Abstract
The purpose of this study was to investigate the effect of atmospheric pressure plasma application on the water contact angle (CA) of zirconia ceramics. Two zirconia ceramics (Katana, Kuraray Noritake Dental and Lava, 3 M ESPE) were used to test the plasma treatment (SAP, surface) with argon gas for one minute (1 L/min). To measure the CA, a drop of water was placed on a zirconia surface, which was observed under optical microscopy, and images were used to calculate the CA (n = 5). The dynamic behavior of the surface wettability was analyzed by collecting the CA data over a 70-hour period. CA data were analyzed by two-way ANOVA and the Tukey test (5%). The CA baseline values were 66° and 68° for Katana and Lava, respectively. After the application of plasma, the CA was reduced significantly to 36° and 31°, respectively. The CAs for Lava zirconia and Katana returned to baseline values after 5 and 15 hours, respectively. The plasma treatment improved the wettability of the zirconia surface, decreasing the CA approximately 50%. The duration of the effect of plasma on zirconia surfaces was at least 5 hours and material-dependent.
Similar content being viewed by others
Background
Zirconia has been used for over 40 years for industrial purposes and for about 20 years in dentistry. Its composition is basically zirconium dioxide (approximately 95%), and it is stabilized with yttrium and enriched with alumina to prevent the leaching of the yttrium oxide. This composition ensures the longevity of zirconia restorations, and the sintering process at 1200–1500°C provides high flexural strength and hardness. Another advantage of using zirconia is its translucency properties that allow light to pass partially through the material, resulting in good esthetic effects [1–3]. Although zirconia has good mechanical properties and biocompatibility, the low surface energy confers poor adhesion of resin cements to their surfaces. Treatment of the surface and the use of adhesive primers have been suggested as methods to overcome the poor adhesion [4–6].
As a surface treatment, atmospheric pressure plasma is an option, since the reactive species created with the interaction between the ions and electrons of argon plasma and the micro-atmosphere around the treatment zone may improve the reactive level of the zirconia surface [7, 8]. When these highly reactive species reach the sample surface, they break weak bonds stabilized with radicals, promoting the formation of polar groups at the surface and opening up the chemical sites to future bonding. In summary, plasma treatment destabilizes the surface, rendering it reactive [9].
A simple technique to measure the reactive level of a surface is the contact angle. It consists of placing water droplets on the surface and observing their shape. If the droplets are spherical, this indicates a low surface energy of the zirconia surface, and hence, poor adhesion. However, if the drop spreads over the surface, this indicates a high surface energy and high adhesion. The measurement of the angle between the surface and the drop of water is called the contact angle [10, 11]. After plasma treatment, the contact angles are commonly lower than that obtained with the natural surface, because of the insertion of polar groups at the surface. As dictated by the first law of thermodynamics, matter seeks a state of lowest possible energy; thus, the instability promoted by the plasma treatment loses efficiency over time because the surface will try to recover the original low surface energy [12–14].
This work supports the idea that new polar groups added on the material surface by atmospheric plasma treatment would result in a decrease of the initial contact angle, and, consequently, improve the bond strength of adhesives, primers and resin cements interacting with zirconia-based materials. It is also necessary to determine the time necessary to reach stability after the onset of plasma treatment, measuring the contact angle until it becomes stable. The purpose of this study was to measure the contact angle formed on zirconia surfaces treated with atmospheric pressure plasma in comparison with that on untreated surfaces. In addition, the contact angle of water droplets was monitored for 70 hours to evaluate the longevity of the effect of plasma on zirconia surfaces. The main research hypothesis tested was that the contact angle on zirconia surfaces is reduced when they are treated with plasma. The second hypothesis was that there are no differences between zirconia ceramics regarding the stability of the contact angle after the application of plasma.
Methods
Contact angle
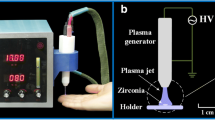
The equipment used to treat the zirconia samples was a Surface plasma tool model SAP - Lab applications (Surface – Engineering and Plasma Solution LTDA, Campinas, SP, Brazil) (Figure 1). Argon was used as the working gas (Praxair 4.8, White Martins Gases Ind. S.A., Rio de Janeiro, RJ, Brazil), with an output of 1.0 liter per minute. Fifteen sintered zirconia plates (10 × 10 × 1 mm) of Katana (Kuraray Noritake, Nagoya, Japan) and Lava (3 M ESPE, Seefeld, Germany) were obtained by using zirconium dioxide stabilized by yttrium oxide. CAD/CAM blocks of both zirconia ceramics were sectioned with a diamond saw (Buehler Ltd, Lake Bluff, IL, USA) and sintered according to the manufacturer’s instructions.
To measure the original contact angle, water droplets of 15 to 20 μL were placed on the zirconia surface, and a digital 300× microscope (Digimicro, Digital Microscope, Shenzhen King Leader Technology Co., Ltd., China) was used to acquire the profile images, as illustrated in Figure 2. The initial contact angle was measured (Wayne Rasband National Institutes of Health, ImageJ 1.47,Bethesda, MD, USA) by 1 measurement per sample of each brand group, with 5 samples per group (n = 5).
The plasma torch, which was 20 mm long, was operated at room temperature (22°C). The distance between the nozzle and the samples was 10 mm, and the time of plasma exposure was 1 minute for each sample. The plasma application was vertical and moved around the specimen. Afterwards, the shape of the water drop was recorded and the contact angle was measured again. A two-way repeated measures analysis of variance (ANOVA) (effect of plasma application and brand) was performed when the contact angle was the variable selected. All statistical tests were performed at a pre-set alpha of 0.05 and followed by Tukey’s post hoc test.
To evaluate the dynamic behavior of the contact angle as a function of the time elapsed after plasma treatment, ten zirconia samples of each brand were treated and kept in a controlled temperature room (approximately 22°C). The contact angles on seven samples of each group were measured every hour for 10 hours. The contact angles on the other zirconia samples were measured at 40, 50, 60 and 70 hours after plasma treatment to ensure that the new surface structure was stable.
Theoretical mathematical simulation
The model assumes an exponential decay in the population of mobile polar groups on the surface, f m . Thus, the population of polar groups, f p , on a surface can be written as:
where f im is the fraction of immobile polar groups and τ is the time decay constant [6].
According to Rangel et al. [14], the contact angle can be expressed by:
Where θp is the contact angle of a pure polar surface and f np and θnpare the fraction of non-polar groups and the contact angle of a pure non-polar surface, respectively, with f p + f np = 1.
However, the combination of the equations (1) and (2) produces:
And developing the substitutions on equation (3) produces:
Thus, with this final equation, it is possible to compare the evolution of the contact angle and the kinetic distribution of polar groups.
Results
Two-way ANOVA indicated that the application of plasma and the type of zirconia ceramic, as well as their interaction, significantly influenced the contact angle results (p < 0.01). Table 1 presents a summary of the statistics for the different experimental groups. The contact angle was significantly lower when the zirconia surface was treated with plasma (p < 0.05). In the comparison of the zirconia ceramics, Katana presented a lower contact angle (p < 0.05).
The original contact angle for Katana zirconia was recovered after 15 hours of plasma exposure (Figure 3), whereas for Lava, this time was 5 hours (Figure 4).
The mobile polar fractions for Katana and Lava were approximately 46% and 40%, respectively. The immobile polar fractions were approximately 76% and 74% for Katana and Lava, respectively. The polar fraction of zirconia ceramics ranged from 33% to 34%, whereas the non-polar fraction was 66% to 67% (Figure 5).
Discussion
The surfaces of all indirect restorative materials are composed of polar and non-polar groups. After plasma exposure, new polar groups are available in the treated area; this improves the wettability of zirconia surfaces. Confirming this statement, Valverde et al. [15] reported higher surface energy levels resulting from the polar components after treatment with non-thermal plasma. In this study, the contact angles decreased approximately 50% when compared to untreated surfaces, because new polar groups formed on the zirconia surface because of exposure to plasma. Therefore, our results support our hypothesis that the contact angle on zirconia surfaces is reduced after plasma treatment.
The plasma torch used in this study was 20 mm long, and the distance between the nozzle and the sample was 10 mm. Each sample was exposed to plasma for 1 minute. This plasma application protocol was used because the distance between the samples and the plasma nozzle should be shorter than the full length of the torch, ensuring that the entire surface of zirconia was totally immersed in the plasma torch. The time of 1 minute of plasma exposure allowed a significant improvement in the hydrophilic character of the surface.
Commercially available primers can also improve the bond strength, and change the contact angles by 30° to 40°. The reason for the use of primers is to raise the inherent inert properties of natural zirconia. The decrease in the contact angle indicates that the surfaces are becoming hydrophilic, thus increasing their potential to bond with acrylate-based resins in restorations. The lower contact angle can be explained by the formation of reactive oxygen species and reactive nitrogen species as a result of plasma treatment and, consequently, their dispersion on the surface [8, 9, 15, 16]. Thus, because of the poor adhesion of most resin cements to zirconia [2, 17], the use of adhesive primers, sandblasting with aluminum oxide, Er:YAG laser irradiation or tribochemical silica coating has been suggested as methods to improve the bond strength [15, 18–20].
The relatively high original contact angle, upper 50˚ (Table 1), indicates the poor adhesion properties of zirconia ceramics. Plasma bonding pretreatment is a possible clinical technique to improve the bond strength of polar resins to zirconia, because of the increase in surface wettability after plasma exposure. Katana zirconia presented a lower contact angle than Lava, even after plasma treatment. ZirCAD is a pre-sintered yttrium-stabilized zirconium oxide CAD/CAM block, whereas Katana is made of non pre-sintered oxidized yttrium ceramic for use as CAD/CAM milling blanks, which influences the contact angle, since the surface may be more porous before and after the sintering process than Lava. Also, the polar fraction (fp) and non-polar fraction (fnp) from the Katana zirconia surface are approximately 34% and 66%, respectively. For Lava, the polar fraction is slightly lower, and thus the non-polar fraction is higher. According to the theoretical simulation, the presence of a majority of non-polar groups in the zirconia surface explains the low hydrophilic properties and the low surface energy of zirconia ceramics [21–24].
The relatively high speed of the aging effect is related to the low percentage of the mobile polar fraction (fm), which is an estimate of the fraction of new polar groups after plasma exposure. Regarding the recovery of hydrophobicity, Katana zirconia maintained the contact angle stability for 12 hours after the plasma treatment, and had 46% of the mobile polar fraction. For Lava, the duration of plasma treatment was 5 hours and 40% of the mobile polar fraction, which may influence the lower duration of the plasma effects. Thus, the second hypothesis stating that there is no difference between zirconia ceramics regarding the duration and stability of contact angle after plasma application was not supported by the evidence, since Lava had a faster hydrophobic recovery and loss of polar groups than Katana.
The model proposed by Chatelier et at. [25] based on exponential fit, allowed the quantitative evaluation of the kinetics of surface reconstruction. The model assumes an exponential decay in time of the population of mobile polar groups on the surface. This theoretical mathematical simulation was performed to investigate the dynamics of polar and non-polar groups at the surface after plasma treatment, to compare with the stability rate. Thus, with this final equation, it is possible to evaluate the evolution of contact angle and the kinetics of the distribution of polar groups. Clinically, it is strongly suggested to carry out the bonding step and the cementation of indirect restorations immediately after the plasma treatment to have a zirconia surface with high surface energy and an excellent condition for bonding with the resin cements.
Conclusions
The treatment of zirconia with plasma reduced the contact angle, increasing the surface wettability, which may improve the bond strength of restorative materials (adhesives, primers or resin cements) to zirconia ceramics. Regarding the recovery of hydrophobicity, the stability of the contact angle on zirconia surfaces depended on the material.
References
Komine F, Saito A, Kobayashi K, Koizuka M, Koizumi H, Matsumura H: Effect of cooling rate on shear bond strength of veneering porcelain to a zirconia ceramic material. J Oral Sci 2010, 52: 647–652. 10.2334/josnusd.52.647
Cavalcanti AN, Foxton RM, Watson TF, Tavares de Oliveira M, Giannini M, Marchi GM: Y-TZP ceramics: key concepts for clinical application. Oper Dent 2009, 34: 344–351. 10.2341/08-79
Piascika J, Swift E, Braswell K, Stoner B: Surface fluorination of zirconia: adhesive bond strength comparison to commercial primers. Dent Mater 2012, 28: 604–608. 10.1016/j.dental.2012.01.008
Blatz M, Richter C, Sadan A, Chiche G, Swift E: Resin bond to dental ceramics. Part II: high-strength ceramics. Journal of Esthetic Restorative Dentistry 2006, 16: 324–328.
Blatz M, Sadan A, Chiche G, Holst S: Influence of surface treatment and simulated aging on bond strengths of luting agents to zirconia. Quintessence Int 2007, 38: 745–753.
Burke T, Fleming G, Nathanson D, Marquis P: Are adhesive technologies needed to support ceramics? An assessment of the current evidence. Journal of Adhesive Dentistry 2007, 4: 7–22.
Murakami T, Niemi K, Gans T, O’Connell D, Graham W: Chemical kinetics and reactive species in atmospheric pressure helium-oxygen plasmas with humid-air impurity. Plasma Source Science and Technologies 2013, 22: 15001–15031. 10.1088/0963-0252/22/1/015001
Lim J, Uhm H, Li S-Z: Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Physics of Plasma 2007, 14: 493–504.
Kong M, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann J: Plasma medicine: an introductory review. New J Phys 2009, 11: 115–140.
Scales P, Grieser F, Furlong N, Healy T: Contact angle changes for hydrophobic and hydrophilic surfaces induced by nonionic surfactants. Colloids and Surface 1986, 21: 55–68.
Ko YC, Ratner D, Hoffman A: Characterization of hydrophilic—hydrophobic polymeric surfaces by contact angle measurements. J Colloid Interface Sci 1981, 82: 25–37. 10.1016/0021-9797(81)90120-X
Wolf R, Sparavigna A: Role of plasma surface treatments on wetting and adhesion. Journal of Engineering 2010, 2: 397–402. 10.4236/eng.2010.26052
Watanabe H, Saito K, Kokubun K, Sasaki H, Yoshinari M: Change in surface properties of zirconia and initial attachment of osteoblastlike cells with hydrophilic treatment. Dent Mater J 2012, 31: 806–814. 10.4012/dmj.2012-069
Rhee K-Y, Park K-S, Hui D, Qiu Y: Effect of oxygen plasma-treated carbon fibers on the tribological behavior of oil-absorbed carbon/epoxy woven composites. Composites Journal 2012, 43: 2395–2399. 10.1016/j.compositesb.2011.11.046
Guilherme V, Paulo C, Malvin J, Fabio L, Ricardo C, Van T, Klaus-Dieter W, da Silva NRFA: Surface characterization and bonding of Y-TZP following non-thermal plasma treatment. J Dent 2013, 41: 51–59. 10.1016/j.jdent.2012.10.002
Barbosa W, Francescantonio M, Aguiar T, Cavalcanti A, Oliveira M, Giannini M: Effect of application of metal primers on the bond strength of resin cements to zirconia. Revista Da Pós-Graduação Da Fousp 2011, 18: 224–228.
Barbosa W, Francescantonio M, Cavalcanti A, Oliveira M, Giannini M: Effect of water storage on bond strength of self-adhesive resin cements to zirconium oxide ceramic. Journal of Adhesive Dentistry 2013, 15: 145–150.
Piwowarczyk A, Lauer H, Sorensen J: The shear bond strength between luting cements and zirconia ceramics after two pre-treatments. Oper Dent 2005, 30: 382–388.
Spohr AM, Borges G, Júnior LH, Mota E, Oshima H: Surface modification of In-Ceram Zirconia ceramic by Nd:YAG laser, Rocatec system, or aluminum oxide sandblasting and its bond strength to a resin cement. Photomed Laser Surg 2008, 26: 203–208. 10.1089/pho.2007.2130
Cavalcanti A, Foxton R, Watson T, Oliveira M, Giannini M, Marchi G: Bond strength of resin cements to a zirconia ceramic with different surface treatments. Oper Dent 2009, 34: 280–287. 10.2341/08-80
Rangel E, Gadioli G, Cruz N: Investigations on the stability of plasma modified silicone surfaces. Plasmas and Polymers 2004, 9: 35–48.
Thompson J, Stoner B, Piascik J, Smith R: Adhesion/cementation to zirconia and other non-silicate ceramics: where are we now? Dent Mater 2011, 27: 71–82. 10.1016/j.dental.2010.10.022
Heikkinen T, Matinlinna J, Vallitu P, Lassila L: Dental zirconia adhesion with silicon compounds using some experimental and conventional surface conditioning methods. Springer Science 2009, 1: 199–202.
Suh B, Chen L, Brown D: Bonding to zirconia: innovative in adhesion. Compendium Supplement Bisco 2010, 31: 2–5.
Chatelier R, Xie X, Gengenbach T, Griesser H: Quantitative analysis of polymer surface restructuring. Langmuir 1995, 11: 2576–2584. 10.1021/la00007a042
Acknowledgements
This study was supported by CNPq (305777/2010-6) and Capes (3110/2010).
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lopes, B.B., Ayres, A.P.A., Lopes, L.B. et al. The effect of atmospheric plasma treatment of dental zirconia ceramics on the contact angle of water. Appl Adhes Sci 2, 17 (2014). https://doi.org/10.1186/2196-4351-2-17
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2196-4351-2-17