Abstract
The occurrence of heavy cyanobacterial blooms has become a worldwide problem, as a consequence of eutrophication of the aquatic ecosystems; furthermore, 60% to 75% of these blooms have been found to be toxic. Microcystins (MCYSTs), the predominant toxins of cyanobacterial blooms, are associated with mortality and illness in both animals and humans. Laboratory-controlled experiments studying the effects of different microcystins on the common carp (Cyprinus carpio) have revealed various histopathological alterations. The aim of the present study is to investigate the effect of chronic or subchronic exposure of fish to microcystins under natural environmental conditions by examining the possible histopathological changes associated with a dense cyanobacterial bloom and determining the microcystin contents of fish tissues. Common carps (C. carpio) were caught from Lake Karla (Greece), during a dense cyanobacterial bloom. The concentration of MCYSTs in the fish liver, kidney and muscle tissues was measured by enzyme-linked immunosorbent assay. The pseudogaster contents were analysed, and a histopathological examination was performed using light and electron microscopy. Severe alterations were detected in the liver and the kidney, suggesting that the toxic effects were caused by various pollutants that were particularly associated with microcystins. The histopathological findings are also discussed, taking into consideration the health conditions of the common carp as a commercial fish species. The mechanisms of expansion of the microcystins and the poisoning of aquatic organisms (e.g. fish) are not yet known in the Lake Karla ecosystem. Future research may focus on identifying the changes caused by microcystins and other factors that exert similar effects on fish tissues, as well as on establishing the overall combined effect of all these factors on fish health.
Similar content being viewed by others
Background
The frequent occurrence of cyanobacterial blooms has been regarded as a serious global public health problem and a major environmental issue. Cyanobacteria can produce several toxic metabolites known as cyanotoxins (Ressom et al. 1994; Blaha et al. 2009). Many species of cyanobacteria produce toxic metabolites, peptides and alkaloids that are a serious threat to the various uses of freshwater lakes and reservoirs. Toxin-producing cyanobacteria that are widely distributed in freshwaters include the planktonic N2-fixing genera of Anabaena, Aphanizomenon, Nostoc, Cylindrospermopsis and the non N2-fixing genera, such as Microcystis, Planktothrix and Oscillatoria (Welker et al. 2004). Among these cyanobacteria, microcystins (MCYSTs) are the most abundant group. Exposure to MCYSTs imposes a health risk on aquatic organisms, wild life, domestic animals and humans through ingesting the cyanobacteria present in the water or the food (Duy et al. 2000; Malbrouck and Kestemont 2006).
Fish, a group of organisms that represents one of the main inhabitants of the aquatic systems, are frequently exposed to MCYSTS both directly and passively, leading to their poisoning and/or their subsequent mortality (Carbis et al. 1996; Ernst 2008).
Fish typically either ingest cyanobacteria or prey that has already fed on cyanobacteria (Tencalla et al. 1994; Fisher and Dietrich 2000). To a lesser extent, they can also absorb the toxins directly from the water (Phillips et al. 1985; Smith et al. 2008). Currently, there is a large body of literature describing experimental studies of cyanobacterial toxins in aquatic systems and their acute toxicity effects; however, there are gaps about the toxicological effects of these toxins under field conditions in environmentally relevant concentrations. Although acute toxicity experiments are useful in the study of toxicokinetics and histopathological changes, such experiments do not represent the mode of exposure in a natural environment (Ferrão-Filho and Kozlowsky-Suzuki 2011). Because the chronic and subchronic effects in aquatic organisms might be more relevant than the acute lethal effects, studies addressing the exposure of biota in cyanobacteria-rich lakes have attracted substantial attention (Ibelings and Chorus 2007; Ibelings and Havens 2008).
It is well known that one cyano-toxigenic mechanism is the potent inhibition of the protein phosphatases 1 and 2A; this inhibition results in the abrogation of the protein phosphorylation status, which is directly associated with the cytotoxic effects and tumour-promoting activity of cyanotoxins (Carmichael 1994; Hooser 2000). In addition, studies of fish pathology have shown that MCYST exposure can produce histopathological effects in the gills, intestine and heart (Atencio et al. 2008; Jiang et al. 2011). Hepatic tumours and severe hepatic haemorrhages, as well as the disruption of the hepatic cytoskeleton and the subsequent, progressive liver necrosis and apoptosis, have been widely reported in fish (Tencalla et al. 1994; Fischer et al. 2000). The degree of these MCYST-induced effects depends on the exposure route. However, most of the studies on MCYSTs have been performed using an IP injection or an oral uptake as the exposure route.
Cyprinus carpio (common carp) is a widespread species found in various freshwater habitats, including almost all freshwater Greek lakes. The cyano-toxicological effects on the common carp are thought to be quite important given its commercial importance as a product of inland fisheries and aquaculture. Planktivorous fish, such as the carp, intentionally ingest cyanobacteria and, in the process, are also exposed to cyanobacterial metabolites through the aquatic food web (Malbrouck and Kestemont 2006).
Although these species are frequently exposed to cyanotoxins in their natural environment, they are also considered to be among the most tolerant in terms of survival. No evidence exists regarding the sublethal effects of cyanotoxins, including pathological alterations. Studies on endemic fish species in Europe, Australia and USA have shown that fish liver histopathology is a useful biomarker of exposure to various contaminants (Au 2004; Feist et al. 2004).
The primary aim of each of these studies was to identify histological changes in selected target organs of bio-indicator fish species with regard to one or more specific contaminants. Regarding cyanotoxins, in most cases, the liver and renal histological alterations were highly important (Fisher and Dietrich 2000; Molina et al. 2005; Lang et al. 2006Li et al. 2007a; Gutierrez-Praena et al. 2011).
In this context, the purpose of the present study is to assess the occurrence and distribution of MCYST in the tissues of the fish species C. carpio, as well as to evaluate the histopathological alterations under cyanobacteria-rich conditions. The histopathological findings were revealed by light and electron microscopy. To our knowledge, this is the first study evaluating the histopathology of a freshwater biomarker under natural conditions to provide information about the public health issue arising from human consumption.
Lake Karla (Central Greece) is a unique example (in Europe) of a shallow lake ecosystem that was dried in the 1960s and has been reconstructed, establishing a ‘new’ ecosystem. During the past 2 years of the lake's rehabilitation (2009 to 2011), frequent and extensive cyanobacterial blooms have occurred that were dominated by toxin-producing species (Oikonomou et al. 2012). During the same period, many episodes of fish mortality were also reported. These events have received intense media coverage due to the lake's importance as a major water reservoir in Greece. Fishing is also among the traditional economic activities in the area, and the common carp is considered to be the main commercial fish species.
Methods
Lake Karla: limnological features of the study area
Lake Karla occupied the lowest part of the Thessaly plain and was considered to be one of the most important wetlands in Greece until 1962. Surface runoff from the watershed and the floodwaters of the Pinios River (discharging via a constructed ditch) supplied the lake with large quantities of freshwater. In 1962, the lake was completely drained to create more land for agriculture. The reconstructed Lake Karla is located on the lower depression of the Thessaly plain, a region of Central Greece (Figure 1). The lake lies between latitude 39°26′49″ and 39°32′03″ N and longitude 22°46′47″ and 23°51′50″ E and has a surface area of 38 km2 with a perimeter of 228 km. The hydrological regime of the lake is determined by the inputs (the rainfall on the lake and the tributary inflows) and the outputs (evaporation). The lake has no natural outflow because the constructed tunnel draining into the Pagasitikos bay is currently closed. Considering the present environmental conditions, Papadimitriou et al. (2011) reported on the water chemistry, and an in-depth analysis of the water column and sediment interactions in Lake Karla has also been published by Jouni (2011). Both studies reported on the highly eutrophic conditions of the lake and highlighted its nutrient-rich content and high chlorophyll-a values. Moreover, there was also an evidence of phosphorous mobilisation from the sediment to the water column. In the same period, Oikonomou et al. (2012) confirmed the occurrence and dominance of toxic cyanobacteria species of Anabaenopsis and Planktothrix, as well as a diverse microbial community indicative of a hypertrophic status.
Map of the re-constructed Lake Karla located in the Thessaly region of Central Greece (Oikonomou et al. 2012 ).
Fish collection and MCYST detection
Thirty specimens of C. carpio with a mean body weight and a mean body length of 1,032 ± 121 g and 30 ± 4.2 cm, respectively, were collected from Lake Karla via two surveys during a period of dense cyanobacterial bloom (May and June 2011) using a trammel net that has a 60-mm inner and 300-mm outer mesh size. Ten C. carpio fish were also obtained from a fish farm (Hatchery station of river Louros-Ioannina, Hellenic Ministry of Agriculture) and were used as controls. To extract the toxins from the fish organs, the fish were sacrificed, and the liver, kidney and muscle tissues were excised, weighed and immediately frozen. Subsequently, all of the tissues were homogenised separately, extracted in 100% methanol (Magalhães et al. 2001), stirred overnight at room temperature and then centrifuged at 1,300 g for 15 min using a Universal 32 centrifuge (DJB Labcare Ltd., Buckinghamshire, England, UK). The supernatants were collected and stored overnight at 4°C. A 5-ml aliquot of each supernatant was concentrated under nitrogen stream to 350 μl to remove the organic solvent. A 100-μl aliquot of the concentrated sample extract was diluted with 900 μl of distilled water according to Sipia et al. (2002). The final sample was clarified using membrane filters (pore size of 0.45 μm and diameter of 4 mm). The sample solutions were immediately analysed by enzyme-linked immunosorbent assay (ELISA). For each ELISA assay, the negative control and the four standards were assayed at least in duplicate. The results are expressed in nanogrammes of MCYST-LR equivalents per gramme of fish tissue.
MCYSTs were also measured in the water of Lake Karla. Two forms of MCYSTs were investigated: dissolved in water (extracellular) and cell-bound in seston (intracellular). For the latter, 1,000 mL of water was filtered on a Whatman GF/C filter (Sigma Aldrich, St. Louis, MO, USA) which was immediately frozen at −20°C. MCYSTs were extracted from the filter after placement in 100% methanol and stirring overnight at room temperature followed by centrifugation at 1,300×g for 15 min. This extraction procedure was repeated three times, and the three supernatants were pooled. The organic solvent was removed by placing the extract under nitrogen stream. The remaining concentrated sample was subjected to ELISA. The results are expressed as microgrammes of cellular MCYST-LR equivalents per litre. For analysis of dissolved MCYSTs, the filtered water was applied directly to ELISA. A commercial Abraxis Microcystin ELISA kit was used (Warminster, PA, USA) following the instructions of the manufacturer.
The recovery of the method was determined by analysing the samples before and after the addition of the pure MCYST and then subtracting the concentration of the MCYSTs present in the sample prior to spiking. The matrix effect (i.e. the effect of animal tissue) was checked by spiking the control tissues with the MCYST-LR standard (2 μg/g). The response was compared to that of the 100% methanol spiked with the same amount of the standard.
Microscopic analyses
Histological samples from the excised tissues, including the liver and the kidney, were subjected to light and electron microscopy. For light microscopy, the samples were initially fixed in a 10% formalin buffer for 24 h at 4°C and were then immediately dehydrated in a graded series of ethanol, immersed in xylene and embedded in paraffin wax using an automatic processor. Sections of 5 to 7 μm were then mounted. After deparaffinisation, the sections were rehydrated, stained with hematoxylin and eosin (Humason 1972) and mounted with Cristal/Mount (Sigma Aldrich, St. Louis, MO, USA). Subsequently, all of the tissues were examined microscopically, and their histological abnormalities were recorded.
For electron microscopy, tissue specimens were prefixed in 2.5% glutaraldehyde diluted in a 0.1-M sodium cacodylate solution for 24 h at 4°C. The specimens were washed in the same buffer before and after fixation. Post-fixation was performed with 1% osmium tetroxide in 0.1-M sodium cacodylate for 2 h at 4°C. The specimens were washed in the same buffer before and after post-fixation and then rinsed in distilled water. Then, the specimens were dehydrated in a graded ethanol series and were subsequently immersed and left overnight in a 1:1 mixture of propylene oxide and the embedding resin. The final step of the embedding took place in capsules containing agar resin. Polymerisation of the resin was completed after 48 h at 60°C. Ultrathin sections (60 to 80 nm) were cut with a Reichert Supernova ultramicrotome (DeKalb, IL, USA). The sections were mounted on a copper grid and stained with uranyl acetate and lead citrate. The tissue sections were examined in a Philips CM10 electron microscope (Amsterdam, The Netherlands).
The common carps are stomachless fish. The short oesophagus connects the posterior pharynx to the anterior part of the intestine, the pseudogaster. Microscopic analysis of the pseudogaster contents was also performed on the examined fish specimens. The pseudogaster was removed from fresh carps, and samples of its contents were collected and kept at 1°C to 5°C until further analysis. Two aliquots of each sample were taken, and one of the aliquots was diluted in distilled water. Dilution was performed in a glass test tube by mixing 330 μl of the sample with 330 μl of distilled water. The non-diluted pseudogaster contents (330 μl) were placed on one end of the slide. The cover slip was placed at a 45° angle on top of the sample to avoid creating air bubbles. Similarly, 330 μl of the diluted pseudogaster contents was placed on the other end of the slide, and optical microscopy images were obtained from both sides of the slides.
Statistics
The data are expressed as the means ± SD. A two-way analysis of variance (ANOVA) was used to compare the means of the MCYSTs contents between the tissues and the sampling periods. The results were considered statistically significant when P < 0.05. When the ANOVA results indicated a significant effect, Tukey's multiple comparison test was used to compare the differences between the means. All statistical analyses were performed with SPSS vers. 20.0 for Windows (Chicago, IL, USA).
Results
MCYST in the fish tissues and lake water
All fish samples of C. carpio examined during the present study contained MCYSTs (Table 1). According to the statistical analysis, there were significant differences between the tissues (n = 20, F = 12.53, P < 0.05). However, there were no significant differences in MCYST concentration between the sampling periods (n = 20, F = 0.05, P > 0.05). The highest MCYST concentration was found in the liver (732 ± 350 ng/g), followed by the kidney (362 ± 207 ng/g), while the lowest concentrations were detected in the muscle tissues (114 ± 25 ng/g) (Table 1). During the second survey, in June, the kidney had a higher concentration of MCYST than the liver tissues (696 ± 258 and 346 ± 156 ng/g, respectively); again, the lowest MCYST values were found in the muscle tissues (108 ± 33 ng/l) (Table 1). The examined tissues of the control C. carpio individuals contained no MCYSTs when examined by ELISA.
The extracellular MCYST values were 2.03 ± 0.32 μg/l and 3.01 ± 0.41 μg/l for May and June, respectively. The respective intracellular MCYST values were 4.19 ± 0.33 μg/l and 5.5 ± 0.29 μg/l (Table 1) for the same time periods as above.
The recovery of MCYST that we obtained from the spiked samples was 77 ± 2.5% for liver, 73 ± 3.2% for kidney and 72.6 ± 2.12% for muscle. The matrix effect was negligible (from 0.08% to 5.2% of the differences between the matrix and the methanol results, with an average of 1.9 ± 0.7%).
Mortality and macroscopic observations
No fish mortalities were observed during the studied period, and the appearance of the examined organisms was macroscopically normal. All of the examined samples (100%) of the fish pseudogaster contents contained various cyanobacterial species, with the dominant species being Microcystis aeruginosa and Planktothrix agarrdii (Figure 2). While examining the test organs, macroscopic lesions were observed in the liver and the kidney. Morphological changes included liver discoloration and brittleness, as well as ecchymoses and petechiae (Figure 3). The kidney appeared brittle and normal in colour. Neither the organ displayed appeared enlarged. No pathological changes were observed in the control samples.
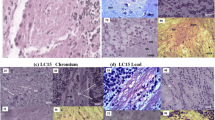
Cyprinus caprio liver. (A) Hydropic degeneration, granular glycogen (circle) and sporadic haemorrhagic symptoms (arrows). (B) Onion-like cells (circle) and sporadic haemorrhagic symptoms (arrows). (C) Capillary fracture (arrow). (D) Control, there are no histopathological degenerations. Bar=62.5 μm. (E) Macroscopic degenerations of the liver. Discoloration (arrow) and petechiaes (star) were observed.
Histopathology under light microscopy
Histopathological changes were observed in the liver and the kidney of the fish specimens in comparison with the control ones. However, no differences were observed among the sampling surveys. Histopathological lesions were detected in the liver and the kidney and were characterised by hydropic degeneration and necrosis. Pathological changes in the fish liver included loss of the architecture structure, onion-like cells, focal necrosis, granular glycogen, nuclei pyknosis (Figure 3) and sporadic haemorrhagic symptoms. The kidney pathology was characterised by the degeneration of the renal tubule, glomerulopathy, glomerular atrophy and a dilated Bowman's capsule (Figure 4). No pathological changes were observed in the muscles.
Ultrastructural histopathological observations
Histopathological alterations were observed in the liver and the kidney of all fish samples except for the control ones. Both organs appeared swollen, with a loss of the parenchymal architecture. An apparent lysis of the membranes of the hepatic cells resulting in necrotic cells was observed, along with vacuolisation and the presence of lipid droplets. The fragmentation of the nuclear envelope, the swelling of mitochondria, the fragmentation and vesicularisation of the endomembrane system, the pyknosis of nuclei and karyorrhexis were also observed in the liver (Figure 5). Nuclei pyknosis, nuclear envelope fragmentation and proliferation of the lysosomes were observed in the kidney (Figure 6).
Ultrastructural observation of Cyprinus carpio 's liver tissue. (A) Nuclear envelop fragmentation (stars) and a swelling mitochondrion (arrow). Bar = 0.37 μm. (B) Fragmentation of endoplasmic reticulum (arrows) and mitochondria internal membrane system (star). Bar = 0.60 μm. (C) Lysis of the hepatic cell's plasma membrane. Mitochondria (arrows) in the extracellular space. Bar = 1.74 μm. (D) Karyorrhexis. Bar = 1.74 μm.
Discussion
The only study before Lake Karla dried out was by Ananiadis (1956). He reported signs of eutrophication and occurrence of few cyanobacterial species. Our data concerning lake water, suggest that the re-constructed Lake Karla is experiencing the occurrence of significant MCYST concentrations. Papadimitriou et al. (2011) previously reported the presence of MCYST concentrations in Lake Karla ranging between 0.75 and 3.90 μg/l, while Oikonomou et al. (2012) and Papadimitriou et al. (2013) confirmed the occurrence of the toxic cyanobacterial species of Anabaenopsis, Planktothrix and Microcystis in Lake Karla. The above cyanobacterial species found in Lake Karla have been shown to produce a variety of toxins and alkaloids with toxic activity in many lakes worldwide (Sivonen and Jones 1999; Sabour et al. 2005; Kurmayer et al. 2005; Hisem 2008). MCYST concentrations (intracellular and extracellular) found in Lake Karla are similar to those reported for other Mediterranean lakes in Turkey (Albay et al. 2003) and in Portugal (Vasconcelos et al. 1996), but generally, lower to those of the temperate lakes in Canada (Kotak et al. 1996) or in Finnish lakes (Lindholm et al. 2003) and also lower than those reported for Brazil lagoons (Magalhães et al. 2001). Extracellular and intracellular MCYST concentrations found in Lake Karla are comparable to those found in another shallow lake in Greece, Lake Pamvotis (Papadimitriou et al. 2012a). It is worth noting that the comparable MCYST concentrations found in Lake Pamvotis, were associated with the bioaccumulation of MCYST in tissues of aquatic organisms (Papadimitriou et al. 2012a).
Studies addressing the chronic effects resulting from the exposure of cyprinids to sublethal concentrations of MCYSTs are quite limited. Our study focuses on the histopathological alterations observed in the C. carpio species found in the eutrophic Lake Karla, thus addressing the effects of the environmentally relevant MCYST concentrations. This study revealed that tissues from all fish samples caught during the sampling surveys contained MCYSTs. This result suggests that the MCYST content in the aquatic environment was adequate to promote MCYST accumulation in the exposed fish. It is well documented that the primary target organ for MCYST accumulation is the liver (Ernst et al. 2006; Papadimitriou et al. 2012a; Moutou et al. 2012), whereas muscle usually presents the lowest MCYST content. The preferential accumulation in the liver may be attributable to the high density of the organic anionic transporters on the surface of hepatocytes, while the process known as presystematic hepatic elimination could prevent or at least minimise the distribution of MCYSTs to other parts of the body (Fischer et al. 2005). However, there is also an evidence that the carp's kidney is the first organ to be affected by MCYST in terms of pathological changes (Fisher and Dietrich 2000).
MCYSTs produced by cyanobacteria in an aquatic environment have been shown to exert adverse effects on fish. Fish are exposed to MCYSTs directly during feeding and/or passively through their continuous contact with their aquatic environment; cyanobacterial metabolites are known to be transported by the aquatic food web (Xie et al. 2004; Smith et al. 2008). Cyanotoxicity can result in two types of structural changes. One is the direct toxic effect of the pollutant, which leads to tissue degeneration and necrosis, and the other is the development of compensatory mechanisms, such as cellular hyperplasia, to address the stressor (Li et al. 2007b). The histopathological changes observed in tissues are the result of histological-level reactions of the fish; thus, histopathology represents a useful tool to assess the degree of toxicity.
According to Palikova et al. (2011), cyanobacteria are regular components of the cyprinid diet, and it appears that M. aeruginosa comprises a significant portion of the carp diet when a bloom formation occurs (Carbis et al. 1997). Nevertheless, carps are not able to avoid the ingestion of toxic cyanobacteria and their toxins in eutrophicated lakes (Tencalla et al. 1994). M. aeruginosa and P. agarrdii found in the pseudogasters of the examined fish were also found in the water of Lake Karla according to the studies of Papadimitriou et al. (2013). This confirms the direct exposure of fish originated from Lake Karla to cyanobacteria and to MCYSTs through feeding. Many previous studies have demonstrated the acute toxicological effects induced by MCYSTs in fish using carps as bioassay models. Laboratory-controlled experiments using different MCYSTs, doses and routes of administration in the common carp (C. carpio) have shown severe histopathological effects (Rabergh et al. 1991; Fisher and Dietrich 2000; Jiang et al. 2011). Jiang et al. (2011) exposed C. carpio individuals to 10 μg/l MC-LR for 14 days, and the results included some liver histopathological changes, such as slight swelling of hepatic cells and partially dissolved parenchymal architecture characterised by vacuolar degeneration with damage of the nuclei (pyknosis, karyolysis). Although the dose of 10 μg/l MC-LR is higher than the MCYST concentrations found in Lake Karla, the histopathological observation found in common carps from Lake Karla are similar. This may be explained by the effect of chronic or subchronic exposure of fish to cyanobacterial blooms containing MCYSTs.
Our histopathological observations seem to be in agreement with previous reports concerning cyanobacterial toxicity (Carbis et al. 1996; Fisher and Dietrich 2000; Malbrouck and Kestemont 2006; Qui et al. 2007; Jiang et al. 2011). Concerning the liver histopathology, the fish exhibited hydropic degeneration and necrosis, in agreement with the findings reported by Rabergh et al. (1991), Snyder et al. (2002), Sugaya et al. (1990), Tencalla et al. (1994), Fisher and Dietrich (2000) and Liu et al. (2002). In contrast to mammals, only a few studies have demonstrated haemorrhagic effects due to cyanobacteria and MCYSTs in fish (Tencala and Dietrich 1997; Tencalla et al. 1994; Jiang et al. 2011). Kidney alterations included degeneration of the renal tubule, glomerulopathy and dilation of Bowman's capsule. According to Carbis et al. (1996), Fischer and Dietrich (2000) and Kotak et al. (1996), the MCYST-induced renal pathology appears to be generally restricted to the proxima in the posterior part of the kidney. Our results seem to confirm these previously published findings.
Very few in vivo studies have been performed regarding the toxic effects of MCYSTs on the ultrastructures of the hepatocytes in the fish, and very little knowledge is available about the kidney's ultrastucture histopathology (Li et al. 2001; Atencio et al. 2008; Trinchet et al. 2011). Our main histological findings were loss of the parenchymal architecture, hepatocyte necrosis with vacuolisation of the cytoplasm and the presence of lipid droplets. Vacuolisation has been reported in isolated hepatocytes from the common carp (Li et al. 2001) after oral exposure to MCYSTs. According to Atencio et al. (2008) and Molina et al. (2005), vacuolisation might indicate an imbalance between the rate of synthesis of substances in the parenchymal cells and the rate of release of these substances into the systemic circulation. Atencio et al. (2008) have observed glomerulopathy with thickening of the basal membrane, an increased number of lysosomes in the proxima tubules and generalised vacuolisation. We also found degenerations that were mainly associated with the irregularity of the endomembrane system, the swelling of mitochondria and the fragmentation of the mitochondria internal membrane system; these findings are more or less consistent with the observations made by Carbis et al. (1996) after exposing common carps to the MCYST.
It is important to note that the cyanobacterial bloom usually contains many different toxins that have been found to be more toxic than the individually purified component toxin(s) (Ibelings and Chorus 2007). Thus, our histopathological observations provide information on a natural model of disease based on the synergies between the toxicity of the various toxins and the other factors, rather than on the quantities of the individual compounds. Moutou et al. (2012) studied the response of the oxidative system in the same fish species under MCYST-rich conditions. They reported that under natural conditions, the response of the GSH/GSSH system and the catalase activity may also depend on other factors besides continuous exposure.
Lang et al. (2006) suggested a categorisation system for the diagnosis of liver histological alterations in terms of the toxicological relevance of these alterations based on the findings in flatfish species. Recently, van Dyk et al. (2011) have applied this system to evaluate pollution-related histopathological changes in the catfish. These categories include non-specific lesions, early toxicopathic non-neoplastic lesions, pre-neoplastic lesions and neoplasms. The alterations listed within each of these categories are widely accepted as useful indicators for monitoring the biological effects of contaminants on fish (Feist et al. 2004). Using the proposed categories as guidelines, it is clear that many of these alterations were also observed in C. carpio during the present study, such as early toxicopathic, non-neoplastic lesions, including hepatocellular necrosis, nuclei pyknosis, vacuolisation and steatosis.
In conclusion, common carps from Lake Karla that were exposed to a cyanobacterial bloom contained high amounts of MCYSTs in the examined tissues. Regarding the risk that MCYST toxicity poses to public health, the World Health Organization (World Health Organization 1998) proposes 0.04 μg MC-LR equivalent/kg per day as the tolerable daily intake for MCYSTs. The estimated daily intake for C. carpio samples from Lake Karla exhibited MCYST amounts above the WHO guideline (World Health Organization 1998); thus, there might be potential harmful effects on human health from the lifelong consumption of contaminated fish that cannot be overlooked.
In general, our results confirmed the hypothesis that chronic exposure to a cyanobacteria-rich environment and, thus, to MCYST can lead to pathological changes in the exposed carps. The potential ecological risk for the species C. carpio, which always dominates eutrophic, hypertrophic lakes and reservoirs, is also demonstrated. However, because MCYSTs almost never occur in nature as single species but rather occur as a mixture of toxins, the present toxicological profile could obviously be the result of multiple cyanotoxins that were present in the lake water and exerted a synergistic effect on the health of C. carpio. In our study, fish were collected during the spring and the early summer; therefore, it is unlikely that the histopathological differences identified would be related to the seasonal variations. Furthermore, we did not estimate the fish age, but all fish samples were of similar body size; thus, it is likely that all fish were of the same age class. However, taking into consideration the different accumulation rates in the different age classes (Papadimitriou et al. 2012b), there is a need for further research regarding the factors, such as seasonal variation and fish age, when histopathological observations are evaluated. Given the number of contradictory results regarding the effects of cyanotoxins in various biomarker studies, histopathological observations could be quite sensitive biomarkers that reflect the cyanotoxicity effects at the cellular level. The results of the current study may contribute to supporting the cause-effect relationship between fish pathology and the pollution levels in the freshwater environment.
Finally, studies about the effects of cyanobacteria and the accumulation of their toxins in the Mediterranean region are of great importance, as the incidence and persistence of toxic blooms in this region are greater than those observed in temperate regions. Monitoring programmes, specifically at environmentally sensitive sites such as the protected Lake Karla, should include the establishment of baseline histopathological data for the endemic and bio-indicator fish species of the region.
Conclusions
The results of the present study contribute to our knowledge on the accumulation of microcystins in fish tissues under algal bloom rich conditions. Carps from Lake Karla that were exposed to a cyanobacterial bloom contained high amounts of MCYSTs in the examined tissues appearing also histopathological changes.
Our results also confirmed that the liver accumulated the highest MCYST concentrations, while the lowest concentrations were detected in the muscle tissues. Histopathological findings could serve as sensitive signals concerning cyanotoxicity. In terms of public health the consumption of carps as lifelong dietary items might cause harmful effects on human health.
References
Albay M, Akcaalan R, Tufekci H, Metcalf J, Beattie K, Codd G: Depth profiles of cyanobacterial hepatotoxins (microcystins) in three Turkish freshwater lakes. Hydrobiologia 2003, 505: 89–95.
Ananiadis CI: Limnological study of Lake Karla. Bull Inst Oceanogr 1956, 1083: 1–19.
Atencio L, Moreno I, Jos A, Pichardo S, Moyano R, Blanco A, Camean A: Dose-dependent antioxidant responses and pathological changes in tenca ( Tinca tinca ) after acute oral exposure to Microcystis under laboratory conditions. Toxicon 2008, 52: 1–12. 10.1016/j.toxicon.2008.05.009
Au D: The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 2004, 48: 817–834. 10.1016/j.marpolbul.2004.02.032
Blaha L, Babica P, Maršálek B: Toxins produced in cyanobacterial water blooms – toxicity and risks. Interdiscip Toxicol 2009,2(2):36–41.
Carbis CR, Mitchell GF, Anderson JW, McCauley I: The effects of microcystins on the serum biochemistry of carp, Cyprinus carpio L, when the toxins are administered by gavage, immersion and intraperitoneal routes. J Fish Dis 1996, 19: 151–159. 10.1111/j.1365-2761.1996.tb00694.x
Carbis CR, Rawlin GT, Grant P, Mitchell GF, Anderson JW, McCauley I: A study of feral carp, Cyprinus carpio L., exposed to Microcystis aeruginosa at Lake Mokoan, Australia, and possible implications for fish health. J Fish Dis 1997, 20: 81–91. 10.1046/j.1365-2761.1997.d01-111.x
Carmichael WW: The toxins of cyanobacteria. Sci Am 1994, 270: 78–86. 10.1038/scientificamerican0194-78
Duy TN, Lam PK, Shaw GR, Connell DW: Toxicology and risk assessment of freshwater cyanobacterial (blue-green algal) toxins in water. Rev Environ Contam Toxicol 2000, 163: 113–186. 10.1007/978-1-4757-6429-1_3
Ernst B: Investigations of the impact of toxic cyanobacteria on fish. Dissertation, University of Kostanz; 2008.
Ernst B, Hoeger SJ, O'Brien E, Dietrich DR: Oral toxicity of the microcystin containing cyanobacterium Planktothrix rubescens in European whitefish ( Coregonus lavaretus ). Aquat Toxicol 2006, 79: 31–40. 10.1016/j.aquatox.2006.04.013
Feist SW, Lang T, Stentiford GD, Kohler A: Biological effects of contaminants: use of liver pathology of the European flatfish dab ( Limanda limanda L.) and flounder ( Platichthys flesus L.) for monitoring. ICES techniques in marine environmental sciences. Volume 38. Copenhagen: ICES; 2004:42.
Ferra˜o-Filho AS, Kozlowsky-Suzuki B: Cyanotoxins: bioaccumulation and effects on aquatic animals. Mar Drugs 2011, 9: 2729–2772. 10.3390/md9122729
Fischer WJ, Hitzfeld BC, Tencalla F, Eriksson JE, Mikhailov A, Dietrich DR: Microcystin-LR toxicodynamics, induced pathology, and immunohistochemical localization in livers of blue-green algae exposed rainbow trout ( Oncorhynchus mykiss ). Toxicol Sci 2000, 54: 365–373. 10.1093/toxsci/54.2.365
Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B: Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol 2005, 203: 257–263. 10.1016/j.taap.2004.08.012
Fisher W, Dietrich D: Pathological and biochemical characterization of 4 MC-induced hepatopancreas and kidney damage in carp ( Cyprinus carpio ). Toxicol Appl Pharmacol 2000, 164: 73–81. 10.1006/taap.1999.8861
Gutierrez-Praena D, Pichardo S, Jos A, Camean AM: Toxicity and glutathione implication in the effects observed by exposure of the liver fish cell line PLHC- 1 to pure cylindrospermopsin. Ecotoxicol Environ Saf 2011, 74: 1567–1572. 10.1016/j.ecoenv.2011.04.030
Hisem D: Toxicity of Heterocytous Cyanobacteria to Model Invertebrate Artemia salina. Is the toxicity to invertebrate specific and environmentally depended?. Bachelor Thesis, Faculty of Science, The University of South Bohemia; 2008.
Hooser SB: Fulminant hepatocyte apoptosis in vivo following microcystin LR administration to rats. Toxicol Pathol 2000, 28: 726–733. 10.1177/019262330002800513
Humason GL: Animal tissue techniques. 3rd edition. San Francisco: W.H. Freeman and Company; 1972.
Ibelings B, Chorus I: Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: a review. Environ Pollut 2007, 150: 177–192. 10.1016/j.envpol.2007.04.012
Ibelings B, Havens KE: Cyanobacterial toxins: a qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. Adv Exp Med Biol 2008, 619: 675–732. 10.1007/978-0-387-75865-7_32
Jiang JL, Gu XY, Song R, Zhang Q, Geng JJ, Wang XY, Yang LY: Time- dependent oxidative stress and histopathological alterations in Cyprinus carpio L. exposed to microcystin-LR. Ecotoxicology 2011, 20: 1000–1009. 10.1007/s10646-011-0646-9
Jouni S: Current trophic status of Lake Karla, Greece, and proposal for remediations. Dissertation, University of Edinburgh; 2011.
Kotak BJ, Semalulu S, Friytz DL, Prepas EE, Hrudey SE, Coppock RW: Hepatic and renal pathology of intraperitoneally administered microcystin-LR in rainbow trout ( Oncorhynchus mykiss ). Toxicon 1996, 34: 517–525. 10.1016/0041-0101(96)00009-8
Kurmayer R, Christiansen G, Gumpenberger M, Fastner J: Genetic identification of microcystin ecotypes in toxic cyanobacteria of the genus Planktothrix. Microbiology 2005,2005(151):1525–1533.
Lang T, Wosniok W, Barsiene J, Broeg K, Kopecka J, Parkoonen J: Liver histopathology in Baltic flounder ( Platichthys flesus ) as indicator of biological effects of contaminants. Mar Pollut Bull 2006, 53: 488–496. 10.1016/j.marpolbul.2005.11.008
Li X, Liu YD, Song LR: Cytological alterations in isolated hepatocytes from common carp ( Cyprinus carpio L.) exposed to microcystin-LR. Environ Toxicol 2001, 16: 517–522. 10.1002/tox.10012
Li L, Xie P, Li S, Qiu T, Guo L: Sequential ultrastructural and biochemical changes induced in vivo by the hepatotoxic microcystins in liver of the phytoplanktivorous silver carp Hypophthalmichthys molitrix . Comp Biochem Phys C 2007, 146: 357–367. 10.1016/j.cbpb.2006.11.006
Li S, Xie P, Li L, Liang G, Zheng L: Tissue distribution of microcystins in bighead carp via interaperitoneal injection. Bull Environ Contamin Toxicol 2007, 79: 297–300. 10.1007/s00128-007-9207-6
Lindholm T, Vesterkvist P, Spoof L, Lundbergniinisto C, Meriluoto J: Microcystin occurrence in lakes in Åland, SW Finland. Hydrobiologia 2003, 505: 129–138.
Liu Y, Song L, Li X: The toxic effects of microcystin LR on embryo-larval and juvenile development of Loach, Misgurnus mizolopis . Toxicon 2002, 40: 395–399. 10.1016/S0041-0101(01)00173-8
Magalhães VF, Soares R, Azevedo S: Microcystin contamination in fish from the Jacarepagua  Lagoon (Rio de Janeiro, Brazil): ecological implication and human health risk. Toxicon 2001, 39: 1077–1085. 10.1016/S0041-0101(00)00251-8
Malbrouck C, Kestemont P: Effects of microcystins on fish. Environ Toxicol Chem 2006, 25: 72–86. 10.1897/05-029R.1
Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Camean A: Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish ( Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon 2005, 46: 725–735. 10.1016/j.toxicon.2005.07.012
Moutou K, Tsikogias S, Papadimitriou T, Kagalou I: Oxidative stress in Cyprinus carpio to analyze microcystin impact in eutrophic shallow lakes: a preliminary study. J Environ Monit 2012,14(8):2195–2203. 10.1039/c2em30129f
Oikonomou A, Katsiapi M, Karayanni H, Moustaka-Gouni M, Kormas K: Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. Sci World J 2012., 504135: 10.1100/2012/504135
Palikova M, Mares J, Kopp R, Hlavkova J, Navratil S, Adamovsky O, Chmelar L, Blaha L: Accumulation of microcystins in Nile Tilapia, Orechromis niloticus L., and effects of a complex cyanobacterial bloom on the dietetic quality of muscles. Bull Environ Contamin Toxicol 2011, 87: 26–30. 10.1007/s00128-011-0279-y
Papadimitriou T, Stampouli Z, Kagalou I: Preliminary results on the cyanotoxicity in the “new” Lake Karla (Thessaly-Greece). In Proceedings of the 12th international conference on environmental science and technology. Rhodes Island; 2011:1416–1423. 8–10 Sept 8-10 Sept
Papadimitriou T, Kagalou I, Stalikas C, Pilidis G, Leonardos I: Assessment of microcystins distribution and biomagnification in tissues of the aquatic food web compartments from a shallow lake and potential risks for public health. Ecotoxicology 2012,21(4):1155–1166. 10.1007/s10646-012-0870-y
Papadimitriou T, Kagalou I, Leonardos I: Seasonally accumulation of microcystins in the various tissues of an endemic and protected fish species ( Rutilus panosi ) with different sizes. Clean-Soil Air Water 2012,40(4):402–407. 10.1002/clen.201000242
Papadimitriou T, Katsiapi M, Kormas KA, Moustaka-Gouni M, I K: Artificially-born “killer” lake: phytoplankton based water quality and microcystin affected fish in a reconstructed lake. Sci Total Environ 2013, 452–453: 116–124.
Phillips MJ, Roberts RJ, Stewart JA, Codd GA: The toxicity of the cyanobacterium Microcystis aeruginosa to rainbow trout, Salmo gairdneri Richardson. J Fish Dis 1985, 8: 339–344. 10.1111/j.1365-2761.1985.tb00953.x
Qui T, Xie P, Ke Z, Li L, Guo L: In situ studies on physiological and biochemical responses of four fishes with different trophic levels to toxic cyanobacterial blooms in a large Chinese lake. Toxicon 2007, 50: 365–376. 10.1016/j.toxicon.2007.04.006
Rabergh CM, Bylund G, Eriksson JE: Histopathological effects of microcystin- LR, a cyclic peptide toxin from the cyanobacterium (blue-green alga) Microcystis aeruginosa , on common carp ( Cyprinus carpio L.). Aquat Toxicol 1991,20(3):131–146. 10.1016/0166-445X(91)90012-X
Ressom R, Soong FS, Fitzgerald J, Turczynowicz L, El Saadi O, Roder D, Maynard T, Falconer IR: Health effects of toxic cyanobacteria (blue-green algae). Canberra: National Health and Medical Council, Australian Government Publishing Service; 1994.
Sabour B, Loudiki M, Oudra B, Vasconcelos V, Oubraim S, Fawzi B: Dynamics and toxicity of Anabaena aphanizomenoides (cyanobacteria) waterblooms in the shallow brackish Oued Mellah (Morocco). Aquat Ecosyst Health Manage 2005, 8: 95–104. 10.1080/14634980590914944
Sipia V, Lahti K, Kankaanpaa H, Vuorinen P, Meriluoto J: Screening for cyanobacterial hepatotoxins in herring and salmon from the Baltic Sea. Aquat Ecosyst Health Manage 2002,5(4):451–456. 10.1080/14634980290001959
Sivonen K, Jones G: Cyanobacterial toxins. In Toxic Cyanobacteria in Water. Edited by: Chorus I, Bartram J. London: E & FN Spon, WHO; 1999.
Smith JL, Boyer GL, Zimba PV: A review of cyanobacterial odours and bioactive metabolites: impacts and management alternatives in aquaculture. Aquaculture 2008, 280: 5–20. 10.1016/j.aquaculture.2008.05.007
Snyder GS, Goodwin AE, Freeman DW: Evidence that channel catfish, Ictalurus punctatus (Rafinesque), mortality is not linked to ingestion of the hepatotoxin microcystin-LR. J Fish Dis 2002, 25: 275–285. 10.1046/j.1365-2761.2002.00374.x
Sugaya Y, Yasuno M, Yanai T: Effects of toxic Microcystis viridis and isolated toxins on goldfish. Jpn J Limnology 1990, 51: 149–153. 10.3739/rikusui.51.149
Tencala F, Dietrich D: Biochemical characterization of microcystin toxicity in rainbow trout ( Oncorhynchus mykiss ). Toxicon 1997,35(4):583–595. 10.1016/S0041-0101(96)00153-5
Tencalla F, Dietrich D, Schlatter C: Toxicity of Microcystis aeruginosa peptide toxin to yearling rainbow trout ( Oncorhynchus mykiss ). Aquat Toxicol 1994, 30: 215–224. 10.1016/0166-445X(94)90059-0
Trinchet I, Djediat C, Huet H, Dao S, Edery M: Pathological modifications following sub-chronic exposure of medaka fish ( Oryzias latipes ) to microcystin-LR. Reprod Toxicol 2011, 32: 329–340. 10.1016/j.reprotox.2011.07.006
van Dyk J, Cochrane M, Wagenaar G: Liver histopathology of the sharptooth catfish Clarias gariepinus as a biomarker of aquatic pollution. Chemosphere 2011,87(4):304–311. 10.1016/j.chemosphere.2011.12.002
Vasconcelos V, Sivonen K, Evans WR, Carmichael WW, Namikoshi M: Hepatotoxic microcystin diversity in cyanobacterial blooms collected in Portuguese freshwaters. Water Res 1996, 30: 2377–2384. 10.1016/0043-1354(96)00152-2
Welker M, Brunke M, Preussel K, Lippert I, von D¨ohren H: Diversity and distribution of Microcystis (Cyanobacteria) oligopeptide chemotypes from natural communities studied by single colony mass spectrometry. Microbiology 2004, 150: 1785–1796. 10.1099/mic.0.26947-0
World Health Organization (WHO): Cyanobacterial toxins: microcystin-LR. Health criteria and other supporting information. In Guidelines for drinking water quality. Volume 2. 2nd edition. Geneva: World Health Organization; 1998:95–110. Addendum to Addendum to
Xie LQ, Xie P, Ozawa K, Honma T, Yokoyama A, Park HD: Dynamics of microcystins-LR and -RR in the phytoplanktivorous silver carp in a sub-chronic toxicity experiment. Environ Pollut 2004, 127: 431–439. 10.1016/j.envpol.2003.08.011
Acknowledgements
Laboratory work was partially financed by the postgraduate taught degree programme ‘Sustainable Management of Aquatic Environment’ by the Department of Ichthyology and Aquatic Environment, University of Thessaly.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AM was responsible for the sampling, analysis and laboratory handling. IK deployed the research protocol, contributed to the analysis of the data, discussion, writing, and reviewing. NP was responsible for the histopathological observations with the optical microscope and contributed in results evaluation. PB was responsible for electron microscopy and ultrastructural histopathological diagnosis, contributed to the data analysis, discussion, writing, and reviewing. EM was responsible for the fish sampling, contributed in analysing the data, discussion of the results, writing and editing. TP made the MCYST detection, contributed to sampling, statistical analysis, discussion, writing and reviewing. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mitsoura, A., Kagalou, I., Papaioannou, N. et al. The presence of microcystins in fish Cyprinus carpio tissues: a histopathological study. Int Aquat Res 5, 8 (2013). https://doi.org/10.1186/2008-6970-5-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-6970-5-8