Abstract
Purpose
Although safe at therapeutic levels, excess intake of acetaminophen can lead to hepatic injury or acute liver failure (ALF). A number of different factors influence metabolism and hepatotoxicity of acetaminophen in patients. Three of the most important are a patient’s physiological response to fasting, alcohol consumption, and chronic acetaminophen consumption. The molecular and enzymatic underpinnings for these processes have been extensively studied. The purpose of this study is to examine and quantify the effects of the noted conditions, provide possible reasons for conflicting clinical observations, and examine dangers associated with uptake of therapeutic doses of acetaminophen.
Methods
In order to gain a better understanding of the transient hepatic changes associated with each physiological and nutritional process, examine risks of ALF associated with individuals based on their unique lifestyle and health issues, and predict improved dosing strategies, a multi-compartmented physiologically-based pharmacokinetic (PBPK) model of acetaminophen metabolism in adult humans was developed. By varying the parameters of this model, changes in metabolism of acetaminophen and its toxic byproducts for a variety of medically relevant conditions were assessed.
Results
Simulated results indicate that in case of chronic ingestion of acetaminophen, the increased rate of glucuronidation plays a significant role in protecting patients from liver damage following uptake of excessive quantities. Analysis of metabolism of acetaminophen in persons who have imbibed excessive amounts of alcohol show that the primary reason for hepatotoxicity in such individuals is decreased availability of glutathione in the liver and not the observed increased production of toxic byproducts. When the glutathione depleting effects of alcohol consumption are combined with those associated with chronic acetaminophen use, intake of slightly higher quantities than the recommended therapeutic doses of acetaminophen can result in initiation of hepatotoxicity.
Conclusions
The results of simulations show that, in healthy and well-fed individuals, chronic uptake of acetaminophen doses even five times the therapeutic recommendations should be safe. However, in persons who have diminished hepatic glutathione regeneration capacities, depending on the magnitude of this deleterious shortcoming, minor overdoses can result in hepatotoxicity. Hence, it can be concluded that for such persons, acetaminophen is just as toxic as any other compound that would generate reactive oxidative species.
Similar content being viewed by others
Background
Acetaminophen (a.k.a. paracetamol, acetyl-para-aminophenol) (APAP) is a popular over-the-counter analgesic and antipyretic drug. By one estimate, 36% of Americans use at least one tablet of APAP per month (Gregory et al., 2010); and in the United Kingdom an average of 55 tablets per person are consumed each year (Jones, 1998).
At therapeutic levels, acetaminophen is generally safe for humans (Thomas, 1993); however, over self-medication (for therapeutic or suicidal intentions) or excess prescription of APAP due to incorrect assessment of risks to patients with unique physiological determinants (McQuade et al., 2012) can result in acetaminophen poisoning, dose-dependent hepatotoxicity, and possibly ALF. Unfortunately, ALF is a common outcome. APAP poisoning is the leading cause of ALF in the United States (Schiødt et al., 2003; Nourjah et al., 2006) and some European countries (Larsen et al., 1995; Bernal, 2003).
The hepatotoxic agent in these cases is a byproduct of APAP metabolism. As part of phase II drug metabolism, the bulk fraction of APAP is glucuronidated (APAP-G) and sulfated (APAP-S) to form conjugates that are more water-soluble than APAP. These compounds are primarily excreted through urine (Bessems and Vermeulen, 2001). Approximately 55 and 30 percent of the administered drug is excreted via urine as APAP-G and APAP-S respectively (Howie et al., 1977).
However, during phase I metabolism, a small fraction of APAP (~5-15%) is oxidized by liver microsomal cytochrome P-450 s (CYP) to form a toxic byproduct N-acetyl-p-benzoquinone imine (NAPQI). The cytochromes involved in oxidation of APAP are CYP2E1 (Raucy et al., 1989; Lee et al., 1996), CYP3A4 (Thummel et al., 1993), CYP2D6 (Dong et al., 2000), CYP1A2 (Raucy et al., 1989), and CYP2A6 (Chen et al., 1998). CYP2E1 is the primary enzyme catalyzing the production of NAPQI at lower APAP concentrations. In CYP2E1 mutant mice, the animals display much greater tolerance to APAP than wild type animals, and only at high concentrations (>600 mg/kg) do they display signs of significant toxicity (Lee et al., 1996). NAPQI produced as a result of uptake of a normal dose of APAP is rapidly detoxified through conjugation with molecules of the glutathione (GSH) antioxidant. However, if the store of GSH in the liver dips below 30-20% of its normal value, NAPQI will begin to accumulate, bind to various liver proteins, and cause liver damage (Mitchell et al., 1973).
A number of different factors influence metabolism and hepatotoxicity of APAP in patients. These include age (Miller et al., 1976), genetics (Ueshima et al., 2006), concurrent uptake of other drugs (Toes et al., 2005), viral infections (Barbaro et al., 1996; Moling et al., 2006), alcohol use (Schiødt et al., 2002), fasting/starvation (Whitcomb and Block, 1994), and tobacco use (Schmidt and Dalhoff, 2003).
This manuscript reports the development and utilization of a multi-compartmented physiologically-based pharmacokinetic (PBPK) model of APAP metabolism in average adult humans to predict the time-course of changes in liver GSH levels following fasting, chronic APAP use, and alcohol consumption. Model simulations have been used to quantify the enhancing or reducing influence of each of the above noted lifestyle choices on the possibility of inducing hepatotoxicity following use of APAP.
Methods
Whole-body PBPK models provide a framework for integrating and interpreting data from disparate sources in order to predict the time-course of xenobiotic metabolism. PBPK models dynamically simulate outcome of metabolism of various therapeutic and/or toxic compounds on the basis of their structure and other important physiological input parameters such as tissue volumes, organ composition, blood flow rate, and system-level clearance rates. Thus, PBPK models are ideal tools for assessing toxicological risks early in the drug development pipeline (Clark et al., 2004).
Mathematical formulation
In the majority of PBPK models, the mammalian body is treated as a series of well stirred homogenous compartments that are connected to one another via arterial and venous blood flow. The rates of biochemical processes, including metabolism, are modeled at different levels of detail depending on the quality and availability of various kinetic and physiological parameters.
In most PBPK models, the transient change in each compound’s concentration in each organ is formulated mathematically as:
where denotes the concentration of compound i in compartment α; represents the concentration of the compound i in the arterial blood; Vα is the volume of compartment α; Qα is the blood flow into compartment α; and are the amounts of drug i that are directly imported and removed from compartment α, respectively. and BP are the tissue plasma partition coefficient and blood to plasma ratio respectively. Blood, skin, gut, and lung are the primary routes of introducing compounds into a system and hence might have a non-zero value for the term . Introduction of a compound into a compartment with a single injection can be represented by the Dirac delta function such that:
where D denotes the dose. For single uptakes δ(t) = 1 at t = t 0 and zero at all other times. For regular periodic uptakes: δ(t) = 1 at t = nT, where n is an integer (n = 0,1,2…) and T is the time interval between uptakes; δ(t) = 0 at all other times.
For non-eliminating tissues . In the eliminating organs, such as liver and kidney, the value of depends on the mode of elimination. If a compound is metabolized then the simplest formulations would involve introduction of first order kinetics such that:
For non-enzymatic or bulk elimination one can use:
In equation 3 the drug is metabolized at a rate dependent on the concentration of drug in the tissue and a constant, k. In equation 4, a fraction of the drug (E < 1) that is entering the tissue is extracted (Poulin and Theil, 2002b). For cases where one would need to account for affinity of a compound binding to a catalyzing enzyme, equation 3 can be changed to:
where K m,i,j represents the Michaelis-Menten coefficient for interaction of drug i with enzyme j, n denotes the number of enzymes in compartment α that can catalyze breakdown of drug i, and V max,i,j represents the maximum rate of metabolism of drug i by enzyme j.
Normal APAP metabolism
A 14-compartment model of human physiology (see Figure 1), where the various tissues are connected by the blood circulatory system, was developed. The collection of ODEs that make up the model was solved by using the Mathematica suite of programs (version 9.0, Wolfram Research Inc., Champaign, IL). The non-drug specific system parameters are from (Luttringer et al., 2003) (see Table 1). The tissue-specific APAP partition coefficients were calculated using the formula proposed by Poulin and coworkers (Poulin and Theil, 2000; Poulin et al., 2001). The BP value for APAP was set to one (Poulin and Theil, 2002a). The model contains two eliminating tissues, liver and kidney. To model APAP metabolism and excretion, equation 4 is used to account for non-enzymatic export of compounds (kidney), and equation 5 accounts for the enzymatic breakdown (liver) of the drug. In the model the latter formulation accounts for three enzymatic processes, APAP glucuronidation, sulfation, and oxidation; the last results in formation of NAPQI. The kinetic parameters for the glucuronidation and sulfation reactions are from (Reith et al., 2009). The average person was assumed to weigh 70 kg. The V max value for the oxidation reaction is based on measurements from (Mitchell et al., 1973). For drug excretion via urine, we set (Larson, 2007). Studies have shown the bioavailability of APAP after consuming pills is about 79% (Ameer et al., 1983); thus, for cases when APAP is taken orally, we used this value for our simulations.
GSH metabolism
For simulations of , we use the equation:
where k II is the bimolecular interaction constant for NAPQI and GSH. represents a coefficient for production GSH in the liver. Synthesis of GSH in the liver has been extensively examined, and a number of different factors that regulate normal and stressed production of GSH have been studied (Griffith, 1999; Lu, 1999; Wu et al., 2004). However, these kinetic alterations have not been fully quantified. This formulation for generation of GSH is similar to those incorporated in other models (Chen and Gillette, 1988). It does not permit to exceed the normal concentration of GSH in the liver , and the rate of regeneration is directly proportional to depletion of GSH levels. The value of was optimized so that a single uptake of APAP greater than 15 g would result in greater than 70% depletion of liver GSH pool and initiate hepatic damage (Rumack, 2002). Although it has been shown that concentration (Smith et al., 1979) and rates of metabolism of GSH vary in different regions of the liver (Kera et al., 1988; Penttilä, 1990), this level of detail has not been incorporated into the model, and as noted earlier, the liver is assumed to be a well-mixed single compartment.
Modeling chronic APAP uptake
When modeling chronic uptake of APAP, uptake of various doses were simulated at regular 6-hour intervals. Experiments have shown that chronic uptake of APAP alters the routes of APAP metabolism. Due to cofactor depletion, clearance via sulfate formation is lowered, while clearance through glucuronidation is increased (Hendrix-Treacy et al., 1986; Gelotte et al., 2007). Since liver concentrations of UDP-glucuronosyltransferase or sulfation cofactors are not explicitly solved in the model, this phenomenon was modeled by multiplying the V max for the glucuronidation and sulfation by coefficients Co g and Co s , respectively. This results in modification of the overall clearance via APAP-G and APAP-S production. The experimentally measured changes in routes of clearance (Gelotte et al., 2007) were used to calculate the maximum values of Co g and Co s . Since the time course of induction of UDP-glucuronosyltransferase and depletion of sulfate stores in liver are not well understood and differ based on diet and numerous other factors, the value of Co g and Co s were made time-dependent such that:
Co g = Co s = 1 and δ(t) = 1 when t ≤ 4320 minutes and δ(t) = 0 when t > 4320. Thus, in the simulations the time course for observed changes in metabolism of APAP is 3 days (4320 minutes). This length of time matches the first time point for which changes in routes of APAP metabolism were observed experimentally (Gelotte et al., 2007). The values for and for different doses of APAP are reported in Table 2.
Experimental data show that diversion of APAP to glucuronidation increases as the dose increases from 1 g to 2 g every 6 hours (Gelotte et al., 2007), but it is not clear if the pattern holds for higher doses. Because of this uncertainty, for chronic ingestion of doses greater than 2 g, levels of induction similar to that of 2 g doses were used.
Effects of alcohol on APAP metabolism
Alcohol ingestion stimulates production of NAPQI through induction of CYP2E1 enzyme and this effect can last up to 5 days in humans after drinking has stopped (Perrot et al., 1989; Takahashi et al., 1993). For simulations of APAP metabolism after drinking alcohol, results from a study (Thummel et al., 2000) that found excessive alcohol consumption (continual blood alcohol concentration of 3 g/L for 200 hours, similar to consuming nearly 14 Liters of 80 proof spirits in less than 9 days) increases CYP2E1 concentration by a factor of 2.14 were used. For simulations of APAP metabolism in alcoholics or binge drinkers, the maximum enhancement in the activity of the CYP2E1 was assumed. Hence, the normal V max for the NAPQI production was multiplied by the above noted value . Half-life for recovery of CYP2E1 activity after alcohol ingestion is about 60 hours (Imai et al., 2011). As with changes associated with chronic APAP use, the CYP induction by ethanol was made to be linearly time dependent such that:
and δ(t) = 1 when t ≤ 7200 minutes and δ(t) = 0 when t > 7200.
Chronic alcoholics also have significantly lower concentrations of hepatic GSH (Lauterburg and Velez, 1988). A number of different causes have been proposed. These include reduced rates of GSH production (Lauterburg et al., 1984), increased efflux of GSH from the liver (Fernandez-Checa et al., 1989; Choi et al., 2000), reduced cysteine production and its diversion to produce taurine (Kim et al., 2003), and increased lipid peroxidative damage resulting from formation of acetaldehyde (Vina et al., 1980). For simulations of APAP metabolism in alcoholics the starting value of steady state hepatic GSH concentration were halved ( (Lauterburg and Velez, 1988; Choi et al., 2000). It has been shown that soon after persons stop consuming alcohol (24 hours, (Choi et al., 2000)), levels of hepatic GSH start to return to normal. Accordingly, when accounting for the alcohol induced reduction of GSH production and increased efflux from the liver, the rate of hepatic GSH replenishment was augmented in a time dependent manner such that and:
where δ(t) = 1 when t ≤ 1440 minutes and δ(t) = 0 when t > 1440.
Effects of fasting on APAP metabolism
When modeling effects of fasting on acetaminophen hepatotoxicity, it is important to account for the fact that hepatic carbohydrate reserves are lower during fasting, and this can result in a significant reduction in rate of APAP glucuronidation (Price et al., 1987; Price and Jollow, 1988; Price and Jollow, 1989). Fasting in rats results in 40% reduction of glucuronidation and 30% reduction in rate of sulfation of APAP (Price and Jollow, 1989). Extreme fasting and uncontrolled diabetes can also result in stabilization of CYP2E1 mRNA (Gonzalez et al., 1991). In rats, CYP2E1 is induced by approximately 30-60% after 24 hr fasting (Hong et al., 1987; Johansson et al., 1988). For simulations of fasting in humans, it was assumed that levels of reduction of APAP-G and APAP-S production are similar to those in rats and . It was also assumed that in fasting individuals the activity of oxidation reactions increases by 50% .
Fasting also reduces the ratio of liver to whole body weight by approximately 20% in fasting rats (Price et al., 1987). Although fasting might reduce the size of human liver, our fasting simulations do not account for this phenomenon and the liver volume remains constant for malnourished and fasting individuals.
Fasting reduces the total GSH quantity in the liver due to a reduced rate of GSH production. For the simulation of acetaminophen metabolism in malnourished/fasting persons, the initial hepatic concentration of GSH was reduced by 25% . This value is based on measurements of GSH levels in malnourished patients (Shi et al., 1982) and agrees with similar GSH reductions in mice (~20%) (Price et al., 1987). Absence of nutrients also reduces the rate of GSH regeneration, and accordingly, we reduced the value for the starvation simulations .
Metabolism in alcoholic, chronic APAP users
Table 3 shows that the effects of the examined nutritional and lifestyle habits on different aspects of APAP metabolism in liver can be complementary or conflicting. When modeling combined effects of two processes that alter different components of a system, such as chronic use of APAP and consumption of alcohol, it might be reasonable to assume that the effects could be combined. However, when processes alter the workings of the same reactions, especially if they are conflicting influences (like chronic APAP use and fasting), it would be unwise to make assumptions about the outcome. For this reason, in this paper we have simulated drug metabolism in chronic APAP users who consume excessive amounts of alcohol but refrained from simulating APAP metabolism in any other combinatorial way, such as malnourished individuals who use the drug on a regular basis.
Results and discussion
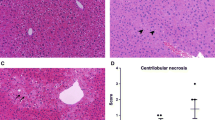
Famous Swiss-German alchemist Philippus Aureolus Paracelsus wrote: “All things are poison and not without poison; only the dose makes a thing not a poison” (Krieger, 2001). This statement is particularly apt for acetaminophen. While small quantities of APAP relieve suffering, relatively slight excess intake by some patients can result in acute liver damage. Significant overdose of APAP causes mitochondrial dysfunction and centrilobular necrosis in the liver and can be lethal (e.g., (McJunkin et al., 1976; Nogen and Bremner, 1978; Price et al., 1991)).
Many drugs are toxic due to production of chemically reactive metabolites that deleteriously alter the normal biochemistry of a patient. The amount of damage that these compounds cause depends on their concentration and metabolic half-life. Some of the toxic byproducts of drug metabolism are so short-lived that they never exit the organs in which they are formed. On the other hand, chemical change of others can be slow enough that they enter the systemic circulation and are transported to other organs. NAPQI, the toxic byproduct of APAP metabolism, is one of the former and does not leave the liver.
NAPQI can bind to sulfhydryl groups of cellular proteins and cause oxidative stress. Experiments have shown that harmful covalent binding of NAPQI to proteins is preceded by significant depletion of glutathione in the liver (Mitchell et al., 1973; Davis et al., 1974) because low doses of NAPQI are rapidly detoxified by glutathione through conversion of NAPQI to glutathionyl acetaminophen (Mitchell et al., 1973; Mitchell et al., 1974). Thus, as long as hepatic concentration of GSH remain sufficiently high, liver proteins are not altered (Jollow et al., 1973; Mitchell et al., 1973), and hepatotoxicity can be averted through replenishment of GSH and introduction of complementary antioxidants (e.g., treatment with N-acetyl-cysteine (Peterson and Rumack, 1977; Prescott et al., 1977)).
A number of factors affect the rate of APAP and NAPQI metabolism in a patient (Larson, 2007). The three that have the most widespread impact on the public are: chronic use of APAP, fasting, and alcohol consumption. In cases where patients have exhibited combinations (e.g. (Whitcomb and Block, 1994)) of above noted conditions, the effects of each factor have not been quantified. This ambiguity about the quantitative effects of each condition has resulted in differing postulates about the safety of APAP and the primary culprit for predisposing some patients to show signs of liver damage following moderate overdoses (4–10 g/day) or even uptake of therapeutic doses (4 g/day) (Whitcomb and Block, 1994; Slattery et al., 1996; Prescott, 2000).
Overall, any systemic perturbation that results in reduced glutathione concentration, induction of CYP enzymes, or reduced rates of sulfation or glucuronidation should be considered for increasing a patient’s susceptibility to hepatotoxicity. Given the uncertainty associated with completeness of patient histories in clinical records and the difficulty of parsing the collected information to quantify the deleterious effects of various daily habits, in silico pharmacological analysis is the sole means by which we can use the available biochemical data to gain a quantitative understanding of kinetics of hepatotoxicity in compromised patients. To this end, we developed a detailed PBPK model of APAP metabolism in humans and used it to simulate generation and detoxification of NAPQI for a number of prevalent scenarios. To date a number of other pharmacokinetic models of APAP and GSH metabolisms have been developed (Chen and Gillette, 1988; Tone et al., 1990; Srinivasan et al., 1994; Chiba and Pang, 1995; Ben-Shachar et al., 2012; Remien et al., 2012; Westerhout et al., 2012) and significantly contributed to our understanding of the dynamics of APAP induced hepatotoxicity. However, each of these studies focused on a specific portion of the metabolic process and did not simulate and examine the combined effects of the noted determining factors on the important aspects of drug metabolism.
Model validation
In order to ensure that the incorporated kinetic parameters are correct, the model’s predictions were compared against clinical measurements (see Figures 2 and 3). The model predictions strongly agree with two sets of measured results (Rawlins et al., 1977; Kennedy and Van Rij, 2006) for the short period after introduction of APAP (Figure 2) and for longer time periods associated with chronic use of APAP (Gelotte et al., 2007) (Figure 3).
Predicted and measured acetaminophen plasma concentrations. The results are for three days of multiple dosing with 1 g every 6 hours (Gelotte et al., 2007) (●)).
Next, depletion of GSH concentrations in the liver following uptake of different doses of APAP were examined (see Figure 4). As noted in the methods section, the regeneration mechanism of GSH in the model has been parameterized so that intake of a single 15 g dose of APAP (generally accepted upper limit of APAP prior to generation of hepatotoxicity (Mitchell et al., 1974; Whitcomb and Block, 1994; Rumack, 2002)) depletes the liver GSH level by approximately 70%, and thus greater doses would dip GSH level below 30% of normal level and initiate liver damage.
Chronic APAP use
Acetaminophen should be used cautiously when taken on a chronic basis because even for some patients without risks, APAP may be hepatotoxic at therapeutic doses (Bolesta and Haber, 2002). Statistical analyses have shown that the odds of developing ALF, following use of therapeutic doses of APAP, are extremely small (0.4 per million adults over the age of 15 per year) (Sabaté et al., 2011). The PBPK model was used to determine if the recorded kinetic characteristics of APAP metabolic pathways can account for this rare phenomenon. The model predicts that under normal conditions, continual uptake of 1 g of APAP every 6 hours results in approximately a 15% reduction in steady state levels of liver GSH (Figure 5). This result agrees with recorded observations (Nuttall et al., 2003). This level of GSH should sufficiently prevent hepatotoxicity in normal patients. Furthermore, as can be seen in Figure 5, the simulations of GSH concentration, following continual uptake of 1, 2 and 5 g every 6 hours show that none of these regimens should result in a reduction of GSH levels bellow 30% of the normal steady state values.
Predicted liver glutathione concentration following chronic ingestion of APAP at 3 different daily doses (1 g/6 hours (black), 2 g/6 hours (blue), and 5 g/6 hours (red)). Solid lines represent simulations with induction of APAP glucuronidation and reduction of APAP sulfation (Gelotte et al., 2007). Dashed curves are for simulations that do not incorporate the observed changes. The gray dashed line marks the minimum level of GSH that is necessary to prevent intrahepatic covalent binding by NAPQI.
Hence, it can be deduced that the reported patients (who based on their collected history are not supposed to be at risk of APAP poisoning) must have some unique physiological characteristic that increases their susceptibility to oxidative damage. One possibility could be that for these individuals routes of APAP metabolism are not changed following chronic uptake of 1–2 g doses of APAP (Gelotte et al., 2007). Based on measured single dose pharmacokinetics of APAP metabolism, the sulfation capacity of the liver sulfatransferases could be saturated and more of the drug would be shunted toward NAPQI production, specially following continual ingestion of high doses APAP. Clinical studies of healthy patients ingesting multiple doses of APAP have shown that the serum sulfate concentration can drop significantly (Hendrix-Treacy et al., 1986). However, recent experimental examinations have shown that at higher doses, chronic uptake of APAP results in an approximately 23% increase in the clearance rate of APAP-G (Gelotte et al., 2007).
Figure 5 shows the predicted transient hepatic concentrations of GSH with and without alterations in APAP metabolism. Chronic uptake of APAP clearly behaves like hormesis. The increased glucuronidation/reduced sulfation of APAP overall has a protective effect. For example, the area under the curves from Figure 5 shows that following uptake of 1, 2 and 5 g of APAP every six hours for 5 days, diversion of APAP metabolism to glucuronidation reduces the need for GSH detoxification by about 1, 10, and nearly 50%, respectively. While induction of UDP-glucuronosyltransferase appears to have a significant beneficial effect on diverting APAP away from NAPQI production, particularly at higher doses, the loss of the 1% improvement cannot explain the observed toxicity following therapeutic doses of APAP.
Other possibilities for the observed phenomenon can be induction of NAPQI- producing CYPs, reduced rates of hepatic GSH generation, or a combination of both. According to model simulations, increasing the rate of CYPs more than 30 times will result in hepatotoxicity following periodic uptake of 1 g of APAP every 6 hours. Such a drastic increase in CYP activity seems highly unlikely, and the mechanism of induction is unclear. One possibility could be that like the behavior observed following interaction between CYPs and alcohol, acetone, or isoniazid (Ryan et al., 1986; Song et al., 1987), over an extended period of time, a compound that has not been considered as a part of a patient’s history slowly induces enzymes such as CYP2E1 through ligand stabilization. Then, a relatively rapid drop in concentration of that compound would result in much greater APAP oxidation. To date, a compound that could so drastically induce the activity of CYPs has not been found.
The model predicts that decreasing the rate of GSH regeneration (i.e., ) by only a factor 5 would make chronic ingestion of 1 g of APAP every 6 hours toxic after about 1 day. This metabolic change seems a lot more plausible as a cause for the observed phenomenon where therapeutic usage of APAP could lead to sever hepatotoxicity. Fasting or poor nutrition is a prime candidate for why a person might present reduced GSH production capabilities. A number of other studies have also suggested that reduced levels of GSH, resulting from poor nutrition, could result in elevated risk of APAP induced hepatotoxicity (e.g., (Whitcomb and Block, 1994; Prescott, 2000)). Some herbal remedies and natural products have been recommended as protection against hepatotoxicity through scavenging of reactive oxidative species and disruption of cell death signaling mechanisms (e.g.,Oz et al., 2005; Chen et al., 2009; Wang et al., 2010; Galal et al., 2012)); however, depending on a variety of factors (like time of ingestion in relation to APAP uptake (Salminen et al., 2012)), the results might vary, and the treatment might actually potentiate hepatotoxicity. Accounting for such heretofore ignored factors could lead to answers about how therapeutic doses of APAP could lead to ALF.
Chronic alcoholism
Activity of many CYPs can be altered in the presence of some drugs and other common biochemical substrates (Hewitt et al., 2007). Analysis of interaction of ethanol with APAP is very complicated, and the resulting conclusions can be controversial (Slattery et al., 1996; Prescott, 2000). For example, in animals, concurrent ingestion of ethanol with APAP actually protects the patient against hepatotoxicity even if prior chronic intake of alcohol has induced the liver’s CYP activities (Sato et al., 1981; Altomare et al., 1984; Thummel et al., 1989). If one assumes that similar mechanisms govern alcohol-APAP interaction in humans as in animals, then alcohol could decrease, increase, or have no effect on the toxicity of APAP, depending on the timing and duration of alcohol consumption.
The deleterious effects of alcohol on maintaining the normal GSH concentrations in hepatocytes are twofold. First, chronic ingestion of alcohol impairs transport of GSH from hepatocyte cytosol to mitochondria while increasing efflux of GSH from the liver (Fernandez-Checa et al., 1989; Choi et al., 2000). There is some controversy about the identity of the causative agent of GSH depletion following alcohol consumption. Some studies have shown that ethanol, rather than its metabolic products, alters in vivo regulatory events and causes the reduction of liver GSH (Speisky et al., 1988). On the other hand, others have shown that although proximate metabolites of ethanol (acetaldehyde and acetate) by themselves appear to have limited effect on GSH levels, hybrid aldehyde adducts (e.g., malondialdehyde-acetaldehyde (Tuma, 2002)) and their role in enhancing lipid peroxidation (Hartley and Petersen, 1997) can deplete GSH.
Second, CYP2E1 is the primary P-450 responsible for metabolism of ethanol (Lieber and DeCarli, 1970;1982) and is induced by the presence of this compound (Koop and Tierney, 1990), principally due to a post-transcriptional mechanism where presence of the substrate stabilizes the enzyme from degradation (Song et al., 1986). Alcohol acts as a competitive inhibitor of the APAP oxidation reaction and while present in the body protects the liver against production of NAPQI. However, after clearance of alcohol from the system, greater availability of CYP2E1 increases (up by a factor of 2) the rate of conversion of APAP to toxic NAPQI molecules (Thummel et al., 2000).
Figure 6 shows the transient hepatic GSH levels following continual uptake of therapeutic doses of APAP after a period of extreme binge drinking (200 hours of continual blood alcohol level of 3 g/L). CYP induction only increases the need for GSH detoxification by approximately 7% more than that in a non-alcoholic person.
Predicted liver glutathione concentrations following chronic ingestion of therapeutic doses of APAP (1 g/6 hours). The black line shows GSH concentration in a normal person. The blue line shows GSH concentration following a period of binge drinking accounting only for the 2.14 times increase of the APAP oxidation pathway (Thummel et al., 2000). The red line shows the model-predicted liver GSH concentration when it accounts for both CYP induction and 50% reduction in steady state concentration of GSH and its regeneration rate. The gray dashed line marks the minimum level of GSH that is necessary to prevent intrahepatic covalent binding by NAPQI.
Given the short half life of deleterious effects of alcohol consumption on hepatic GSH levels (~12 hours (Choi et al., 2000)), the most dangerous period for a person drinking and chronically taking APAP would be the first 12–18 hours after drinking has stopped. During this period, if alcohol consumption reduces the hepatic steady state concentration of GSH and the rate of its regeneration to half the normal values, then consecutive uptake of supra-therapeutic doses of APAP can be toxic. Interestingly, the model predicts that as long as the GSH production capacity of liver return to normal within a reasonable period of time (24–36 hours), and the detoxifying capacity of the liver is not challenged by other toxins, then chronic use of therapeutic doses of APAP should not result in liver injury.
This result is in strong agreement with some of the published arguments that have reasoned that CYP induction cannot form the primary basis for the strong link between APAP induced hepatotoxicity and use of alcohol (Prescott, 2000). The main reason for increased cases of APAP-induced hepatotoxicity is reduced availability of GSH. While depletion of hepatic GSH is a rare mode of toxicity for drugs (examples in (Kostrubsky et al., 2007; Dykens et al., 2008)), it is common for a variety of toxicants such as carbon tetrachloride (Jaeschke et al., 2013). It can also occur in individuals consuming certain herbal medications (Senadhi et al., 2012) like pennyroyal oil (Chitturi and Farrell, 2000). Accordingly, one can argue that APAP is not unique in its toxicity to alcoholics and that for these individuals exposure to any compound that would be metabolized in the liver to produce reactive oxidative species could result in hepatotoxicity.
Fasting and malnutrition
Malnutrition is one of the primary instigators of hepatotoxicity following moderate (4–10 g/day) overdose of APAP (Whitcomb and Block, 1994). The PBPK model predictions agree with these observations. Figure 7 shows the model-predicted hepatic GSH levels following ingestion of 4 g of APAP. The results indicate that the combined effect of reduced hepatic GSH levels and its rate of regeneration along with augmented enzymatic activities could make a single dose uptake of 4 g of APAP harmful. When the outcomes resulting from changes to enzyme activities are compared to those associated with reduced GSH levels, the deleterious effects seem to be of the same magnitude (Figure 7, green and blue lines respectively). This result is significant because it indicates that treatments that only resupply the liver with antioxidants might not be enough to significantly reduce the risk of hepatotoxicity.
Predicted liver glutathione levels following ingestion of a 4 g dose of APAP. The black line represents metabolism in a healthy well-fed person. The red line represents metabolism in a malnourished individual. The blue line represents GSH levels if only the GSH levels and its regeneration rates are altered as a result of malnutrition. The green line represents the outcome if fasting only change the activity of APAP metabolizing enzymes. The gray dashed line marks the minimum level of GSH that is necessary to prevent intrahepatic covalent binding by NAPQI.
Conclusions
The number of ALF cases in United States attributed to acetaminophen have been continually rising (Larson et al., 2005; Nourjah et al., 2006), and there has been a lot of debate about safety of this popular drug. A number of different factors have been suggested as the primary cause of hepatotoxicity in patients. In this study, we conducted a quantitative examination of the effects of chronic APAP use, alcohol consumption, and malnutrition on increasing the risks of liver damage. The results of our simulations show that there is a hormesis-like protective behavior following chronic consumption of APAP. The shunting of metabolism to the glucuronidation pathway reduces production of toxic byproducts at higher doses, and this could have a significant protective effect.
Our simulations show that alcohol drinkers who are chronic acetaminophen users have an increased risk of liver damage particularly within the first day following an episode of binge drinking. However, these individuals are at risk from any compound that could be activated to act as an oxidizing agent, and therefore APAP is not unique in its toxicity.
Finally, our analysis of APAP metabolism in fasting patients show that they are at a much greater risk of hepatotoxicity, resulting from a mild overdose (4–10 g), than well-fed individuals. For these individuals, a combination of factors, including shunting of drug metabolism to oxidative pathways and reduced rate of glutathione metabolism, exacerbate the problem and complicate the treatment choices since focusing on only one of the above causes might not fully mitigate the problem.
Abbreviations
- ALF:
-
Acute liver failure
- APAP:
-
Acetaminophen
- NAPQI:
-
N-acetyl-p-benzoquinone imine.
References
Altomare E, Leo MA, Lieber CS: Interaction of acute ethanol administration with acetaminophen metabolism and toxicity in rats fed alcohol chronically. Alcohol Clin Exp Res 1984, 8: 405–408. 10.1111/j.1530-0277.1984.tb05688.x
Ameer B, Divoll M, Abernethy DR, Greenblatt DJ, Shargel L: Absolute and relative bioavailability of oral acetaminophen preparations. J Pharm Sci 1983, 72: 955–958. 10.1002/jps.2600720832
Barbaro G, Di Lorenzo G, Soldini M, Parrotto S, Bellomo G, Belloni G, Grisorio B, Barbarini G: Hepatic glutathione deficiency in chronic hepatitis C: quantitative evaluation in patients who are HIV positive and HIV negative and correlations with plasmatic and lymphocytic concentrations and with the activity of the liver disease. Am J Gastroenterol 1996, 91: 2569.
Ben-Shachar R, Chen Y, Luo S, Hartman C, Reed M, Nijhout HF: The biochemistry of acetaminophen hepatotoxicity and rescue: a mathematical model. Theoretical Biol Med Model 2012, 9: 55. 10.1186/1742-4682-9-55
Bernal W: Changing patterns of causation and the use of transplantation in the United kingdom. Semin Liver Dis 2003, 23: 227–237.
Bessems JG, Vermeulen NP: Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 2001, 31: 55–138. 10.1080/20014091111677
Bolesta S, Haber SL: Hepatotoxicity associated with chronic acetaminophen administration in patients without risk factors. Ann Pharmacother 2002, 36: 331.
Chen R, Gillette JR: Pharmacokinetic procedures for the estimation of organ clearances for the formation of short-lived metabolites. Acetaminophen-induced glutathione depletion in hamster liver. Drug Metab Dispos 1988, 16: 373–385.
Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, Nelson SD: Oxidation of Acetaminophen to Its Toxic Quinone Imine and Nontoxic Catechol Metabolites by Baculovirus-Expressed and Purified Human Cytochromes P450 2E1 and 2A6. Chem Res Toxicol 1998, 11: 295–301. 10.1021/tx9701687
Chen X, Sun C-K, Han G-Z, Peng J-Y, Li Y, Liu Y-X, Lv Y-Y, Liu K-X, Zhou Q, Sun H-J: Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significanly correlated with cytochrome P450 suppression. World J Gastroenterol WJG 2009, 15: 1829. 10.3748/wjg.15.1829
Chiba M, Pang K: Glutathione Depletion Kinetics With Acetaminophen - A Simulation Study. Drug Metab Dispos 1995, 23: 622–630.
Chitturi S, Farrell GC: Herbal hepatotoxicity: an expanding but poorly defined problem. J Gastroenterol Hepatol 2000, 15: 1093–1099. 10.1046/j.1440-1746.2000.02349.x
Choi DW, Kim SY, Kim SK, Kim YC: Factors involved in hepatic glutathione depletion induced by acute ethanol administration. J Toxicol Environ Health A 2000, 60: 459–469. 10.1080/00984100050079520
Clark LH, Setzer RW, Barton HA: Framework for evaluation of physiologically-based pharmacokinetic models for use in safety or risk assessment. Risk Anal 2004, 24: 1697–1717. 10.1111/j.0272-4332.2004.00561.x
Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B: The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys 1988, 264: 253. 10.1016/0003-9861(88)90592-9
Davis DC, Potter WZ, Jollow DJ, Mitchell JR: Species differences in hepatic glutathione depletion, covalent binding and hepatic necrosis after acetaminophen. Life Sci 1974, 14: 2099–2109. 10.1016/0024-3205(74)90092-7
Dong H, Haining RL, Thummel KE, Rettie AE, Nelson SD: Involvement of human cytochrome P450 2D6 in the bioactivation of acetaminophen. Drug Metab Dispos 2000, 28: 1397–1400.
Dykens JA, Jamieson JD, Marroquin LD, Nadanaciva S, Xu JJ, Dunn MC, Smith AR, Will Y: In Vitro Assessment of Mitochondrial Dysfunction and Cytotoxicity of Nefazodone, Trazodone, and Buspirone. Toxicol Sci 2008, 103: 335–345. 10.1093/toxsci/kfn056
Fernandez-Checa JC, Ookhtens M, Kaplowitz N: Effects of chronic ethanol feeding on rat hepatocytic glutathione. Relationship of cytosolic glutathione to efflux and mitochondrial sequestration. J Clin Investig 1989, 83: 1247. 10.1172/JCI114008
Galal RM, Zaki HF, Seif E-NMM, Agha AM: Potential Protective Effect of Honey Against Paracetamol-induced Hepatotoxicity. Arch Iran Med 2012, 15: 674.
Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT: Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther 2007, 81: 840–848. 10.1038/sj.clpt.6100121
Gonzalez FJ, Ueno T, Umeno M, Song BJ, Veech RL, Gelboin HV: Microsomal ethanol oxidizing system: transcriptional and posttranscriptional regulation of cytochrome P450, CYP2E1. Alcohol Alcohol (Oxford, Oxford) Suppl 1991, 1: 97.
Gregory B, Larson AM, Reisch J, Lee WM: Acetaminophen dose does not predict outcome in acetaminophen-induced acute liver failure. J Invest Med 2010, 58: 707.
Griffith OW: Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biol Med 1999, 27: 922. 10.1016/S0891-5849(99)00176-8
Hartley DP, Petersen DR: Co‒Metabolism of Ethanol, Ethanol‒Derived Acetaldehyde, and 4‒Hydroxynonenal in Isolated Rat Hepatocytes. Alcohol Clin Exp Res 1997, 21: 298–304. 10.1111/j.1530-0277.1997.tb03764.x
Hendrix-Treacy S, Wallace SM, Hindmarsh KW, Wyant GM, Danilkewich A: The effect of acetaminophen administration on its disposition and body stores of sulphate. Eur J Clin Pharmacol 1986, 30: 273–278. 10.1007/BF00541527
Hewitt NJ, Lecluyse EL, Ferguson SS: Induction of hepatic cytochrome P450 enzymes: methods, mechanisms, recommendations, and in vitro-in vivo correlations. Xenobiotica 2007, 37: 1196–1224. 10.1080/00498250701534893
Hong J, Pan J, Gonzalez FJ, Gelboin HV, Yang CS: The induction of a specific form of cytochrome P-450 (P-450j) by fasting. Biochem Biophys Res Commun 1987, 142: 1077–1083. 10.1016/0006-291X(87)91525-7
Howie D, Adriaenssens PI, Prescott LF: Paracetamol metabolism following overdosage: application of high performance liquid chromatography. J Pharm Pharmacol 1977, 29: 235–237. 10.1111/j.2042-7158.1977.tb11295.x
Imai H, Kotegawa T, Ohashi K: Duration of drug interactions: putative time courses after mechanism-based inhibition or induction of CYPs. Expert Rev Clin Pharmacol 2011, 4: 409. 10.1586/ecp.11.30
Jaeschke H, Williams CD, McGill MR, Xie Y, Ramachandran A: Models of drug-induced liver injury for evaluation of phytotherapeutics and other natural products. Food Chem Toxicol 2013, 55: 279–289.
Johansson I, Ekstroem G, Scholte B, Puzycki D, Joernvall H, Ingelman-Sundberg M: Ethanol-, fasting-, and aceton-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry 1988, 27: 1925–1934. 10.1021/bi00406a019
Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. II Role covalent bind vivo. J Pharmacol Exp Ther 1973, 187: 195–202.
Jones AL: Mechanism of action and value of N-acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. Clin Toxicol 1998, 36: 277–285. 10.3109/15563659809028022
Kennedy JM, Van Rij AM: Drug absorption from the small intestine in immediate postoperative patients. British J Anaesth 2006, 97: 171–180. 10.1093/bja/ael117
Kera Y, Penttilä KE, Lindros KO: Glutathione replenishment capacity is lower in isolated perivenous than in periportal hepatocytes. Biochem J 1988, 254: 411.
Kim SK, Seo JM, Jung YS, Kwak HE, Kim YC: Alterations in hepatic metabolism of sulfur-containing amino acids induced by ethanol in rats. Amino Acids 2003, 24: 103–110.
Koop DR, Morgan ET, Tarr GE, Coon MJ: Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem 1982, 257: 8472–8480.
Koop DR, Tierney DJ: Multiple mechanisms in the regulation of ethanol-inducible cytochrome P450IIE1. BioEssays News Rev Mol Cell Dev Biol 1990, 12: 429. 10.1002/bies.950120906
Kostrubsky SE, Strom SC, Ellis E, Nelson SD, Mutlib AE: Transport, Metabolism, and Hepatotoxicity of Flutamide, Drug–Drug Interaction with Acetaminophen Involving Phase I and Phase II Metabolites. Chem Res Toxicol 2007, 20: 1503–1512. 10.1021/tx7001542
Krieger RI: Handbook of pesticide toxicology. San Diego: Academic Press; 2001.
Larsen FS, Kirkegaard P, Rasmussen A, Hansen BA: The Danish liver transplantation program and patients with serious acetaminophen intoxication. Transplant Proc 1995, 27: 3519–3520.
Larson AM: Acetaminophen hepatotoxicity. Clin Liver Dis 2007, 11: 525–548. vi vi 10.1016/j.cld.2007.06.006
Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO: Acetaminophen‒induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005, 42: 1364–1372. 10.1002/hep.20948
Lauterburg BH, Davies S, Mitchell JR: Ethanol suppresses hepatic glutathione synthesis in rats in vivo. J Pharmacol Exp Ther 1984, 230: 7–11.
Lauterburg BH, Velez ME: Glutathione deficiency in alcoholics: risk factor for paracetamol hepatotoxicity. Gut 1988, 29: 1153–1157. 10.1136/gut.29.9.1153
Lee SST, Buters JTM, Pineau T, Fernandez-Salguero P, Gonzalez FJ: Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem 1996, 271: 12063–12067. 10.1074/jbc.271.20.12063
Lieber CS, DeCarli LM: Hepatic microsomal ethanol-oxidizing system. J Biol Chem 1970, 245: 2505–2512.
Lu SC: Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999, 13: 1169–1183.
Luttringer O, Theil FP, Poulin P, Schmitt-Hoffmann AH, Guentert TW, Lavé T: Physiologically based pharmacokinetic (PBPK) modeling of disposition of epiroprim in humans. J Pharm Sci 2003, 92: 1990–2007. 10.1002/jps.10461
McJunkin B, Barwick KW, Little WC, Winfield JB: Fatal massive hepatic necrosis following acetaminophen overdose. JAMA J Am Med Assoc 1976, 236: 1874–1875. 10.1001/jama.1976.03270170040024
McQuade DJ, Aknuri S, Dargan PI, Wood DM: Management of acute paracetamol (acetaminophen) toxicity: a standardised proforma improves risk assessment and overall risk stratification by emergency medicine doctors. Emerg Med J 2012, 29: 961–964. 10.1136/emermed-2011-200889
Miller RP, Roberts RJ, Fischer LJ: Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther 1976, 19: 284.
Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB: Acetaminophen-induced hepatic necrosis. IV Protec role glutathione. J Pharmacol Exp Ther 1973, 187: 211–217.
Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H: Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 1974, 16: 676.
Moling O, Cairon E, Rimenti G, Rizza F, Pristerá R, Mian P: Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin Ther 2006, 28: 755–760. 10.1016/j.clinthera.2006.05.002
Nogen AG, Bremner JE: Fatal acetaminophen overdosage in a young child. J Pediatr 1978, 92: 832. 10.1016/S0022-3476(78)80169-3
Nourjah P, Ahmad SR, Karwoski C, Willy M: Estimates of acetaminophen (paracetomal)‒associated overdoses in the United States. Pharmacoepidemiol Drug Safe 2006, 15: 398–405. 10.1002/pds.1191
Nuttall SL, Khan JN, Thorpe GH, Langford N, Kendall MJ: The impact of therapeutic doses of paracetamol on serum total antioxidant capacity. J Clin Pharm Ther 2003, 28: 289–294. 10.1046/j.1365-2710.2003.00493.x
Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJS, Chen TS: Diverse antioxidants protect against acetaminophen hepatotoxicity. J Biochem Mol Toxicol 2005, 18: 361–368. 10.1002/jbt.20042
Penttilä KE: Role of cysteine and taurine in regulating glutathione synthesis by periportal and perivenous hepatocytes. Biochem J 1990, 269: 659.
Perrot N, Nalpas B, Yang CS, Beaune PH: Modulation of cytochrome P450 isozymes in human liver, by ethanol and drug intake. Eur J Clin Investig 1989, 19: 549. 10.1111/j.1365-2362.1989.tb00273.x
Peterson RG, Rumack BH: Treating acute acetaminophen poisoning with acetylcysteine. JAMA J Am Med Assoc 1977, 237: 2406–2407. 10.1001/jama.1977.03270490046025
Poulin P, Schoenlein K, Theil FP: Prediction of adipose tissue: plasma partition coefficients for structurally unrelated drugs. J Pharm Sci 2001, 90: 436–447. 10.1002/1520-6017(200104)90:4<436::AID-JPS1002>3.0.CO;2-P
Poulin P, Theil FP: A priori prediction of tissue:plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci 2000, 89: 16–35. 10.1002/(SICI)1520-6017(200001)89:1<16::AID-JPS3>3.0.CO;2-E
Poulin P, Theil FP: Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci 2002, 91: 129–156. 10.1002/jps.10005
Poulin P, Theil FP: Prediction of pharmacokinetics prior to in vivo studies. II Generic physiol based pharm model drug dispos. J Pharm Sci 2002, 91: 1358–1370. 10.1002/jps.10128
Prescott LF: Paracetamol, alcohol and the liver. Br J Clin Pharmacol 2000, 49: 291–301.
Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT: Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977, 2: 432.
Price LM, Poklis A, Johnson DE: Fatal acetaminophen poisoning with evidence of subendocardial necrosis of the heart. J Forensic Sci 1991, 36: 930.
Price VF, Jollow DJ: Mechanism of decreased acetaminophen glucuronidation in the fasted rat. Biochem Pharmacol 1988, 37: 1067–1075. 10.1016/0006-2952(88)90512-6
Price VF, Jollow DJ: Effect of glucose and gluconeogenic substrates on fasting-induced suppression of acetaminophen glucuronidation in the rat. Biochem Pharmacol 1989, 38: 289–297. 10.1016/0006-2952(89)90039-7
Price VF, Miller MG, Jollow DJ: Mechanisms of fasting-induced potentiation of acetaminophen hepatotoxicity in the rat. Biochem Pharmacol 1987, 36: 427–433. 10.1016/0006-2952(87)90346-7
Raucy JL, Lasker JM, Lieber CS, Black M: Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys 1989, 271: 270–283. 10.1016/0003-9861(89)90278-6
Rawlins MD, Henderson DB, Hijab AR: Pharmacokinetics of paracetamol (acetaminophen) after intravenous and oral administration. Eur J Clin Pharmacol 1977, 11: 283–286. 10.1007/BF00607678
Reith D, Medlicott NJ, Kumara De Silva R, Yang L, Hickling J, Zacharias M: simultaneous modelling of the Michaelis-Menten kinetics of paracetamol sulphation and glucuronidation. Clin Exp Pharmacol Physiol 2009, 36: 35–42. 10.1111/j.1440-1681.2008.05029.x
Remien CH, Adler FR, Waddoups L, Box TD, Sussman NL: Mathematical modeling of liver injury and dysfunction after acetaminophen overdose: early discrimination between survival and death. Hepatology 2012, 56: 727–734. 10.1002/hep.25656
Rumack BH: Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin toxicol 2002, 40: 3. 10.1081/CLT-120002882
Ryan DE, Koop DR, Thomas PE, Coon MJ, Levin W: Evidence that isoniazid and ethanol induce the same microsomal cytochrome P-450 in rat liver, an isozyme homologous to rabbit liver cytochrome P-450 isozyme 3a. Arch Biochem Biophys 1986, 246: 633. 10.1016/0003-9861(86)90319-X
Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, Guarner C, Forné M, Solà R: Paracetamol in therapeutic dosages and acute liver injury: causality assessment in a prospective case series. BMC Gastroenterol 2011, 11: 80. 10.1186/1471-230X-11-80
Salminen WF, Yang X, Shi Q, Greenhaw J, Davis K, Ali AA: Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol 2012, 50: 1439–1446. 10.1016/j.fct.2012.01.027
Sato C, Nakano M, Lieber CS: Prevention of acetaminophen-induced hepatotoxicity by acute ethanol administration in the rat: comparison with carbon tetrachloride-induced hepatoxicity. J Pharmacol Exp Ther 1981, 218: 805–810.
Schiødt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stribling R, Crippin JS, Flamm S, Somberg KA: Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transplant Surg 2003, 5: 29–34.
Schiødt FV, Lee WM, Bondesen S, Ott P, Christensen E: Influence of acute and chronic alcohol intake on the clinical course and outcome in acetaminophen overdose. Aliment Pharmacol Ther 2002, 16: 707–715. 10.1046/j.1365-2036.2002.01224.x
Schmidt LE, Dalhoff K: The impact of current tobacco use on the outcome of paracetamol poisoning. Aliment Pharmacol Ther 2003, 18: 979–985. 10.1046/j.1365-2036.2003.01789.x
Senadhi V, Arora D, Arora M, Marsh F: A rare cause of drug-induced hepatitis in an immunocompromised patient and the role of glutathione. World J Hepatol 2012, 4: 248. 10.4254/wjh.v4.i8.248
Shi EC, Fisher R, McEvoy M, Vantol R, Rose M, Ham JM: Factors influencing hepatic glutathione concentrations: a study in surgical patients. Clin Sci (London England 1979) 1982, 62: 279.
Slattery JT, Nelson SD, Thummel KE: The complex interaction between ethanol and acetaminophen. Clin Pharmacol Ther 1996, 60: 241. 10.1016/S0009-9236(96)90050-8
Smith MT, Loveridge N, Wills ED, Chayen J: The distribution of glutathione in the rat liver lobule. Biochem J 1979, 182: 103.
Song BJ, Gelboin HV, Park SS, Yang CS, Gonzalez FJ: Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem 1986, 261: 16689–16697.
Song BJ, Matsunaga T, Hardwick JP, Park SS, Veech RL, Yang CS, Gelboin HV, Gonzalez FJ: Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol 1987, 1: 542–547. 10.1210/mend-1-8-542
Speisky H, Kera Y, Penttilä KE, Israel Y, Lindros KO: Depletion of hepatic glutathione by ethanol occurs independently of ethanol metabolism. Alcohol Clin Exp Res 1988, 12: 224–228. 10.1111/j.1530-0277.1988.tb00184.x
Srinivasan RS, Bourne DW, Putcha L: Application of physiologically based pharmacokinetic models for assessing drug disposition in space. J Clin Pharmacol 1994, 34: 692–698. 10.1002/j.1552-4604.1994.tb02025.x
Takahashi T, Lasker JM, Rosman AS, Lieber CS: Induction of cytochrome P‒4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA. Hepatology 1993, 17: 236–245.
Thomas SH: Paracetamol (acetaminophen) poisoning. Pharmacol Ther 1993, 60: 91–120. 10.1016/0163-7258(93)90023-7
Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT: Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by Human CYP3A4. Biochem Pharmacol 1993, 45: 1563–1569. 10.1016/0006-2952(93)90295-8
Thummel KE, Slattery JT, Nelson SD, Lee CA, Pearson PG: Effect of ethanol on hepatotoxicity of acetaminophen in mice and on reactive metabolite formation by mouse and human liver microsomes. Toxicol Appl Pharmacol 1989, 100: 391–397. 10.1016/0041-008X(89)90287-1
Thummel KE, Slattery JT, Ro H, Chien JY, Nelson SD, Lown KE, Watkins PB: Ethanol and production of the hepatotoxic metabolite of acetaminophen in healthy adults&ast. Clin Pharmacol Ther 2000, 67: 591–599. 10.1067/mcp.2000.106574
Toes MJ, Jones AL, Prescott L: Drug interactions with paracetamol. Am J Ther 2005, 12: 56–66. 10.1097/00045391-200501000-00009
Tone Y, Kawamata K, Murakami T, Higashi Y, Yata N: Dose-dependent pharmacokinetics and first-pass metabolism of acetaminophen in rats. J Pharmacobiodyn 1990, 13: 327–335. 10.1248/bpb1978.13.327
Tuma DJ: Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med 2002, 32: 303. 10.1016/S0891-5849(01)00742-0
Ueshima Y, Tsutsumi M, Takase S, Matsuda Y, Kawahara H: Acetaminophen Metabolism in Patients with Different Cytochrome P‒4502E1 Genotypes. Alcohol Clin Exp Res 2006, 20: 25a-28a.
Vina J, Estrela JM, Guerri C, Romero F: Effect of ethanol on glutathione concentration in isolated hepatocytes. Biochem J 1980, 188: 549.
Wang AY, Lian LH, Jiang YZ, Wu YL, Nan JX: Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J Gastroenterol WJG 2010, 16: 384. 10.3748/wjg.v16.i3.384
Westerhout J, Ploeger B, Smeets J, Danhof M, De Lange ECM: Physiologically Based Pharmacokinetic Modeling to Investigate Regional Brain Distribution Kinetics in Rats. AAPS J 2012, 14: 543–553. 10.1208/s12248-012-9366-1
Whitcomb DC, Block GD: Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA J Am Med Assoc 1994, 272: 1845–1850. 10.1001/jama.1994.03520230055038
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND: Glutathione metabolism and its implications for health. J Nutr 2004, 134: 489–492.
Acknowledgements
The work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. The project (12-SI-004) was funded by the Laboratory Directed Research and Development program at LLNL. This material is also based upon work supported by the S.D. Bechtel, Jr. Foundation and by the National Science Foundation under Grant No. 0952013. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the S.D. Bechtel, Jr. Foundation or the National Science Foundation. LLNL-JRNL-640155
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
AN and DMN developed the PBPK model and validated the results. AN analyzed the data and drafted the manuscript. BJS provided toxicological expertise, information on metabolism of glutathione in liver, and some text for the manuscript. SEW and FCL supervised the study. All authors have read the manuscript and approve of its content.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Navid, A., Ng, D.M., Stewart, B.J. et al. Quantitative In Silico analysis of transient metabolism of acetaminophen and associated causes of hepatotoxicity in humans. In Silico Pharmacol. 1, 14 (2013). https://doi.org/10.1186/2193-9616-1-14
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-9616-1-14