and [MMTOI], respectively (Table 3). The negative values of ΔS* for [PAMTOI] and [MMTOI] imply that the activated complex in the rate-determining step represents an association rather than a dissociation step, indicating that a decrease in disordering takes place on going from reactants to the activated complex [25, 26]. This also indicated that the activated complex was in a higher order state than at the initial state.
The average values for the change in the free energy of adsorption (ΔGads) were calculated using the following equation:
where R is the molar gas constant (J K−1 mol−1) and T is the temperature. The value 55.5 in the above equation is the concentration of water in the solution (mol L−1). The equilibrium constant (K) has been replaced by the following equation:
where θ is the degree of coverage on the metal surface and C is the concentration of inhibitors.
By plotting log K against 1/T, the value of ΔGads was calculated (ΔGads = −2.303× R × Slope) from the slope of the straight line obtained (Figure 6). The values of the free energy of adsorption at 200 ppm of [PAMTOI] and [MMTOI] were found to be −38.15 and −34.11 kJ mol−1, respectively. The negative values of ΔGads are consistent with the spontaneity of the adsorption process on the N80 steel surface. Generally, the change in free energy values of −20 kJ mol−1 or less negative are associated with an electrostatic interaction between charged molecules and charged metal surface (physisorption); those above −40 kJ mol−1 or more negative involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate covalent bond (chemisorption) [27, 28]. In the present study, the ΔGads values obtained for both inhibitors on N80 steel in 15% HCl solution were higher than −20 kJ mol−1 but less than −40 kJ mol−1 (Table 3); this indicates that the adsorption is neither typical physisorption nor typical chemisorption, but it is of a complex mixed type, that is the adsorption of inhibitor molecules on the N80 steel surface in the present study involves both physisorption and chemisorption. Lebrini et al. [29] studied some triazole derivatives as corrosion inhibitors of mild steel in 1 M HClO4. The Gibbs free energy of adsorption of these molecules was reported to be around −34 kJ mol−1. They concluded that the adsorption mechanism of these molecules on steel involved two types of interactions, chemisorption and physisorption. A similar conclusion was found by Ozcan [30], who studied the use of cystine as a corrosion inhibitor on mild steel in sulfuric acid. Thus, adsorption of the studied inhibitors at the surface of N80 steel is not pure physisorption, but it is a combination of physisorption as well as chemisorption.
Potentiodynamic polarization study
Figures 7 and 8 show the polarization curves of N80 steel in 15% HCl solution in the absence and presence of different concentrations (20, 100, and 200 ppm) of [PAMTOI] and [MMTOI], respectively. The polarization curves remain almost the same in the absence and presence of both inhibitors, but in the presence of inhibitors, the curves shifted towards the lower current density as compared to the blank solution. The shift in current density to a lower value is high with increasing concentration of the inhibitors. The electrochemical corrosion parameters from the polarization curves of [PAMTOI] and [MMTOI] are presented in Table 4. It is apparent from Table 4 that Icorr decreases considerably in the presence of both inhibitors and η increases with the increase in the inhibitor concentration due to the increase in the blocked fraction of the electrode surface by adsorption. The variation in the values of βa and βc in the presence of both inhibitors may indicate that both the anodic and cathodic processes are controlled. A minor shift in Ecorr values towards the negative direction was obtained in the presence of both inhibitors, indicating the mixed nature of the inhibitors. Generally, if the displacement in Ecorr is >85 mV with respect to Ecorr in the uninhibited solution, the inhibitor can be seen as a cathodic or anodic type [31, 32]. In our study, the maximum displacement is 22 mV in [PAMTOI] and 18 mV in [MMTOI], which indicates that both inhibitors can be arranged as a mixed-type inhibitor.
Electrochemical impedance spectroscopy
Nyquist plots of N80 steel in 15% HCl in the presence and absence of different concentrations (20, 100, and 200 ppm) of [PAMTOI] and [MMTOI] at 298 K are shown in Figures 9 and 10, respectively. All Nyquist plots obtained were semicircle in nature; the diameter of the semicircles increased with increase in inhibitor concentration, and the shape was maintained throughout the tested concentration, indicating that almost no change in the corrosion mechanism occurred due to inhibitor action. The values of electrochemical Cdl were calculated at the frequency fmax, at which the imaginary component of the impedance is maximal (−Zi) using the following equation:
The electrochemical parameters (Rct, Cdl, and η) calculated from the Nyquist plots using the equivalent circuit (Figure 11) in the presence and absence of inhibitors are presented in Table 5. In the EIS study, an increase in the Rct value is observed with increasing inhibitor concentration, suggesting that the charge transfer process is retarded due to a decrease in the uncovered surface available for corrosion reaction. In this case, the higher the inhibitor concentration, the lower is the associated corrosion rate. Due to the non-homogeneity or roughness of the metal surface, the observed semicircles of capacitive loops were depressed into Zi which is often referred to as frequency dispersion [33]. It is worth mentioning that the Cdl value is affected by imperfections of the surface. The Cdl values were found to decrease with increase in concentration of inhibitor solutions. This behavior is generally seen for a system where inhibition occurred due to the formation of a surface film by the adsorption of inhibitor on the metal surface [34]. The decrease in Cdl, which results from a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer, suggests that the inhibitor molecules act by adsorption at the metal/solution interface [35]. Inhibition efficiencies obtained from weight loss, potentiodynamic polarization curves, and EIS were found to be in good agreement.
FTIR analysis of corrosion products
In order to evaluate the protective layer formed on the steel surface in the presence of inhibitors and also to provide new bonding information on steel surface, a FTIR study was done. The FTIR spectra of pure and metal surface products after the corrosion test of [PAMTOI] and [MMTOI] are shown in Figures 12 and 13, respectively. FTIR absorption band positions of the pure sample of [PAMTOI] with respect to that of its metal surface product (Figure 12) were observed to have shifted from 3,450 to 3,419 cm−1, 2,925 to 2,924 cm−1, 2,856 to 2,847 cm−1, 1,744 to 1,723 cm−1, 1,646 to 1,619 cm−1, 1,458 to 1,455 cm−1, 1,397 to 1,339 cm−1, and 883 to 870 cm−1 corresponding to -NH, C-H aromatic, C-H methylene, C=O, C=N, C-N, C=S, and C-S stretching vibrations, respectively. FTIR absorption band positions of the pure sample of [MMTOI] with respect to that of its metal surface product (Figure 13) were observed to have shifted from 3,445 to 3,416 cm−1, 2,925 to 2,920 cm−1, 2,857 to 2,850 cm−1, 1,741 to 1,729 cm−1, 1,647 to 1,636 cm−1, 1,560 to 1,550 cm−1, 1,160 to 1,145 cm−1, 1,487 to 1,465 cm−1, and 835 to 802 cm−1 corresponding to -NH, C-H aromatic, C-H methylene, -C=O, -C=N, -C=C, -C-S, -C=S, and morpholine stretching vibrations, respectively [36]. The major shift in the position of bands corresponding to C=N and -NH indicates involvement of these groups in adsorption, and the minor shift in position of bands of the other groups indicates the interaction of the inhibitor molecule with the atoms of the metal surface.
SEM micrographs of metal surface
Figure 14a shows the scanning electron microscopy (SEM) microphotographs of the polished N80 steel sample (magnification ×1,000). Figure 14b shows the SEM microphotographs of N80 steel (magnification ×1,000) when exposed to 15% HCl solution at room temperature in the absence of inhibitors. Figure 14b also shows that the steel surface appears to be very rough in the absence of inhibitors. This is due to the formation of uniform flake-type corrosion products on the metal surface. No pitting and other separate phases are visible in microphotograph. Figure 14c,d shows the microphotographs of the metal surface when exposed to the acid medium in the presence of 200 ppm of [PAMTOI] and [MMTOI], respectively, at the same magnification. Comparing the microphotograph of the steel surface without an inhibitor with the photographs of the exposed surface in the presence of inhibitors, the steel was found to be covered with a semiglobular-type protective film of compounds uniformly spread over the surface. The protective film is formed due to adsorption of the inhibitor molecules on the N80 steel surface.
Experimental
N80 steel sample preparation
The corrosion studies were performed on mild steel samples with the following composition (wt.%): C, 0.31; Mn, 0.92; Si, 0.19; P, 0.01; S, 0.008; Cr, 0.20; and Fe, remainder. The N80 steel coupons having the size dimension 3.0 cm × 3.0 cm × 0.1 cm were mechanically cut and abraded with emery papers of different grades (120, 220, 400, 600, 800, 1,500, and 2,000) for weight loss experiment. For electrochemical measurements, mild steel coupons having the dimension 1.0 cm × 1.0 cm × 0.1 cm were mechanically cut and abraded in the same manner as before, with an exposed area of 1 cm2 (the rest covered with araldite resin) with a 3-cm-long stem. Prior to the experiment, specimens were washed with distilled water, degreased in acetone, dried, and stored in a vacuum desiccator.
Test solutions
For weight loss study, the test solutions (15% HCl, wt.%) were prepared by dilution of analytical-grade 37% HCl (Rankem, Faridabad, India), and the required concentrations of inhibitors were calculated before the 15% HCl solutions have been made up. The concentrations of the studied inhibitors ranging from 20 to 200 ppm by weight in 15% HCl were prepared. All solutions were prepared in double-distilled water.
Conclusions
[PAMTOI] and [MMTOI] both act as good corrosion inhibitors for the corrosion of N80 steel in 15% HCl solution. The inhibition efficiency values increase with the inhibitor concentration, but decreases with increasing temperature for the corrosion of N80 steel in 15% HCl solution. The adsorption of [PAMTOI] and [MMTOI] on the N80 steel surface obeys the Langmuir adsorption isotherm. The variation in the values of βa and βc (Tafel slopes) and the minor negative shift in the values of the corrosion potential (Ecorr) indicate that both tested inhibitors are of mixed type but predominantly control the cathodic reactions. EIS measurements show that Rct increases and Cdl decreases in the presence of inhibitors. The FTIR data for the synthesized product and those for the metal surface product suggested the adsorption of the inhibitor molecules on the surface of N80 steel.
Change history
15 July 2020
The Editor-in-Chief has retracted this article [1] because of significant textual overlap with articles with overlapping authors [2, 3, 5, 8]. Additionally, there appear to be a number of overlapping images.
References
Vishwanatham S, Haldar N: Corros Sci. 2008, 50: 2999. 10.1016/j.corsci.2008.08.005
Abd El-Maksoud SA, Fouda AS: Mater Chem Phys. 2005, 93: 84. 10.1016/j.matchemphys.2005.02.020
Migahed MA, Nassar IF: Electrochim Acta. 2008, 53: 2877. 10.1016/j.electacta.2007.10.070
Mohammed AA, Ibrahim M: Corros Sci. 2011, 53: 873. 10.1016/j.corsci.2010.10.022
Nataraja SE, Venkatesha TV, Manjunatha K: Corros Sci. 2011, 53: 2651. 10.1016/j.corsci.2011.05.004
Ghareba S, Sasha O: Corros Sci. 2010, 52: 2104. 10.1016/j.corsci.2010.02.019
Aljourani J, Raeissi K, Golozar MA: Corros Sci. 2009, 51: 1836. 10.1016/j.corsci.2009.05.011
Emregal CK, Mustfa H: Corros Sci. 2006, 48: 797. 10.1016/j.corsci.2005.03.001
Muralidharan S, Quraishi MA, Iyer SVK: Corros Sci. 1995, 37: 1739. 10.1016/0010-938X(95)00068-U
Bentiss F, Lebrini M, Lagrenee M, Traisnel M, Elfarouk A, Vezin H: Electrochim Acta. 2007, 52: 6865. 10.1016/j.electacta.2007.04.111
Cruz J, Martinez R, Genesca J, Garcia-Ochoa E: J Electroanal Chem. 2004, 566: 111. 10.1016/j.jelechem.2003.11.018
Khaled KF, Babic-Samradzija K, Hackerman N: Electrochim Acta. 2005, 50: 2515. 10.1016/j.electacta.2004.10.079
Roberge PR: Corrosion inhibitors: handbook of corrosion engineering. New York: McGraw-Hill; 1999.
Olivares-Xometl O, Likhanova NV, Domínguez-Aguilar MA, Arce E, Dorantes H, Arellanes-Lozada P: Mater Chem Phys. 2008, 110: 344. 10.1016/j.matchemphys.2008.02.010
Quartarone G, Battilana M, Bonaldo L, Tortato T: Corros Sci. 2008, 50: 3467. 10.1016/j.corsci.2008.09.032
Ebenso EE: Bull Electrochem. 2003, 19: 209.
Gardner GS, Saukaitis AJ: US Patent. 1957, 2: 807,585.
Quraishi MA, Ahamad I, Singh AK, Shukla SK, Lal B, Singh V: Mater Chem Phys. 2008, 112: 1035. 10.1016/j.matchemphys.2008.07.011
Abdulghani AJ, Abbas NM: Bioinorg Chem Appl. 2011. 10.1155/2011/706262
Putilova IN, Balezin SA, Barannik VP: Metallic corrosion inhibitors. New York: Pergamon; 1960.
Emregül KC, Hayvalı M: Corros Sci. 2006, 48: 797. 10.1016/j.corsci.2005.03.001
Shorky H, Yuasa M, Issa SI, El-Baradie RMHY, Gomma GK: Corros Sci. 1998, 40: 2173. 10.1016/S0010-938X(98)00102-4
Mu GN, Zhao TP, Liu M, Gu T: Corrosion. 1996, 52: 853. 10.5006/1.3292077
Schweinsberg DP, George GA, Nanayakkara AK, Steiner DA: Corros Sci. 1988, 28: 33. 10.1016/0010-938X(88)90004-2
Olivares O, Likhanova N, Gomez B: Appl Surf Sci. 2006, 252: 2894. 10.1016/j.apsusc.2005.04.040
Al-Juaid SS: Chemistry and Technology of Fuels and Oils. 2011, 47: 58. 10.1007/s10553-011-0257-4
Dehri I, Ozcan M: Mater Chem Phys. 2006, 98: 316. 10.1016/j.matchemphys.2005.09.020
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A: Corros Sci. 2008, 50: 2172. 10.1016/j.corsci.2008.06.020
Lebrini M, Traisnel M, Lagrene M, Mernari B, Bentiss F: Corros Sci. 2007, 50: 473.
Ozcan M: J Solid State Electrochem. 2008, 12: 1653. 10.1007/s10008-008-0551-1
Ashassi-Sorkhabi H, Majidi MR, Seyyedi K: Appl Surf Sci. 2004, 225: 176. 10.1016/j.apsusc.2003.10.007
Li XH, Deng SD, Fu H: Corros Sci. 2009, 51: 1344. 10.1016/j.corsci.2009.03.023
Lebrini M, Lagrenée M, Vezin H, Traisnel M, Bentiss F: Corros Sci. 2007, 49: 2254. 10.1016/j.corsci.2006.10.029
Rosenfield IL: Corrosion inhibitors. New York: McGraw-Hill; 1981.
MaCafferty M, Hackerman N: J Electrochem Soc. 1972, 119: 146. 10.1149/1.2404150
Silverstein RM, Bassler GC, Morrill TC: Spectrometric identification of organic compounds. 4th edition. New York: Wiley; 1991.
Acknowledgements
Financial assistance from the Indian School of Mines, Dhanbad, under the ‘Faculty Research Scheme’ to M. Yadav is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MY carried out the data treatment and drafted the manuscript. US carried out the synthesis of the inhibitors and the gravimetric and electrochemical measurements. PY studied the effect of temperature and carried out the thermodynamic parameter calculations. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yadav, M., Sharma, U. & Yadav, P. RETRACTED ARTICLE: Corrosion inhibitive properties of some new isatin derivatives on corrosion of N80 steel in 15% HCl. Int J Ind Chem 4, 6 (2013). https://doi.org/10.1186/2228-5547-4-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-6