Abstract
Nanosized ZnO was prepared through hydrothermal process and characterized by scanning electron microscopy, X-ray diffraction and laser-induced breakdown spectra measurement techniques. The as-prepared nanosized ZnO was used to investigate the decolorization/degradation of crystal violet, a cationic dye which is extensively used in dyeing/textile industries, under visible light through adsorption studies of the dye solution with ZnO in the dark. The results show that the adsorption of CV on ZnO takes about 200 min to reach equilibrium, and the equilibrium time at a certain concentration of the dye seems to be independent of temperatures that are used for the preparation of ZnO samples. The adsorption data follow the pseudo-first-order kinetic model (Lagergren), and the adsorption pattern follows the Langmuir model.
Prepared ZnO (300°C) was found to be a more efficient photocatalyst among others including pristine ZnO, to decolorize/degrade the dye. The decolorization rate is increased with the decreasing of the initial dye concentration and reached at a limiting value. The catalyst loading also influences the decolorization/degradation of the dye, and decolorization rate is increased with increasing the catalyst loading and reached at a limiting value. ZnO was found to be stable under visible light irradiation at solution pH = 6. The photocatalytic degradation of the dye followed zero-order kinetics, and the Langmuir-Hinshelwood mechanism was found to be valid.
Similar content being viewed by others
Background
Crystal violet (CV) is a triarylmethane dye which is extensively used in industries such as textile/dyeing, ball-point pen, paper, leather, additives, foodstuffs, cosmetics, and analytical chemistry [1, 2]. CV is also used in biological staining. CV binds to DNA, so it has been used in cell viability assays in biochemistry. However, this binding to DNA will cause replication errors in living tissue, possibly leading to mutations and cancer. Toxicological investigations indicate that crystal violet has carcinogenic and mutagenic effects in rodents [3]. It has also been linked to increased risk of human bladder cancer. Therefore, it is treated as a serious pollutant in wastewater and difficult to treat by common removal methods such as coagulation and biodegradation, but few studies on its treatment have been attempted [1, 4]. The release of wastewater containing the dye poses a dramatic source of water pollution, eutrophication, and perturbation of aquatic life. Therefore, a method is highly required of treating wastewater containing CV.
In the last few decades, much attention has been paid to investigate degradation of organic pollutants mediated by TiO2 particles in aqueous suspension under UV-light irradiation [5–12]. Recent studies indicate that ZnO can also be used as a promising photocatalyst to degrade such types of organic pollutants under visible/solar irradiation [13–16]. However, artificial UV light and TiO2 particles tend to be somewhat expensive, and the UV component in sunlight reaching the earth surface and available to excite TiO2 is relatively small (ca. 3% to 5%), whereas solar spectrum has about 40% visible region.
Organic pollutants like dyestuffs have the ability to absorb visible light. The electron transfer process from dye to semiconductor, especially ZnO has been found to be very effective. Initially, the dye molecules adsorb on ZnO surface and excited through absorption light. The excited dye (D*) injects an electron to the conduction band of ZnO where it is scavenged by O2 to form active oxygen radicals. The consequence of electron ejection into the conduction band results hole–electron pair formation. These hole–electron pairs are responsible of generating other reactive oxygen species like .OH, HO2, H2O2, and also . These active radicals are responsible for photodegradation or mineralization of organic compounds. The mechanism can be attributed as follows:
Recently, we reported [13] a comparative study of decolorization of Ponceau S (PS), an anionic dye, in pristine ZnO and prepared ZnO suspensions. The ZnO was prepared through the hydrothermal method. We found that the prepared ZnO (300°C) showed better efficiency to decolorize/degrade Ponceau S. In this report, CV, a cationic dye, has been taken as an example of wastewater pollutants in different industries. Photocatalytic degradation of CV has been reported recently in the presence of TiO2[1, 8–11] under UV irradiation. So far, no attempt has been taken to use ZnO instead of TiO2 as a photocatalyst to decolorize/degrade CV. However, ZnO takes part in photocorrosion under UV radiation at lower solution pH (approximately 6.0). Therefore, the present study focuses on decolorization/degradation of CV in aqueous suspension of ZnO under visible light irradiation with a variety of conditions. In addition, an adsorption study of CV on ZnO is also discussed.
Results and discussion
Adsorption of CV from solution on ZnO
Adsorption studies of CV solution (2.50 × 10−5 M) with ZnO (0.20 g/40.0 mL) in dark are shown in Figure 1. The results show that the adsorption of CV on ZnO takes about 200 min to reach equilibrium. The equilibrium time at a certain concentration seems to be independent of temperatures that are used for the preparation of ZnO samples. The adsorption data have also been fitted to pseudo-first-order (Lagergren) and the pseudo-second-order kinetic equations of the adsorption processes (data not shown). The values of correlation coefficients (r2) for the pseudo-first-order kinetic model are between 0.994 to 0.998 and those for the pseudo-second-order kinetic model between 0.963 to 0.989. These results seem to suggest that adsorption of CV from solution on ZnO favors the pseudo-first-order adsorption model. Results further suggest that the commercial ZnO has been made at a temperature higher than 300°C.
The shapes of adsorption isotherms (30°C) indicate that adsorption of CV from solution on ZnO will reach plateau at its certain concentration as shown in Figure 2. In the range of CV concentration up to 2.50 × 10−5 M, Ce/(x/m)e vs Ce plots are linear (data not shown) which suggests the Langmuir model, and the corresponding correlation coefficient are satisfactory (r2 = 0.992 to 0.998). However, plots of ln(x/m)e vs lnCe (data not shown) suggest the Freundlich model and show straight lines, but r2 values vary between 0.967 and 0.984, r2 = 0.967 for ZnO prepared at 300°C. Therefore, it is suggested that the adsorption pattern of CV on ZnO favors the Langmuir model that also was observed for Ponceau S, an anionic dye, on ZnO [13].
The kinetic data (k) and Langmuir parameters (Qm and KL) (Table 1) are likely to suggest that increasing the heating temperatures during the preparation of ZnO samples to higher values causes agglomeration of ZnO particles, decreasing thereby the active adsorption sites. Accordingly, adsorption of CV on ZnO falls as the temperature of heating is raised during the preparation of ZnO samples. The amount of CV adsorbed (25% ([CV] = 2.50 × 10−5 M, Temp. = 30°C, pH = 6, ZnO = 0.20 g/ 40.0 mL), Qm = 0.31 mg/g) at a particular time and concentration is the highest in the case of ZnO prepared at 300°C.
Adsorption of a dye on a semiconducting metal oxide surface, as on the surface of ZnO, is a prerequisite for the successful photodecolorization of the dye. Consequently, the adsorption studies were necessary.
The better adsorption characteristics of the CV from solution on ZnO made at 300°C, were the criteria for selecting this ZnO sample for studying the visible light-influenced degradation. The higher value of KL-H compared to KL (Table 1) suggests that there is some surface activation by the visible light used in this study. This activation should be considerably low to promote valence band electrons to the conduction band as the band gap energy for ZnO is 3.2 eV (λmax ≈ 388 nm).
ZnO-mediated photodecolorization CV
The initial pH, in the range 3.75 to 6.02, of CV solutions did not affect on its UV–vis spectra. The spectrum of the CV solution before illumination with visible light (Figure 3) has characteristic prominent peaks at 590, 302, and 250 nm corresponding to conjugated triphenyl methane chromophore, diphenyl methyl type, φ2C = (φ, -C6H5), and benzene moieties, respectively. The decrease in absorbance at 590 nm with illumination is due to the breakdown of the chromophore responsible for the characteristic color of CV. This breakdown, caused by the visible light illumination of CV in the aqueous suspension of ZnO particles, leads to the formation of intermediates like benzophenone and benzene derivatives [17]. Some of the benzene derivatives are benzoic acid and aniline derivatives. All of these compounds are finally mineralized to CO2, H2O, and . The hypsochromic shift of the peak at 590 nm of the chromophore to about 575 nm is of particular significance as it indicates N-dimethylation process [18] that ultimately generates ions [19, 20].
Figure 3 shows that the characteristic peaks gradually disappear with the time of illumination. When the illumination was continued for about 360 min ([CV] = 2.00 × 10−5 M), none of the peaks could be detected. The residual spectrum with a peak near about 200 nm disappears when very dilute solution of NaNO3 is used as reference in the place of water. This suggested the presence of ions, as one of the products formed during visible light degradation of CV in the ZnO suspension.
Figure 4 shows how decolorization and disappearance of total organic carbon (TOC) progress with time of illumination of CV in ZnO suspension. The decrease of TOC with time of illumination is the actual mineralization of CV, that is, the conversion of CV molecules to CO2, H2O, N2, and ions. TOC measures CO2 formed from the carbon atoms of CV molecules. Accordingly, TOC measures complete degradation of CV molecules. With ZnO = 0.20 g/40 mL and [CV] = 2.00 × 10−5 M, it needs about 360 min for complete mineralization of CV molecules in the suspension. On the other hand, for complete decolorization, it takes about 200 min; therefore, it is concluded that decolorization is faster than degradation.
Influence of ZnO on decolorization of CV
The initial rate of decolorization of CV also increases with the increasing of the concentration of ZnO in the suspension (data not shown). However, this increase seems to be linear up to about 0.15 g ZnO/40 mL. Subsequently, deceleration of the initial rate of decolorization of CV starts. In our previous studies, it has also been reported that the initial rate of decolorization of an anionic dye, Ponceau S, increases with increasing of ZnO in suspension [13].
Figure 5 shows that after 5 min, about 28% decolorization takes place with 0.20 g ZnO. This decolorization is about 35% after 5 min when 0.40 g ZnO is used. Moreover, with 0.20 g ZnO, decolorization increases to about 43% after 10 min, 51% after 15 min, and about 62% after illumination for 30 min. Respectively, under identical times of illumination, with 0.40 g ZnO, about 46%, 55%, and 64%, decolorization takes place. These results suggest that increasing the time of illumination is much useful than increasing the concentration of ZnO in the suspension. The use of higher concentration, along with longer duration of illumination, does not appear to be that profitable (Table 2). For an example, after 60 min of illumination, there is about 5% enhancement of decolorization when 0.40 g ZnO/40 mL is used instead of 0.20 g ZnO/40 mL (Table 2). The results in Table 2 further show that like PS [13], the increase in turbidity of the suspension, due to increased concentration of ZnO, does not affect the decolorization that much. The reason for this may be ascribed to the continuous stirring of the suspensions in the open air. This replaces ZnO particles with adsorbed dyes at the surface, exposed to the visible light and having the dye molecules decolorized, by fresh particles with adsorbed dyes from the bulk. The speed of agitation may have been sufficiently high to help overcome the retarding effect of the higher ZnO concentration on the movement of the bulk particles to the surface.
Thus, the excess surface sites, provided by the increase in concentration of ZnO, for adsorption of fixed and a small number of CV molecules should have been useful [21]. There had been no loss of these surface sites due to aggregation when ZnO concentration increases. This loss of surface sites could be one of the dominant factors [20] that are responsible for the deceleration of the initial rate of CV decolorization with the increase in ZnO concentration in the suspension.
Various parameters related to CV decolorization (Table 2) are somewhat lower in comparison to those of PS, an anionic dye [13]. One of the inherent causes is likely to be that CV has adsorption parameters lower than those of PS.
Influence of concentration of CV
The trend in the decolorization of CV solution with its higher concentrations (Figure 6) seems to be similar to that with increasing amounts of ZnO (Figure 5), and the situation arises because of a small number of adsorption sites on ZnO in comparison to the increasingly higher number of CV molecules. The initial rate of decolorization of CV in solution by visible light in the presence of ZnO seems to remain linear up to the concentration of CV much less than 1.0 × 10−5 M (Figure 7). As like other dyes, inner filter effect [13, 22, 23] is also quite prominent in the case of CV as its concentration in solution increases. The data of variation of initial CV concentrations, [CV]i, and initial rates, Ri, of disappearance of CV color from ZnO suspension, were applied to the Langmuir-Hinshelwood equation in the following form:
kLH is a proportionality constant and provides a measure of intrinsic reactivity of CV adsorbed on ZnO. On the whole, kLH offers an idea of the apparent rate constant of the reaction involving adsorbed CV with oxidizing radicals formed at the ZnO surface. It is the zero-order surface reaction constant [24]. KLH is the apparent adsorption equilibrium constant (so called Langmuir-Hinshelwood constant). Ri-1 vs [CV]i-1 is a nicely fitted straight line (Figure 8). KLH and kLH values are 7.70 × 103 L/mol and 2.37 × 10−6 mol/min, respectively (Table 1). The higher KLH value, compared to that of KL (4.16 × 103 L/mol; Table 1), suggests some surface activation of ZnO surface by visible light.
Conclusions
Adsorption of CV on ZnO aqueous suspension follows the pseudo-first-order kinetic model (Lagergren) while the adsorption pattern follows the Langmuir model; it is a prerequisite condition for semiconductor metal oxide-mediated photodegradation. Decolorization results show that ZnO can efficiently decolorize/degrade CV in the aqueous suspension under visible light irradiation. ZnO prepared at 300°C shows superior photocatalytic activity compared to the ZnO samples treated at 500°C and 700°C and also to the pristine ZnO. The prepared ZnO has been found to be quite stable under visible light illumination at a pH of approximately 6, and there is some activation of the surfaces of ZnO particles by this light. In addition, remediation of industrial effluents containing some azo dyes by the prepared ZnO under visible light is underway.
Methods
Materials
The dye CV, also known as gentian violet 10B, was obtained from local dye suppliers. The dye shows an absorption maximum at 590 nm. The structure of the dye is shown in Figure 9. Pristine zinc oxide (>99.9% purity, MZ-500 with a surface area of 38.4 m2/g) was from Tayca Corporation, Japan. Zinc sulfate, ammonium carbonate, sodium hydroxide, and hydrochloric acid were purchased from BDH (Dubai, United Arab Emirates). All the chemicals were used without further purification. The pH of the solutions was adjusted using dilute solutions of HCl or NaOH. Deionized water was used throughout the experiment.
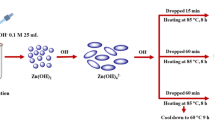
ZnO was prepared through hydrothermal method. The prepared ZnO was characterized using a scanning electron microscope (SEM; model S-3400N, Hitachi, Tokyo, Japan), X-ray diffraction (XRD; RINT 2200, Rigaku Corporation, Tokyo, Japan) and laser-induced breakdown spectroscopy (model LAB-170, pulsed Nd:YAG, Spectra-Physics Inc., Santa Clara, CA, USA) measurement. Preparation procedure has been described in detail in our previous study [13]. Briefly, ZnCO3 was collected as a precipitate upon the addition of ammonium carbonate solution into zinc sulfate solution at 60°C. The precipitate was filtered and washed with water to remove sulfate ions and dry it at 105°C in an oven. The dry ZnCO3 was further heated in a muffle furnace (OSK, Isuzu Seisa kusu Co., Ltd., Japan) at different temperatures to get desired thermally treated ZnO samples.
Adsorption studies
ZnO portions of 0.20 g were soaked overnight in water in stoppered bottles. The volume was made up to 40 mL by adding water and CV solution so that the desired concentration and an initial pH, approximately pH 6, of the dye solution were maintained. The bottles with their contents were shaken in a thermostat shaker (NTS-4000AL, Tokyo Rika Kikai Co., Ltd, Tokyo, Japan) at 30°C. Samples were withdrawn at definite intervals of time and centrifuged, and their dye concentrations were determined by measuring absorbance of the clear solution at 590 nm (UV-visible spectrophotometer, UV-160A, Shimadzu Corporation, Kyoto, Japan).
Procedure of decolorization
An experimental setup for photodecolorization of CV has been reported in our previous study [13, 14]. Briefly, the visible light illumination source comprised two 40-W fluorescent lamps, each with a length of 20 cm, placed side by side. The lamp house was a wooden box with the internal surface covered with aluminum foil. The temperature inside the box was maintained at 30°C by regulated flow of cool air through it. The air entered the box at the bottom and flowed out at the top. The approximate intensity of the visible light at the position of the reactor was approximately 1.8 × 10−4 W/cm2 as measured using a spectroradiometer (model IL-588, International Light Technologies, Peabody, MA, USA).
Fifty-milliliter beakers, with an internal diameter of 4.0 cm, were used as reactors. Each reactor was placed on the magnetic stirrer, and the distance of the solution surface from the lower part of the lamps was kept fixed.
ZnO portions of 0.20 g were taken in each of the several reactors containing 10 mL of water each. They were left overnight, and CV solutions of desired concentrations were added to the reactors in each to reach the total volume of 40.0 mL. The contents of the reactors were agitated ultrasonically for 5 min prior to subjecting them to visible light illumination for definite times. The weight of each reactor was recorded. During the illumination, the suspensions were continuously stirred magnetically while the air passed through the lamp house. After illumination, the weights of the reactors were checked, and the weights were made up by the addition of water. Clear solutions, obtained after centrifugation, were analyzed spectrophotometrically at 590 nm to find out the concentration of the remaining CV in each reactor. The clear solutions (0 dye concentrations) were analyzed for free Zn2+ ions by AAS (Shimadzu, Kyoto, Japan) with reference to the standard ZnSO4 solution.
Percent decolorization and mineralization
Decolorization or color removal of CV was calculated as (Xt/Xo) × 100, where Xt is the absorbance at time ‘t’ and Xo is that at t = 0. On the other hand, the percent color remaining was [(Xo − Xt ) / Xo] × 100.
In the case of mineralization/degradation, Xo is the TOC (ppm) of CV at t = 0, and Xt (ppm) is its TOC at time ‘t’. The total organic carbon analyzer (CPH/CPN and 638 - 4241C, Shimadzu) was used for this purpose.
The initial rate of decolorization
The equation Ri = k[CV]i was used to determine the initial rate (Ri) by multiplying the apparent first-order rate constant k with the initial concentration of CV, [CV]i. k was determined from the slope of the plot ln([CV]i/[CV]t) vs time.
References
Senthilkumaar S, Porkodi K: Heterogeneous photocatalytic decomposition of Crystal Violet in UV-illuminated sol–gel derived nanocrystalline TiO 2 suspensions. J. Colloid Interface Sci. 2005, 288: 184–189. 10.1016/j.jcis.2005.02.066
Habibi MH, Hassanzadeh A, Mahdevi S: The effect of operational parameters on the photocatalytic degradation of three textile azo dyes in aqueous TiO 2 suspensions. J. Photochem. Photobiol. A. 2005, 172: 89–96. 10.1016/j.jphotochem.2004.11.009
Littlefield NA, Blackwell BN, Hewitt CC, Gaylor DW: Chronic toxicity and carcinogenicity studies of gentian violet in mice. Fundam. Appl. Toxicol. 1985, 5: 902–912. 10.1016/0272-0590(85)90172-1
Sahoo C, Gupta A, Pal A: Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO 2 . Dyes. Pigm. 2005, 66: 189–196. 10.1016/j.dyepig.2004.09.003
Fox MA, Dulay MT: Heterogeneous photocatalysis. Chem. Rev. 2000, 93: 341–357.
Fujishima A, Rao TN, Tryk DA: Titanium dioxide photocatalysis. J. Photochem. Photobiol. C. Photochem. Rev. 2000, 1: 1–21. 10.1016/S1389-5567(00)00002-2
Gaya UI, Abdullah AH: Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C. Photochem. Rev. 2008, 9: 1–12. 10.1016/j.jphotochemrev.2007.12.003
Chen CC, Fan HJ, Jang CY, Jan JL, Lin HD, Lu CS: Photooxidative N-de-methylation of crystal violet dye in aqueous nano-TiO 2 dispersions under visible light irradiation. J. Photochem. Photobiol. A. Chem. 2006, 184: 147–154. 10.1016/j.jphotochem.2006.04.008
Chen CC, Mai FD, Chen KT, Wu CW, Lu CS: Photocatalyzed N-de-methylation and degradation of crystal violet in titania dispersions under UV irradiation. Dyes. Pigm. 2007, 75: 434–442. 10.1016/j.dyepig.2006.06.040
Ju Y, Fang J, Liu X, Xu Z, Ren X, Sun C, Yang S, Ren Q, Ding Y, Yu K, Wang L, Wei Z: Photodegradation of crystal violet in TiO 2 suspensions using UV–vis irradiation from two microwave-powered electrodeless discharge lamps (EDL-2): Products, mechanism and feasibility. J. Hazard. Mater. 2011, 185: 489–1498. 10.1016/j.jhazmat.2010.09.058
Samira S, Raja A, Mohan C, Modak JM: Photocatalytic Degradation of Crystal Violet (C.I. Basic Violet 3) on NanoTiO 2 Containing Anatase and Rutile Phases (3:1). J. Thermodynam. Cat. 2012, 3: 5.
The CM, Mohamed AR: Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: a review. J. Alloys. Compd. 2011, 509: 1648–1660. 10.1016/j.jallcom.2010.10.181
Moslem M, Habib MA, Mahmood AJ, Islam TSA, Ismail IMI: Zinc oxide-mediated photocatalytic decolourization of Ponceau S in aqueous suspension by visible light. Inter. Nano. Lett. 2012, 2: 30. 10.1186/2228-5326-2-30
Habib MA, Shahadat MT, Bahadur NM, Ismail IMI, Mahmood AJ: Synthesis and characterization of ZnO-TiO2 nanocomposites and their application as photocatalysts. Inter. Nano. Lett. 2013, 3: 5. 10.1186/2228-5326-3-5
Elamin N, Elsanousi A: Synthesis of ZnO Nanostructures and their photocatalytic activity. J. Appl. Indust. Sci. 2013, 1: 32–35.
Tian C, Zhang Q, Wu A, Jiang M, Liang Z, Jiang B, Fu H: Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48: 2858–2860. 10.1039/c2cc16434e
Saquib M, Muneer M: TiO 2 -mediated photocatalytic degradation of a triphenylmethane dye (genetian violet), in aqueous suspensions. Dyes. Pigm. 2003, 56: 37–49. 10.1016/S0143-7208(02)00101-8
Li X, Liu G, Zhao J: Two competitive primary processes in the photodegradation of cationic triarylmethane dyes under visible irradiation in TiO 2 dispersion. New J. Chem. 1999, 23: 1193–1196. 10.1039/a906765e
Lachheb H, Puzenat E, Houas A, Ksibi M, Elaloui E, Guillard C, Herrmann J-M: Photocatalytic degradation of various types of dyes (Alizarins, Crocein Orange G, Methyl red, Congo red, Methylene blue) in water by UV- irradiated titania. Appl. Catal. B. Environ. 2002, 39: 75–90. 10.1016/S0926-3373(02)00078-4
Hu C, Yu JC, Hao Z, Wong PK: Photocatalytic degradation of triazine-containing azo dyes in aqueous TiO 2 suspensions. Appl. Catal. B. Environ. 2003, 42: 47–55. 10.1016/S0926-3373(02)00214-X
Kang SF, Liao CH, Po ST: Decolorization of textile wastewater by photo-Fenton oxidation Technology. Chemosphere 2000, 41: 1287–1294. 10.1016/S0045-6535(99)00524-X
Chen D, Roy AK: Photodegradation kinetics of 4- nitrophenols in TiO 2 suspension. Water Res. 1998, 32: 3223–3234. 10.1016/S0043-1354(98)00118-3
Neppollian B, Sankar MV, Murugesan V: Semiconductor assisted photodegradation of textile dye. J. Sci. Ind. Res. 2002, 6: 224–230.
Lagrasta C, Bellobono IR, Bonardi M: Photobleaching and photomineralization of azobenzene and substituted azobenzenes in aqueous solution by photocatalytic membranes immobilizing titanium dioxide. J. Photochem. Photobiol. A. Chem. 1997, 110: 201–205. 10.1016/S1010-6030(97)00179-2
Acknowledgment
Muhammad Muslim gratefully acknowledges the grant of study leave and deputation by the Ministry of Education, and his UGC Ph.D. Fellowship by Bangladesh University Grants Commission (UGC).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MM is a PhD student and his work is the basis of the paper. AJM and TSAI are the supervisor and co-supervisor, respectively. MAH, MTS, MNI and IMII have helped with the instrumental analysis and final preparation of the paper. All the authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Habib, M.A., Muslim, M., Shahadat, M.T. et al. Photocatalytic decolorization of crystal violet in aqueous nano-ZnO suspension under visible light irradiation. J Nanostruct Chem 3, 70 (2013). https://doi.org/10.1186/2193-8865-3-70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-70