Abstract
A novel lectin from the large globiferous pedicellariae of the toxopneustid sea urchin, Toxopneustes pileolus, was isolated by a combination of gel permeation chromatography and affinity chromatography techniques. On an SDS-PAGE gel, single bands were detected with relative molecular weights of 28 and 170 kDa in the presence and absence of 2-mercaptoethanol, respectively, suggesting that this lectin is present as a homohexamer. The 170-kDa lectin was named sea urchin lectin-III (SUL-III). The N-terminal partial amino acid sequence of the intact 28-kDa subunit of SUL-III was determined as follows: RCPQPAALPYRIAQIGNRFL. Agglutination of rabbit erythrocytes by SUL-III was most effectively inhibited by L-rhamnose. SUL-III induced mitogenic stimulation on murine splenocytes. These results suggest that SUL-III may be a novel L-rhamnose-binding lectin with potent bioactivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Sea urchins are common marine organism belonging to the phylum Echinoderma. Echinoids have globular or flatted bodies covered by regularly arranged spines and delicate triple-jawed pedicellariae. Approximately 200 species of echinoids are found around the coast of Japan. Six species of Japanese urchins serve as important source of food, whereas several species are dangerous to humans (Nakagawa et al. 2003). Envenomation is caused by stings from either spines or pedicellariae. The toxopneustid sea urchins, Toxopneustes pileolus, Tripneustes gratilla, and Lytechinus variegatus, have extremely well-developed globiferous pedicellariae that contain biologically active substances (Alender et al. 1965; Feigen et al. 1966; Fujiwara 1935; Kimura et al. 1975; Mebs 1984; Mendes et al. 1963; Nakagawa et al. 1991,1992). Some of these active substances produce deleterious effects such as severe pain, syncope, and respiratory distress (Fujiwara 1935; Walker 1988; Auerbach 1991). In our research on lectin components as bioactive substances, we have been investigating mitogenicity and/or chemotoxicity in the globiferous pedicellariae of the toxopneustid sea urchin T. pileolus (Nakagawa et al. 1996,1997).
Lectins are (glyco)proteins possessing at least one noncatalytic domain that recognize and bind reversibly to specific carbohydrates inside and outside cells (Drickamer 1988; Kilpatric 2002; Sharon and Lis 2004). In recent years, several lectins have also been isolated from various marine invertebrates, including echinoderms (Belogortseva et al. 1998; Dam et al. 1992; Dresch et al. 2008; Giga et al. 1987; Hatakeyama et al. 1994; Himeshima et al. 1994; Kawagishi et al. 1994; Marques and Barracco 2000; Nair et al. 2000; Suzuki et al. 1990). We have purified D-galactose-specific lectins SUL-I, SUL-II, and SUL-IA from the large globiferous pedicellariae of T. pileolus (Nakagawa et al. 1996,1997; Satoh et al. 2002; Edo et al. 2012). These findings indicate the presence of multiple lectins in the large globiferous pedicellariae of T. pileolus. SULs may be useful tools for studies on biological functions of cells (Fusetani and Kem 2009). Therefore, in this study, we attempted to isolate a novel lectin from the large globiferous pedicellariae of T. pileolus.
Methods
Isolation of a pedicellarial lectin
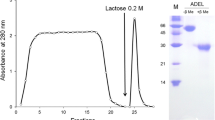
Sixty specimens of T. pileolus (8 to 11 cm in diameter) were collected along the coast of Tokushima Prefecture, Shikoku Island, Japan, from December 2008 to December 2009. The venom protein was extracted from the large flower-like globiferous pedicellariae as reported previously (Nakagawa et al. 1996). Briefly, for the first step of purification, the venom protein was applied to a Superdex 200 (prep grade, GE Healthcare, Uppsala, Sweden) gel filtration column (1.6 × 50 cm) equilibrated with 0.15 M NaCl solution containing 100 mM D-galactose and was eluted with the same solution at a flow rate of 8 ml/h (Figure 1A). Fractions of 2 ml each were collected and analyzed for absorption at 280 nm and screened for agglutinating activity. The final purification was achieved using an immobilized D-galactose column (2 ml) equilibrated with 150 mM phosphate buffer solution (Na2HPO4 73 mM, KH2PO4 37 mM, NaCl 40 mM) (PBS) (pH 7.2). The gel chromatographic fraction (the P-II fraction) was rinsed and washed with the same buffer and eluted with same buffer containing 100 mM D-galacotose at a flow rate of 20 ml/h (Figure 1B). Elution fractions (2 ml) were collected and analyzed for absorption at 280 nm and screened for agglutinating activity. Each of the second peaks was pooled and analyzed for electrophoresis, and then used as the purified lectin. Protein assays were performed employing the method of Bradford (1976) using bovine serum albumin as a standard.
Isolation of a novel lectin from the large globiferous pedicellariae of T. pileous. The isolation procedure is described in detail in the 'Methods’ section. (A) The first purification step used Superdex 200. (B) The second purification step used immobilized D-galactose. Inset panels show SDS-PAGE under reducing condition of gel filtration chromatographic fractions and affinity chromatographic fractions. M = mol. wt. markers.
Electrophoresis
Blue native polyacrylamide gel electrophoresis (BN-PAGE) was run as described by Schägger and von Jagow (1991) using a 4% to 16% gradient gel. Sodium dodecyl sulfate (SDS)-PAGE was carried out by the method of Laemmli (1970) using a 10% to 20% gradient gel. Protein sample were heated in the presence of 2-mercaptoethanol for 4 min at 98°C. The gels were stained with Coomassie brilliant blue.
Assay of agglutinating activity
Agglutination and sugar inhibition assay were performed in accordance with the method described previously (Nakagawa et al. 1996) using 2% rabbit erythrocytes suspension in microtiter plates. A total of 30 μl of a 2% (v/v) suspension of erythrocytes in 6.4 mM phosphate-buffer saline (PBS) was added to 50 μl of serial twofold dilutions of the sample. The plates were incubated at room temperature for 1 h. The results are expressed as the minimum concentration of the sample (μg/ml) required for positive agglutination. Agglutination inhibition was expressed as the minimum concentration of each sugar required for inhibition of agglutinating activity by the purified lectin.
Mitogenic activity
Mitogenic activity on the murine splenocytes was determined by cell culture assay using 3(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Nakagawa et al. 1997). Splenocytes (5 × 106 cells per milliliter) with or without concanavalin A (1 μg/ml) as the positive control, and the lectin fractions were plated in flat-bottomed microplates and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 68 h. A total of 10 μl of MTT tetrazolium salt solution (5 mg/ml) was then added to each well, and formazan in the cells was extracted with 10% SDS after 4 h. The optical density of each well was measured spectrophotometrically using a microplate reader (Bio-Rad Lab., Model 680, Tokyo, Japan) at 570 nm.
N-terminal amino acid sequencing
Approximately 3 μg of the sample protein was subjected to SDS-PAGE, followed by electroblotting onto a polyvinylidene difluoride membrane. The membrane was then stained with Ponceau S and destained. The protein band was excised and subjected to automated Edman degradation using the Shimadzu Model PPSQ-10 protein sequencer (Shimadzu Corp., Kyoto, Japan).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). The statistical analyses were performed using SPSS version 16.0 software package (SPSS, Chicago, Inc., IL., USA). The statistical analysis of the results was performed by Dunnett's multiple comparison test when various experimental groups were compared to the control groups, and Student's t test was used for paired or unpaired groups. P < 0.05 was considered statistically significant.
Results
Purification of sea urchin lectin-III
A pedicellarial lectin from T. pileolus was purified by gel chromatography and affinity chromatography (Figure 1A,B). As shown in Figure 1B, the gel chromatographic fraction (the P-II fraction) with potent agglutinating activity was applied to an immobilized D-galactose column equilibrated with 150 mM PBS (pH 7.2). SDS-PAGE analysis of the unbound fraction (the IDG-I fraction) identified two bands corresponding to proteins with molecular weights of 23 and 25 kDa (Figure 1B). On the other hand, the bound fraction (the IDG-II fraction) contained a single band corresponding to a protein with a molecular weight of 28 kDa. The IDG-I fraction did not induce agglutination with rabbit erythrocytes at higher concentrations up to 400 μg/ml. However, the IDG-I fraction at a concentration of 50 μg/ml exhibited agglutination activity in 10 mM Tris–HCl buffer (pH 7.5)-0.15 M NaCl (TBS) containing 20 mM CaCl2 (Hatakeyama et al. 1994). Thus, agglutination induced by the IDG-I fraction was Ca2+-dependent (data not shown). In contrast, the IDG-II fraction induced agglutination of rabbit erythrocytes at a concentration of 1.25 μg/ml in a Ca2+-independent manner (data not shown).
As shown in Figure 2, the IDG-II fraction contained a single band corresponding to a molecular weight of 28 and 170 kDa under reducing condition and nonreducing condition (without 2-mercaptoethanol), respectively. Thus, the purified lectin appears to be a hexameric protein that shows Ca2+-independent agglutinating activity. This lectin was named sea urchin lectin-III (SUL-III). The N-terminal amino acid of intact 28-kDa subunit of SUL-III was arginine. The sequence of 20 residues from the N-terminus was determined as follows: RCPQPAALPYRIAQIGNRFL. The recovery of SUL-III in terms of protein content was 3.6% of the pedicellarial venom.
Sugar-binding specificity of SUL-III
As shown in Table 1, the effects of mono- and oligosaccharides on agglutination by SUL-III were examined. Although SUL-III was expected to exhibit affinity for carbohydrate containing galactose residues, the extent of agglutination inhibition differed for each sugar used. The agglutinating activity of SUL-III was most effectively inhibited by L-rhamnose and, to a lesser extent, by lactulose (Galβ1 → 4Fru) and lactose (Galβ1 → 4Glc), suggesting that the hydroxy group at C-1, C-2, and C-4 of the pyranose ring structure may influence sugar binding to the lectin (Table 1).
Mitogenic activity of SUL-III
We found previously that pedicellarial venom exhibited mitogenic stimulation of murine splenocytes (Nakagawa et al. 1997). Therefore, in this study, the mitogenic activities of the IDG-I fraction and SUL-III were also investigated. As shown in Figure 3, the IDG-I fraction and SUL-III stimulated mitogenic responses of murine splenocytes, in the dose range of 0.31 to 1.25 μg/ml, respectively. SUL-III caused potent mitogenic responses in murine splenocytes in comparison with the IDG-I fraction. At a lower dose of 0.31 μg/ml, SUL-III had maximum mitogenic activity on murine splenocytes. However, in the dose range of 0.62 to 1.25 μg/ml, SUL-III showed a significant decrease in mitogenic activity. In contrast, the IDG-I fraction induced the sustained mitogenic activity in the dose range of 0.31 to 1.25 μg/ml (Figure 3).
Mitogenic effects of the IDG-I fraction and IDG-II fraction (SUL-III) on murine splenocytes. Splenocytes (5 × 106 cells per milliliter) were incubated with the IDG-I fraction or SUL-III for 68 h, and then, the incubation was continued with MTT for 4 h in a CO2 humidified atmosphere. Data show the mean ± SD of three experiments with determinations in triplicate. *P < 0.05, **P < 0.01, compared with the negative control. @@P < 0.01, statistically different between two groups according Student's unpaired t test.
Comparison of the partial amino acid sequence of SUL-III and catfish lectin
As shown in Table 2, sequence analysis of intact 28 kDa of SUL-III (170 kDa) determined N-terminal sequence from Arg-1 to Leu-20. SUL-III did not show sequence homology to SUL-I (32 kDa), SUL-IA (32 kDa), SUL-II (23 kDa), and Contractin A (18 kDa) from T. pileolus venom. However, SUL-III was related to SAL (31.7 kDa), which is a rhamnose-binding lectin from catfish (Silurus asotus) roe (Table 2). Thus, the structure of SUL-III as a hexameric protein may be unique.
Discussion
Sea urchins belonging to the Toxopnesutidae possess extremely well-developed globiferous pedicellariae, which contain biologically active substances. The venomous globiferous pedicellariae of sea urchins T. pileolus and T. gratilla have been studied in some detail (Fujiwara 1935; Okada et al. 1955; Alender et al. 1965; Kimura et al. 1975). Some of the bioactive substances produce deleterious and pharmacological effects (Nakagawa et al. 1982; Nakagawa et al. 1991; Kuwabara 1994). In the course of purification studies on the pedicellarial venom of T. pileolus, we have found that the venom contains lectin components (Nakagawa et al. 1996,1997).
In the present study, we have isolated a novel lectin, SUL-III, from the large globiferous pedicellariae of T. pileolus, using Superdex 200 and immobilized D-galactose (Figures 1 and 2). Using SDS-PAGE, SUL-III migrated as a single band corresponding to relative molecular weights of 28 and 170 kDa under reducing condition and nonreducing condition, respectively (Figure 2). Therefore, SUL-III was considered to be a homohexameric protein. The N-terminal amino acid sequence of SUL-III was analyzed as follows: RCPQPAALPYRIAQIGNRFL. SUL-III induced agglutinating activity of rabbit erythrocytes at a concentration of 1.25 μg/ml in Ca2+-independent manner. The agglutination with rabbit erythrocytes by SUL-III was most effectively inhibited by L-rhamnose (Table 1). Thus, SUL-III appears to be a L-rhamnose-binding lectin. On the other hand, the IDG-I fraction gave two bands corresponding to 23 and 25 kDa protein on SDS-PAGE under reducing condition (Figure 1B). The IDG-I fraction did not cause agglutination with rabbit erythrocytes at concentrations up to 400 μg/ml, whereas it showed agglutination at a concentration of 50 μg/ml in the presence of 20 mM CaCl2 (Hatakeyama et al. 1994). This is the first finding about Ca2+-dependent lectin fraction (the IDG-I fraction) from the large globiferous pedicellariae of T. pileolus. Further studies may also find some novel lectins from the IDG-I fraction. In this study, we showed that the IDG-I fraction and SUL-III had mitogenic stimulation on murine splenocytes (Figure 3). SUL-III exhibited potent mitogenic activity in comparison with the IDG-I fraction. However, in the dose range of 0.62 to 1.25 μg/ml, SUL-III showed a significant decrease in mitogenic activity. The dual response to SUL-III suggests that it may have multiple functions on murine splenocytes (Satoh et al. 2002; Nakagawa et al. 2003).
Previously, we isolated toxins and lectins from the globiferous and large globiferous pedicellariae, such as Contractin A (Nakagawa et al. 1991), UT841 (Zhang et al. 2001), SUL-I, and SUL-II (Nakagawa et al. 1999; Suzuki-Nishimura et al. 2001; Satoh et al. 2002). More recently, it has also been reported that SUL-1A, a D-galactose-specific lectin, as well as SUL-I and SUL-II could be purified from the large globiferous pedicellariae of T. pileolus (Edo et al. 2012). SUL-IA is a monomeric protein with a molecular weight of 32 kDa. As shown in Table 2, SUL-III did not show sequence homology to Contractin A, SUL-I, SUL-IA, and SUL-II. However, SUL-III showed sequence homology to SAL, a rhamnose-binding lectin from catfish eggs (Hosono et al. 1999). This homology was equivalent to 22% of the 35 N-terminal amino acid residues from SAL. Rhamnose-binding lectins (RBLs) including SAL seem to serve as part of a defense system (Hosono et al. 1999; Watanabe et al. 2008). Thus, SUL-III may play a role in innate immunity. Further studies on detailed structure of SULs may advance the understanding of biological functions of pedicellarial lectins from T. pileolus.
It has been reported that various carbohydrate recognition proteins, including lectins, are involved in the immune response (Kilpatric 2002). RBLs have been isolated from various kinds of fish and invertebrates and been found to interact with various bacteria, suggesting RBLs are involved in inflammatory reactions (Watanabe et al. 2008). In this study, SUL-III showed strong mitogenic activity when added to the culture of murine splenocytes (Figure 3). The present results suggest that SUL-III may be a useful tool as a novel mitogen. SUL-III also exhibited effective chemotactic activity on guinea-pig neutrophils (unpublished data). Chemotaxis and phagocytosis by leukocytes play an important role in the defense reactions to infection and injury in vertebrates. Recently, it has been shown that SUL-I induces dendritic cell maturation from human monocytes (Takei and Nakagawa 2006). Thus, it seems likely that SUL-III is a valuable tool for analyses of the inflammatory and immunity reactions of cells, such as SUL-I (Takei and Nakagawa 2006) and SUL-IA (Edo et al. 2012). Investigation of the structural features of SUL-III is needed for resolution of the physiological significance of SULs from T. pileolus. Our data suggest that SULs from T. pileolus could become useful probes for investigating the process involved in cell functions.
Conclusions
A novel lectin, SUL-III was isolated from the large globiferous pedicellariae of T. pileolus, by gel permeation chromatography and affinity chromatography. SDS-PAGE under nonreducing condition showed that SUL-III is a homohexameric protein with a molecular weight of 170 kDa. The agglutinating activity of SUL-III was effectively inhibited by L-rhamnose. SUL-III was shown to have potent mitogenic stimulation on murine splenocytes. The N-terminal partial amino acid sequence of intact 28-kDa subunit of SUL-III was determined up to 20 residues. SUL-III showed sequence homology to SAL, a rhamnose-binding lectin from catfish (Silus asotus) eggs. These results suggest that SUL-III may be a useful L-rhamnose-binding lectin as research tools, which is one of the multiple lectins from the globiferous pedicellariae of T. pileolus.
References
Alender CB, Feigen GA, Tomita JT: Isolation and characterization of sea urchin toxin. Toxicon 1965, 3: 9–17.
Auerbach PS: Marine envenomations. N Engl J Med 1991, 325: 486–493.
Belogortseva NI, Molchanova VI, Kurika AV, Skobun AS, Glazkova VE: Isolation and characterization of new GalNAc/Gal-specific lectin from sea mussel Crenomytilus grayanus . Comp Biochem Physiol C 1998, 119: 45–50.
Bradford MM: A rapid and sensitive methods for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72: 248–254.
Dam TK, Sarkar M, Ghosal J, Choudhury A: A novel galactosyl-binding lectin from the plasma of the blood clam, Anadara granosa (L) and a study of its combining site. Mol cell Biochem 1992, 117: 1–9.
Dresch RR, Lerner CB, Mothes B, Trindade VMT, Henriques AT, Vozari-Hampe MM: Biological activities of ACLI and physicochemical properties of ACL-II, lectins isolated from the marine sponge Axinella corrugata . Comp Biochem Physiol B 2008, 161: 365–370.
Drickamer K: Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem 1988, 263: 9557–9560.
Edo K, Sakai H, Nakagawa H, Hashimoto T, Shinohara M, Ohura K: Immunomodulatory activity of a pedicellarial venom lectin from the toxopneustid sea urchin, Toxopneustes pileolus . Toxin Rev 2012, 31: 54–60.
Feigen GA, Santz E, Alender CB: Studies on the mode of action of sea urchin toxin-I. Conditoins affecting release of histamine and other agents from isolated tissues. Toxicon 1966, 4: 161–175.
Fujiwara T: On the poisonous pedicellariae of Toxoneustes pileolus (Lamark). Anot Zool Jpn 1935, 15: 62–69.
Fusetani N, Kem W: Marine toxins: an overview. Marine Toxins as Research Tools. In Progress in Molecular and Subcellular Biology. Volume 46. Edited by: Fusetani N, Kem W. Springer, Berlin; 2009:1–44.
Giga Y, Ikai A, Takahashi K: The complete amino acid sequence of ehinoidin, a lectin from the coelomic fluid of the sea urchin Anthrocidaris crassispina : homologies with mammarlian and insect lectins. J Biol Chem 1987, 262: 6197–6203.
Hatakeyama T, Kohzaki H, Nagatomo H, Yamasaki N: Purification and characterization of four Ca2+-dependent lectins from the marine invertebrate, Cucumaria echinata . J Biochem 1994, 116: 209–214.
Himeshima T, Hatakeyama T, Yamasaki N: Amino acid sequence of a lectin from the sea cucumber, Sticopus japnicus , and its structural relationship to the C-type animal lectin family. J Biochem 1994, 115: 689–692.
Hosono M, Ishikawa K, Mineki R, Murayama K, Numata C, Ogawa Y, Takayanagi Y, Nitta K: Tandem repeat structure of rhamnose-binding lectin from catfish ( Silurus astous ) eggs. Biochim Biophys Acta 1999, 1472: 668–675.
Kawagishi H, Yamawaki M, Iobe S, Usui T, Kimura A, Chib S: Two lectin from the maine sponge Halichondria okadai . An N-acetyl-sugar specific lectin (HOL-I) and an N-acetyllactosamine-specific lectin (HOL-II). J Biol Chem 1994, 269: 1375–1379.
Kilpatric DC: Animal lectin: a historical introduction and overview. Biochim Biophys Acta 2002, 1572: 187–197.
Kimura A, Hayashi H, Kuramoto M: Studies of urchi-toxins: separation, purification and pharamacological actions of toxinic substances. Jpn J Pharamacol 1975, 25: 109–120.
Kuwabara S: Purification and properties of peditoxin and the structure of its prosthetic group, pedoxin, from the sea urchin Toxopneustes pileolus (Lamarck). J Biol Chem 1994, 269: 26734–26738.
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacterriophage T4. Naure 1970, 227: 680–685.
Marques MRF, Barracco MA: Lectins as non-self recognition factors in crustanceans. Aquaculture 2000, 191: 23–44.
Mebs D: A toxin from the sea urchin Tripneustes gratilla . Toxicon 1984, 22: 306–307.
Mendes EG, Abbud L, Umiji S: Cholinergic action of homogenates of sea urchin pedicellariae. Science 1963, 139: 408–409.
Nair SV, Pearce S, Green PL, Mahajan D, Newton RA, Raftos DA: A collectin-like protein from tunicates. Comp Biochem Physiol B 2000, 125: 279–289.
Nakagawa H, Kimura A, Takei M, Endo K: Histamine release from rat mast cells induced by an extract from the sea urchin Toxopnesutes pileolus . Toxicon 1982, 20: 1095–1097.
Nakagawa H, Tu A, Kimura A: Purification and characterization of Contractin A from the pedicellarial venom of sea urchin, Toxopneustes pileolus . Arch Biochem Biophys 1991, 284: 279–284.
Nakagawa H, Yanagihara N, Izumi F, Wada A, Kimura A: Inhibition of nicotinic acetylcholine receptor-mediated secretion and synthesis of catecholamines by sea urchin toxin in cultured bovine adrenal medullary cells. Biochem Pharmacol 1992, 44: 1779–1785.
Nakagawa H, Hashimoto T, Hayashi H, Shinohara M, Ohura K, Tachikawa E, Kashimoto T: Isolation of a novel lectin from the globiferous pedicellariae of the sea urchin Toxopneustes pileolus . Adv Exp Med Biol 1996, 391: 213–223.
Nakagawa H, Yamaguchi C, Hayashi H: Biologically active substances from sea urchins. J Natural Toxins 1997, 6: 193–202.
Nakagawa H, Yamaguchi C, Tomiyoshi F, Hayashi H: A novel mitogenic lectin from the globiferous pedicellariae of the sea urchin, Toxopneustes pileolus . J Chem Soc Pak 1999, 21: 305–310.
Nakagawa H, Tanigawa T, Tomita K, Tomihara Y, Araki Y, Tachikawa E: Recent studies on the pathological effects of purified sea urchin toxins. J Toxicol Toxin Rev 2003, 22: 633–649.
Okada K, Hashimoto T, Miyauchi Y: A preliminary report on the poisonous effect of Toxopneustes toxin upon the heart of oyster. Bull Mar Biol Asamushi 1955, 7: 133–140.
Satoh F, Nakagawa H, Yamada H, Nagasaka K, Nagasaka T, Araki Y, Tomihara Y, Nozaki M, Sakuraba H, Ohshima T: Fishing for bioactive substances from scorpionfish and some sea urchins. J Nat Toxins 2002, 11: 297–304.
Schägger H, von Jagow G: Blue native electrophoresis for isolation of membrane protein complexs in enzymatically active form. Anal Biochem 1991, 199: 223–231.
Sharon N, Lis H: History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 2004, 14: 53–62.
Suzuki T, Takagi T, Furukohori T, Kawamura K, Nakauchi M: A calcium-dependent galactose-binding lectin from the tunicate Plyandrocarpa misakiensis . J Biol Chem 1990, 265: 1274–1281.
Suzuki-Nishimura T, Nakgawa H, Uchida MK: D-galactose-specific sea urchin lectin sugar-specifically inhibited histamine release induced by Datura stramonium agglutinin: differences between sugar specific effects plan lectins. Jpn J Pharmacol 2001, 85: 443–452.
Takei M, Nakagawa H: A sea urchin lectin, SUL-I, from the toxopneustid sea urchin induces DC maturation from human monocytes and drives Th1 polarization in vitro . Toxicol Appl Pharamacol 2006, 213: 27–36.
Walker MJA: Coelenterate and echinoderm toxins: Mechanisms and actions. Marine Toxins and Venoms. In Handbook of Natural Toxins. Volume 3. Edited by: Tu AT. Marcel Dekker, New York; 1988:279–325.
Watanabe Y, Shiina N, Shinozaki F, Yokoyama H, Kominami J, Nakamura-Tsuruta S, Hirabayashi J, Sugahara K, Kamiya H, Matsubara H, Ogawa T, Muramoto K: Isolation and characterization of L-rhamnose-binding lectins, which binds to microsporidian Glugea plecoglossi , from ayu ( Plecoglossus altivelis ) eggs. Dev Comp Immunol 2008, 26: 543–550.
Zhang Y, Abe J, Siddiq A, Nakagawa H, Honda S, Wada T, Ichida S: UT841 purified from sea urchin ( Toxopneustes pileolus ) venom inhibits time-dependent (45)Ca(2+) uptake in crude synaptosome fraction from chick brain. Toxicon 2001, 39: 1223–1229.
Acknowledgements
We would like to thank Mr. Hideho Nagata and Mr. Hiroshi Nagata for the collections of T. pileolus specimens, and Ms. Shizuka Satoh and Ms. Yuri Kotake for the assistance with the purification of pedicellarial lectin from T. pileolus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HS conducted the purification of sea urchin lectin. KE conducted the assay of mitogenic activity. HN participated in the study design and coordination. MS participated in the interpretation of the results. RN participated in the assay of agglutination. KO participated in the study design. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sakai, H., Edo, K., Nakagawa, H. et al. Isolation and partial characterization of a L-rhamnose-binding lectin from the globiferous pedicellariae of the toxopneustid sea urchin, Toxopneustes pileolus. Int Aquat Res 5, 12 (2013). https://doi.org/10.1186/2008-6970-5-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-6970-5-12