Abstract
Yellow stripe trevally (Selaroides leptolepis) is one of the abundant dark-fleshed fish species caught in Southern Thailand. However, the pelagic fish have high content of dark flesh associated with the high lipid and myoglobin contents contributing to the difficulties in making high-quality surimi. However, setting or heating at an appropriate temperature can improve the property of gel by avoiding the degradation but enhancing cross-linking of proteins. Therefore, the objective of this study was to investigate the gel-forming ability of surimi from yellow stripe trevally prepared under different heating conditions. Surimi gels were prepared under different heating conditions. Textural properties, whiteness, expressible moisture content, trichloroacetic acid (TCA)-soluble peptide content, protein pattern and microstructure of gels were determined. Additionally, the optimal temperature of muscle transglutaminase (TGase) was determined using monodansyl cadaverine incorporation method. Kamaboko gel with prior setting at 40°C (K40) exhibited the highest breaking force, followed by another kamaboko gel having setting temperature of 25°C, directly heated gel and modori gel, respectively (p < 0.05). For deformation, both kamaboko gels showed the lower values than both directly heated gel and modori gel (p < 0.05). The lowest expressible moisture content and whiteness were found in K40 sample (p < 0.05). The optimal temperature of yellow stripe trevally muscle transglutaminase was found to be 40°C. The highest TCA-soluble peptide content with decreased myosin heavy chain was found in modori surimi gel (p < 0.05). K40 sample had finer and more ordered fibrillar structure with smaller voids than other gels. Yellow stripe trevally could be used as the new raw material for surimi which yielded the gel with high deformation. Setting at 40°C is a promising means to improve the properties of surimi gel. Conversely, the incubation of sol at temperature close to 60°C should be avoided to prevent gel weakening.
Similar content being viewed by others
Background
Thailand is one of the largest surimi producers in Southeast Asia. About 16 surimi factories are located in Thailand, with a total production of 96,500 to 113,500 metric tons of surimi per year of which 80% is exported to Japan and Korea and the remainder to Singapore and other countries (Hong & Eong [2005]). In general, lean fish have been used for surimi production. Due to the limited fish resources, especially lean fish, pelagic dark-fleshed fish have been paid more attention as a potential alternative raw material for surimi production (Chaijan et al. [2004]). Dark-fleshed fish make up 40% to 50% of the total fish catch in the world (Hultin & Kelleher [2000]), and the catch of those species in the Gulf of Thailand was approximately 844.2 metric tons in 2006 (Department of Fisheries [2006]). Due to the abundance and lower price, these pelagic fish can be used for surimi production. However, those pelagic fish have high content of dark flesh associated with the high lipid and myoglobin contents (Chaijan et al. [2004]). Those components contribute to the difficulties in making high-quality surimi (Chen [2002]; Ochiai et al. [2001]). The presence of sarcoplasmic proteins of dark muscle also contributes to the poorer gelation (Haard et al. [1994]). Sarcoplasmic proteins are able to bind with myofibrillar proteins, thus interfering the formation of strong gel network. In addition, lipid oxidation seems to be a distinct problem in surimi made from some dark-fleshed fish (Lanier [2000]; Wu et al. [2000]).
Setting has been reported to play an essential role in the formation of protein cross-links mediated by endogenous transglutaminase (TGase) (Kumazawa et al. [1995]; Seki et al. [1990]). In general, setting has been applied to enhance gel strength of surimi. However, setting response can be varied, depending on fish species, and is related to habitat temperature of fish species (Shimizu et al. [1981]; Araki & Seki [1993]; Morales et al. [2001]). Benjakul and Visessanguan ([2003]) reported that surimi from Priacanthus tayenus and P. macracanthus showed the maximised setting at 40°C and 25°C, respectively. Nevertheless, the incubation of surimi paste at 60°C to 65°C mainly resulted in gel weakening or ‘modori’ phenomenon (Benjakul et al. [2003a]). This phenomenon is caused by endogenous heat-activated proteases (An et al. [1996]; Benjakul et al. [1997]). Generally, gel weakening is varying with fish species. Dark-fleshed fish generally had a high proteolytic activity, resulting in high susceptibility to gel weakening (Shimizu et al. [1992]).
Yellow stripe trevally (Selaroides leptolepis) is one of the abundant dark-fleshed fish species caught in Southern Thailand. Therefore, the use of this pelagic fish for surimi production is one of the challenges in transforming the underutilised fish into value-added products, particularly surimi. This species has been used for protein hydrolysate production with antioxidative activity (Klompong et al. [2007]). However, no information regarding the gel properties of surimi from this species has been reported. Therefore, the objective of this study was to investigate the gel-forming ability of surimi from yellow stripe trevally (S. leptolepis) prepared with different heating conditions.
Methods
Chemicals
Sodium dodecyl sulphate (SDS), Coomassie Blue R-250, N,N,N′,N′-tetramethyl ethylene diamine and all chemicals for electrophoresis were procured from Bio-Rad Laboratories (Hercules, CA, USA). Trichloroacetic acid was purchased from Merck (Darmstadt, Germany). Monodansyl cadaverine (MDC), N,N′-dimethylated casein (DMC) and dithiothreitol (DTT) were obtained from Sigma Chemical Co., Ltd. (St. Louis, MO, USA). All chemicals were of analytical grade.
Fish collection and mince preparation
Yellow stripe trevally (S. leptolepis) with an average weight of 65 to 75 g were caught from Songkhla coast along the Gulf of Thailand from June to July 2011. The fish, off-loaded approximately 12 h after capture, were placed in ice with a fish/ice ratio of 1:2 (w/w) and transported to the Department of Food Technology, Prince of Songkla University, Hat Yai within 30 min. The fish were immediately washed, gutted, filleted and de-skinned. Fish flesh was minced to uniformity using a mincer with a hole diameter of 5 mm. Fish and mince were kept in ice during preparation.
Surimi and surimi gel preparation
Surimi was prepared according to the method of Benjakul and Visessanguan ([2003]) with slight modifications. Fish mince was washed with cold water (4°C) at a water/mince ratio of 3:1 (v/w). The mixture was stirred gently for 4 min, and washed mince was filtered with a layer of nylon screen. The washing process was repeated twice. For the third washing, cold 0.2% NaCl solution was used. Finally, the washed mince was subjected to centrifugation using a Model CE 21 K basket centrifuge (Grandiumpiant, Belluno, Italy) pre-cooled with ice at a speed of 700 × g for 10 min. The temperature of dewatered mince was approximately 8°C to 10°C. To the dewatered mince, 4% sucrose and 4% sorbitol were added and mixed uniformly using a kneader (Crypto Peerless Ltd., Birmingham, England). Prior to mixing, the kneader bowl was kept at −20°C for 1 h, in which the temperature of surimi was maintained below 10°C throughout the mixing process. The mixture (500 g) was packed in a polyethylene bag and frozen using an air-blast freezer (−20°C). Frozen surimi was stored at −20°C for not longer than 1 month.
To prepare the gels, the frozen surimi was tempered at 4°C for 3 to 4 h until the core temperature reached 0°C. The sample was then cut into small pieces, and the moisture content was adjusted to 80% by the addition of iced water. The mixture was added with 2.5% (w/w) NaCl and chopped for 5 min in a walk-in cold room at 4°C to obtain the homogeneous sol. The sol was then stuffed into a polyvinylidine casing with a diameter of 2.5 cm, and both ends of the casing were sealed tightly (Benjakul et al. [2003b]). The sol was then incubated at 40°C for 30 min, followed by heating at 90°C for 20 min in a temperature-controlled water bath (Memmert Gmbh Co. KG, Schwabach, Germany). The obtained gel was referred to as ‘kamaboko gel-40°C (K40)’. The gel prepared by incubating the sol at 25°C for 3 h, followed by heating at 90°C for 20 min, was referred to as ‘kamaboko gel-25°C (K25)’. Modori gel was prepared by incubating the sol at 60°C for 30 min, followed by heating at 90°C for 20 min (Benjakul et al. [2010]). A directly heated gel was prepared by heating the sol at 90°C for 20 min. After heating, all gels were immediately cooled in iced water for 30 min and stored for 24 h at 4°C prior to analysis.
Determination of gel properties
Textural analysis
Textural analysis of gel samples was performed using a Model TA-XT2i texture analyser (Stable Micro Systems, Godalming, Surrey, UK). Gels were equilibrated and evaluated at room temperature (28°C to 30°C). Five cylinder-shaped samples with a length of 2.5 cm were prepared and subjected to determination. Breaking force (gel strength) and deformation (elasticity/deformability) were measured using the texture analyser equipped with a spherical plunger (diameter 5 mm, depression speed of 60 mm/min).
Determination of whiteness
All gels were subjected to whiteness measurement using a Hunterlab (ColorFlex, Hunter Associates Laboratory, Reston, VA, USA). Illuminant C was used as the light source of measurement. L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were measured, and whiteness was calculated as described by NFI ([1991]) as follows:.
Determination of expressible moisture content
Expressible moisture content was measured according to the method of Benjakul et al. ([2003c]) with slight modifications. A gel sample with a thickness of 0.5 cm was weighed (X in grams) and placed between two pieces of Whatman filter paper no. 1 (Whatman International Ltd., Maidstone, England) at the top and three pieces of the same type of filter paper at the bottom. The standard weight (5 kg) was placed on the top of the sample and maintained for 2 min. The sample was then removed and weighed again (Y in grams). Expressible moisture content was calculated and expressed as percentage of sample weight as follows:.
Determination of TCA-soluble peptide content
Trichloroacetic acid (TCA)-soluble peptide content was determined according to the method of Benjakul et al. ([2010]). To 3 g of finely chopped gel samples, 27 ml of cold 5% (w/v) TCA were added and homogenised for 2 min using an IKA homogeniser (IKA Labortechnik, Selangor, Malaysia) at a speed of 11,000 rpm. The homogenate was allowed to stand in ice for 1 h and centrifuged at 8,000 × g for 10 min. TCA-soluble peptides in the supernatant were measured according to the Lowry method (Lowry et al. [1951]) and expressed as micromole tyrosine per gram sample (Morrissey et al. [1993]).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
Protein patterns of surimi and surimi gels were determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli ([1970]). To prepare the protein sample, 27 ml of 5% (w/v) hot SDS (85°C) solution was added to the sample (3 g). The mixture was then homogenised at a speed of 11,000 rpm for 2 min. The homogenate was incubated at 85°C for 1 h to dissolve total proteins. The sample was centrifuged at 8,000 × g for 20 min at room temperature (26°C to 28°C) using a centrifuge (Model MIKRO20, Hettich ZENTRIFUGEN, Tuttlingen, Germany). Protein concentration in the supernatant was determined as per the method of Lowry et al. ([1951]). Solubilised samples were mixed at a 1:1 (v/v) ratio with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4% SDS, 20% glycerol and 10% β ME) and boiled for 3 min. Samples (15 μg protein) were loaded onto the polyacrylamide gels comprising a 10% running gel and a 4% stacking gel and subjected to electrophoresis at a constant current of 15 mA/gel using a Mini Protein III unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, the gel was stained with 0.02% (w/v) Coomassie Blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid and destained with 50% (v/v) methanol and 7.5% (v/v) acetic acid. A protein standard (Bio-Rad Laboratories, Inc., Richmond, CA, USA) containing myosin (206 kDa), β-galactosidase (116 kDa), phosphorylase B (97.4 kDa), serum albumin (66.2 kDa) and ovalbumin (45 kDa) was used to estimate the molecular weight of the proteins.
Scanning electron microscopy
Microstructure of surimi gels prepared with different heating conditions was determined using a scanning electron microscope (JE0L JSM-5800 LV, Tokyo, Japan). Samples with a thickness of 2 to 3 mm were fixed with 2.5% (v/v) glutaraldehyde in 0.2 M phosphate buffer (pH 7.2) for 2 h. The samples were then rinsed for 1 h in distilled water before being dehydrated in ethanol with serial concentrations of 50%, 70%, 80%, 90% and 100% (v/v). Dried samples were mounted on a bronze stub and sputter-coated with gold (Sputter coater, SPI-Module, West Chester, PA, USA). The specimens were visualised with a scanning electron microscopy (SEM) at an acceleration voltage of 15 kV.
Characterisation of endogenous TGase in yellow stripe trevally muscle
Preparation of TGase crude extract
Fish flesh was homogenised with four volumes of extraction buffer (10 mM NaCl, 2 mM DTT, 10 mM Tris–HCl, pH 7.5). The homogenate was centrifuged at 16,000 × g using a refrigerated centrifuge (Beckman Coulter, Allegra 25R, Palo Alto, CA, USA) for 20 min at 4°C. Subsequently, the supernatant obtained was centrifuged at 18,000 × g for 60 min at 4°C. The supernatant was used as ‘crude TGase extract’.
Study on temperature profile of TGase
TGase activity was measured in terms of the incorporation of MDC into DMC according to the procedure of Takagi et al. ([1986]) with a slight modification. To study the temperature profile, the assay was performed at various temperatures (20°C, 25°C, 30°C, 35°C, 40°C, 45°C, 50°C, 55°C, 60°C, 65°C and 70°C) for 30 min. The reaction mixture containing 100 μl of 2 mg/ml DMC, 100 μl of 0.5 mM MDC, 100 μl of 0.2 M DTT, 0.4 ml of 0.1 M CaCl2 and 2.4 ml of 0.1 M Tris–HCl (pH 7.5) was prepared. The reaction was initiated by the addition of 0.2 ml of crude TGase extract, and the mixture was incubated at different temperatures for 30 min. The reaction was terminated by the addition of 0.2 ml of 1.0 M ammonium sulphate. Fluorescence intensity of MDC incorporated into DMC was measured with a spectrofluorophotometer (RF-1501, Shimadzu, Kyoto, Japan) at excitation and emission wavelengths of 350 and 480 nm, respectively. Blanks were prepared in the same manner, except ammonium sulphate which was added prior to the addition of crude extract. TGase activity was calculated after blank subtraction and expressed as the increase in fluorescence intensity upon the incorporation of MDC into DMC.
Statistical analysis
All experiments were run in triplicate. Data were subjected to analysis of variance. Comparison of means was carried out by Duncan's multiple-range tests (Steel & Torrie [1980]). Statistical analysis was performed using the Statistical Package for Social Science (SPSS 17.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Properties of gel from surimi of yellow stripe trevally prepared using different heating conditions
Textural property
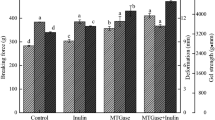
Breaking force and deformation of surimi gels with various heating conditions are shown in Figure 1. Different heating conditions rendered gels with different properties. Among all gels tested, kamaboko gel with setting at 40°C had the highest breaking force, followed by another kamaboko gel (K25) (p < 0.05). Breaking force of K40 was 37.96%, 134.99% and 118.2% higher than that of K25, modori and directly heated samples, respectively. Breaking force of directly heated gel was lower than that of kamaboko gel but was higher than that of modori gel (p < 0.05). The results indicated that protein-protein interactions stabilised by strong bonds were established in kamaboko gels. For deformation, it was noted that kamaboko gels, either with prior setting at 25°C or 40°C, showed the lower values than both directly heated gel and modori gel (p < 0.05). The result suggested that kamaboko gels had slightly lower elasticity than other two gels. The decreased deformation of kamaboko gels was concomitant with their higher breaking force. The rigid and hard gel governed by the strong network generally losses its elasticity (Park et al. [2005]). An increment of protein-protein interactions decreases the water-protein interactions thereby resulting in a decrease in elasticity (Tanaka [1981]). It was noted that the gel of surimi from yellow stripe trevally showed the high deformation (16.33 to 19.10 mm) compared with those reported in surimi from other fish species including bigeye snapper (P. macracanthus) (8 to 10 mm) (Benjakul et al. [2002]), frigate mackerel (6 to 10 mm) and Indian mackerel (9 to 10 mm) (Chaijan et al. [2010]).
Breaking force (a) and deformation (b) of surimi gels from yellow stripe trevally using different heating conditions. DH, directly heated gel; MD, modori gel; and K25 and K40, kamaboko gels with setting at 25°C and 40°C, respectively. Bars represent the standard deviation (n = 3). Different letters (a, b, c and d in Figure 1a) and (a and b in Figure 1b) on the bars indicate significant differences (p < 0.05).
Setting played an important role in cross-linking of gel network, especially by non-disulphide covalent bonds induced by endogenous TGase (Kumazawa et al. [1995]; Seki et al. [1990]). The differences in setting response at different temperatures were presumed to be due to the differences in protein and TGase stability. TGase was found to mediate myosin cross-linking via the formation of ∈ϵ-(γ-glutamyl) lysine linkage (Seki et al. [1990]). When comparing the setting at high temperature (40°C) and medium temperature (25°C), the superior gel was obtained in the former. Protein molecules of yellow stripe travelly might undergo unfolding at 40°C at a greater extent. As a result, more exposure of those reactive residues favoured TGase mediated reaction. Tsukamasa and Shimizu ([1991]) found that heat susceptibility of myosin heavy chain (MHC) was a factor affecting TGase associated setting phenomenon in fish muscle. Additionally, at sufficiently high temperature during setting, hydrophobic interaction might be more enhanced (Niwa [1992]). Without setting, protein cross-linking induced by endogenous TGase might not be enhanced. Furthermore, gradual alignment and aggregation via some weak bonds occurred to some degree. This was reflected by the lower breaking force in directly heated gel in comparison with kamaboko gels. For modori gel, the lowest breaking force was obtained (p < 0.05). This result was in agreement with several reports for surimi from other fish species including sardine (Chaijan et al. [2004]) and Indian mackerel (Chaijan et al. [2010]). Gel weakening or modori phenomenon has been known to be caused by heat-activated proteases, which are active in temperature range of 60°C to 65°C (An et al. [1996]; Benjakul et al. [1997]). The lower breaking force of modori gel indicated that endogenous heat-activated proteases were involved in degradation of surimi proteins at 60°C used in this study.
Whiteness
Whiteness of gels prepared from yellow stripe travelly surimi with different heating conditions was in the range of 73.18 to 74.41 (Table 1). Slight differences in whiteness were observed in different gels (p < 0.05). Directly heated gel exhibited the highest whiteness compared with those prepared by two-step heating (p < 0.05). This could be due to non-enzymatic browning, which might take place at higher extent with a longer exposure time used for two-step heating. During heating, not only metmyoglobin formation may cause a decreased whiteness of gels but the Maillard browning reaction may also affect the colour of gels (Whistler & Daniel [1985]). Yellow stripe trevally is a dark-fleshed pelagic fish and has high lipid content. Lipid oxidation products including aldehydes formed during gelation could be participated in the Maillard reaction (Chaijan et al. [2007]). Nevertheless, gels of yellow stripe trevally surimi showed the higher whiteness than those from surimi of other dark-fleshed fish including sardines (Chaijan et al. [2004]) and Indian mackerel (Chaijan et al. [2010]). Whiteness is an important factor determining the quality and acceptability of surimi gels (Yoon et al. [1997]).
Expressible moisture content
The lowest expressible drip was found in kamaboko gels, both K40 and K25 (p < 0.05), indicating the highest water holding capacity (Table 1). Modori gel and directly heated gel had the highest expressible drip (p < 0.05), suggesting that protein network of the gel was lower in water-binding capacity (Niwa [1992]). Generally, the lowered expressible moisture content was in accordance with the increased breaking force (Figure 1a). During direct heating, rapid unfolding of proteins possibly resulted in more intense coagulation. More water was released from the gel network, and the protein dispersion becomes very uneven (Niwa [1992]). Benjakul et al. ([2010]) also reported that kamaboko gel from goatfish surimi showed the higher water holding capacity than modori gel. Therefore, the improved water holding capacity of yellow stripe trevally surimi gel could be achieved via appropriate setting prior to heating.
Protein pattern
SDS-PAGE protein patterns of surimi, surimi sol and surimi gels with different heating conditions under reducing conditions are shown in Figure 2a. MHC constituted as the major protein in surimi and surimi sol as indicated by the highest band intensity. The decrease in MHC was noticeable in all gels. However, MHC was more retained in directly heated gel. The result indicated that MHC might undergo either polymerisation or degradation to a lowest extent compared with other gels. The decrease in MHC after heating was generally due to the polymerisation or degradation (Benjakul & Visessanguan [2003]).
SDS-PAGE patterns (a) and TCA-soluble peptide content (b) of Surimi gels from yellow stripe trevally using different heating conditions. DH, directly heated gel; MD, modori gel; and K25 and K40, kamaboko gels with setting at 25°C and 40°C, respectively. M, marker; Sur, surimi; Sol, surimi sol; MHC, myosin heavy chain; and AC, actin. Bars represent the standard deviation (n = 3). Different letters (a, b and c) on the bars indicate significant differences (p < 0.05).
Setting at 25°C or 40°C prior to heating at 90°C might allow the protein cross-linking mediated by endogenous TGase to take place effectively, especially when setting was performed at 40°C. It was noted that protein bands with MW of 120 to 130 kDa were formed in K40 sample, suggesting that the degradation of MHC occurred to some extent. Therefore, polymerisation and degradation of MHC occurred simultaneously, especially during the setting at 40°C. However, polymerisation more likely took place to a higher degree than degradation. The ratio of polymerisation to degradation, which varied among the fish species, directly determined the final gel quality. MHC was most susceptible to cross-linking during setting (Benjakul & Visessanguan [2003]). Benjakul and Visessanguan ([2003]) reported the decrease in MHC of surimi gel from bigeye snapper, particularly when the setting at high temperature was implemented. Benjakul et al. ([1997]) reported that MHC was also more prone to proteolytic degradation than other muscle proteins, including actin, troponin and tropomyosin. In modori gel, the disappearance of MHC with the occurrence of new protein bands of MW approximately 110 to 120 kDa suggested the degradation of MHC during incubation at 60°C. The degradation of MHC in the modori gel was coincidental with the decreased breaking force (Figure 1a). High degradation of MHC was reported in surimi gel from dark-fleshed fish including mackerel (Benjakul et al. [2002]). Alkaline proteases have been reported to contribute to the protein degradation in surimi gels (Boye & Lanier [1988]; Toyohara et al. [1987]).
TCA-soluble peptide content
TCA-soluble peptide content of surimi gels prepared by different heating conditions is shown in Figure 2b. The highest content of TCA-soluble peptides was found in modori gel (p < 0.05), which was in agreement with the highest degradation of proteins as shown in SDS-PAGE (Figure 2a). This indicated that the proteolysis was maximised in modori gel. The highest degradation of muscle proteins led to the marked weakening of the gel as indicated by the lowest breaking force of this gel (Figure 1a). Dark-fleshed fish had a high proteolytic activity, resulting in poorer gelation characteristics and high susceptibility to modori (Shimizu et al. [1992]). Shimizu et al. ([1992]) also reported that the poor gel-forming properties of muscle from dark-fleshed species are caused by the presence of heat-stable proteases, which are active in degrading myosin during heating at temperature ranges of 50°C to 70°C. The directly heated gel had the lower TCA-soluble peptide content than modori gel (p < 0.05). Lower TCA-soluble peptide content was also found in kamaboko gels in comparison with modori gel. Bigeye snapper surimi gel, prepared by setting at 40°C, also contained the degradation products (Benjakul et al. [2004]). Takeda and Seki ([1996]) also found some proteolysis in walleye pollack paste during setting at 25°C. Polymerised proteins found during setting might be less susceptible to degradation by endogenous proteases. As a consequence, the lower degradation took place in kamaboko gel with prior setting. K25 samples had the higher TCA-soluble peptide content than K40 samples (p < 0.05). Polymerised proteins might make gel network more resistant to hydrolysis by proteases. Without incubation or setting, the low TCA-soluble peptide content was observed. This result suggested that slow heating at temperature enhancing proteolytic activity might cause the protein degradation and loss in gel property. Since increased breaking force and deformation were obtained with setting at 25°C and 40°C, it was suggested that cross-linking occurred to a greater extent than proteolysis. As a consequence, properties of surimi produced from the yellow stripe travelly could be improved by prior setting.
Microstructure
The microstructures of surimi gels prepared by heating at different conditions are illustrated in Figure 3. Kamaboko gel, particularly K40, showed the higher interconnected three-dimensional protein networks. More compact and denser gel network with finer strand observed in K40 sample was coincidental with the higher breaking force (Figure 1a) and higher water holding capacity (Table 1). K25 sample showed similar microstructure but exhibited lower interconnected three-dimensional protein network with larger voids. However, K25 exhibited denser protein network than directly heated gel and modori gels. The directly heated and modori gels had loose protein network with larger voids. This was in agreement with the lower breaking force with less water holding capacity of directly heated and modori gels. The finer and ordered gel network with smaller voids was observed in gels with the highest gel strength as compared with the very loose network with larger voids observed in the gels with lower gel strength (Balange & Benjakul [2009]). Rawdkuen and Benjakul ([2008]) reported that kamaboko gel from bigeye snapper with high breaking force and deformation was finer and denser than others, while the gel structure of lizardfish with poorer gel property consisted of a large number of pores and loose structure. Jafarpour and Gorczyca ([2008]) reported changes in the polygonal structures of kamaboko gel from the threadfin bream surimi as a result of cross-linking between sarcoplasmic protein and myofibrillar protein. However, no association between the area or the number of polygonal structures per square millimetre and gel strength of the resultant kamaboko was found.
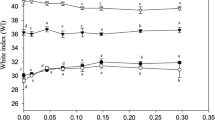
Endogenous transglutaminase activity as affected by temperatures
Activity of endogenous TGase from yellow stripe trevally muscle as a function of temperature determined by MDC incorporation method is shown in Figure 4. The activity increased as the temperature increased and reached the maximum at 40°C (p < 0.05). The decrease in activity was observed at temperature higher than 40°C. The loss in activity at higher temperature was caused by the thermal denaturation of TGase. Benjakul and Visessanguan ([2003]) reported that TGase from P. tayenus muscle showed the highest activity at 40°C, whereas that from P. macracanthus muscle had the optimum temperature at 25°C. The optimum temperature for TGase activity varies with fish species. Red sea bream liver TGase had an optimum temperature of 55°C (Yasueda et al. [1994]), whereas walleye pollack liver TGase showed the maximum activity at 50°C (Kumazawa et al. [1996]). Therefore, temperature was crucial to maximise the setting phenomenon. Temperature of 40°C used for prior setting of K40 sample in this study was coincidental with the optimum temperature for endogenous TGase. As a result, yellow stripe trevally TGase remaining in surimi catalysed the cross-linking of muscle proteins to a greater extent at 40°C than at a lower temperature (25°C), leading to higher breaking force of K40 sample.
The activity of endogenous TGase at 60°C was found to be very low as compared to that found at 40°C. The loss in activity at higher temperature was caused by the thermal denaturation of TGase, which consequently resulted in lowered cross-linking of muscle proteins. Additionally, the degradation at 60°C was pronounced. As a consequence, the lowest breaking force of modori gel was obtained. However, the rate of TGase mediated cross-linking of MHC was primarily dependent on the conformation of substrate at a given temperature rather than on the optimum temperature of TGase (Araki & Seki [1993]; Kamath et al. [1992]). Thus, TGase activity played a role in cross-linking of protein and gel property.
Conclusion
Yellow stripe trevally could be used as the new raw material for surimi which yielded the gel with high deformation. Setting temperature showed significant effect on textural properties and cross-linking of myofibrillar proteins. Kamaboko (K40) gel exhibited the highest breaking force with the lowest expressible moisture content (p < 0.05), corresponding to the optimum temperature of TGase activity. Therefore, setting at 40°C is a promising means to improve gel quality of surimi from yellow stripe trevally. Conversely, the incubation of sol at temperature close to 60°C should be avoided to prevent gel weakening.
Abbreviations
- DMC = N:
-
N′-dimethylated casein
- DTT:
-
Dithiothreitol
- K25:
-
Kamaboko gel having setting temperature of 25°C
- K40:
-
Kamaboko gel with prior setting at 40°C
- MDC:
-
Monodansyl cadaverine
- MHC:
-
Myosin heavy chain
- SDS:
-
Sodium dodecyl sulphate
- SDS-PAGE:
-
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- SPSS:
-
Statistical Package for Social Sciences
- TGase:
-
Transglutaminase
- TCA:
-
Trichloroacetic acid.
References
An H, Peters MY, Seymours TA: Roles of endogenous enzymes on surimi gelation. Trends Food Sci Technol 1996, 7: 321–327. 10.1016/0924-2244(96)10035-2
Araki H, Seki N: Comparison of reactivity of transglutaminase to various fish actomyosin. Nippon Suisan Gakkaishi 1993, 59: 711–716. 10.2331/suisan.59.711
Balange AK, Benjakul S: Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocolloids 2009, 23: 1693–1701. 10.1016/j.foodhyd.2009.01.007
Benjakul S, Visessanguan W: Transglutaminase-mediated setting in bigeye snapper surimi. Food Res Int 2003, 36: 253–266. 10.1016/S0963-9969(02)00167-9
Benjakul S, Seymour TS, Morrissey MT, An H: Physicochemical changes in Pacific whiting muscle proteins during iced storage. J Food Sci 1997, 62: 729–733. 10.1111/j.1365-2621.1997.tb15445.x
Benjakul S, Visessanguan W, Riebroy S, Ishizaki S, Tanaka M: Gel-forming properties of surimi produced from bigeye snapper, Priacanthus tayenus and P. macracanthus, stored in ice. J Sci Food Agric 2002, 82: 1442–1451. 10.1002/jsfa.1207
Benjakul S, Visessanguan W, Leelapongwattana K: Purification and characterization of heat-stable alkaline proteinase from bigeye snapper (Priacanthus macracanthus) muscle. Comp Biochem Phys B 2003, 134: 579–591.
Benjakul S, Visessanguan W, Tueksuban J: Heat-activated proteolysis in lizardfish (Saurida tumbil) muscle. Food Res Int 2003, 36: 1021–1028. 10.1016/j.foodres.2003.07.005
Benjakul S, Visessanguan W, Tueksuban J: Changes in physico-chemical properties and gel-forming ability of lizardfish (Saurida tumbil) during post-mortem storage in ice. Food Chem 2003, 80: 535–544. 10.1016/S0308-8146(02)00339-4
Benjakul S, Visessanguan W, Chantarasuwan C: Effect of high-temperature setting on gelling characteristic of surimi from some tropical fish. Int J Food Sci Technol 2004, 39: 671–680. 10.1111/j.1365-2621.2004.00825.x
Benjakul S, Yarnpakdee S, Visessanguan W, Phatcharat S: Combination effects of whey protein concentrate and calcium chloride on the properties of goatfish surimi gel. J Texture Stud 2010, 41: 341–357. 10.1111/j.1745-4603.2010.00228.x
Boye SW, Lanier TC: Effects of heat-stable alkaline protease activity of Atlantic menhaden (Brevoorti tyrannus) on surimi gels. J Food Sci 1988, 53: 1340–1343. 10.1111/j.1365-2621.1988.tb09272.x
Chaijan M, Benjakul S, Visessanguan W, Faustman C: Characteristics and gel properties of muscles from sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) caught in Thailand. Food Res Int 2004, 37: 1021–1030. 10.1016/j.foodres.2004.06.012
Chaijan M, Benjakul S, Visessanguan W, Lee S, Faustman C: The effect of freezing and aldehydes on the interaction between fish myoglobin and myofibrillar proteins. J Agric Food Chem 2007, 55: 4562–4568. 10.1021/jf070065m
Chaijan M, Panpipat W, Benjakul S: Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chem 2010, 121: 85–92. 10.1016/j.foodchem.2009.12.007
Chen HH: Decolouration and gel-forming ability of horse mackerel mince by air-flotation washing. J Food Sci 2002, 67: 2970–2975. 10.1111/j.1365-2621.2002.tb08847.x
Department of Fisheries: Production by species for whole marine fishery 2002–2006. In Fishery statistics capture product yearbook 2006. Department of Fisheries, Bangkok; 2006.
Haard NF, Simpson BK, Pan BS: Sarcoplasmic proteins and other nitrogenous compounds. In Seafood proteins. Edited by: Sikorski ZE, Pan BS, Shahidi F. Chapman & Hall, New York; 1994:13–39.
Hong GK, Eong YS: Maximizing utilization of fish catch for human consumption. 2005. Paper presented at the regional workshop on low value and “trash fish” in the Asia-Pacific Region, Hanoi, Vietnam, 7–9 June 2005
Hultin HO, Kelleher SD: Surimi processing from dark muscle fish. In Surimi and surimi seafood. Edited by: Park JW. Marcel Dekker, New York; 2000:59–77.
Jafarpour A, Gorczyca EM: Characteristics of sarcoplasmic proteins and their interaction with surimi and kamaboko gel. J Food Sci 2008, 74: N16-N22.
Kamath GG, Lanier TC, Foegeding EA, Hamann DD: Nondisulfide covalent cross-linking of myosin heavy chain in “setting” of Alaska pollock and Atlantic croaker surimi. J Food Biochem 1992, 16: 151–172. 10.1111/j.1745-4514.1992.tb00443.x
Klompong V, Benjakul S, Kantachote D, Shahidi F: Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem 2007, 102: 1317–1327. 10.1016/j.foodchem.2006.07.016
Kumazawa Y, Numazawa T, Seguro K, Motoki M: Suppression of surimi gel setting by transglutaminase inhibitors. J Food Sci 1995, 60: 715–717. 10.1111/j.1365-2621.1995.tb06213.x
Kumazawa Y, Nakanishi K, Yasueda H, Motoki M: Purification and characterization of transglutaminase from walleye pollack liver. Fish Sci 1996, 62: 959–964.
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227: 680–685. 10.1038/227680a0
Lanier TC: Surimi gelation chemistry. In Surimi and surimi seafood. Edited by: Park JW. Marcel Dekker, New York; 2000:237–265.
Lowry QH, Rosebrough NJ, Farr LA, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 1951, 193: 256–275.
Morales OG, Ramirez JA, Vivanco DI, Vazquez M: Surimi of fish species from the Gulf of Mexico: evaluation of the setting phenomenon. Food Chem 2001, 75: 43–48. 10.1016/S0308-8146(01)00181-9
Morrissey MT, Wu JW, Lin D, An H: Protease inhibitor effects on torsion measurements and autolysis of Pacific whiting surimi. J Food Sci 1993, 58: 1050–1054. 10.1111/j.1365-2621.1993.tb06109.x
NFI: A manual of standard methods for measuring and specifying the properties of surimi. National Fisheries Institute, Washington, DC; 1991.
Niwa E: Chemistry of surimi gelation. In Surimi technology. Edited by: Lanier TC, Lee CM. Marcel Dekker, New York; 1992:389–427.
Ochiai Y, Ochiai L, Hashimoto K, Watabe S: Quantitative estimation of dark muscle content in the mackerel meat paste and its products using antisera against myosin light chains. J Food Sci 2001, 66: 1301–1305. 10.1111/j.1365-2621.2001.tb15205.x
Park SH, Cho SY, Kimura M, Nozawa H, Seki N: Effects of microbial transglutaminase and starch on the thermal gelation of salted squid muscle paste. Fish Sci 2005, 71: 896–903. 10.1111/j.1444-2906.2005.01043.x
Rawdkuen S, Benjakul S: Whey protein concentrate: autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chem 2008, 106: 1077–1084. 10.1016/j.foodchem.2007.07.028
Seki N, Uno H, Lee NH, Kimura I, Toyoda K, Fujita T, Arai K: Transglutaminase activity in Alaska pollack muscle and surimi and its reaction with myosin B. Nippon Suisan Gakkaishi 1990, 56: 125–132. 10.2331/suisan.56.125
Shimizu Y, Machida R, Takanemi S: Species variations in the gel forming characteristics of fish meat paste. Nippon Suisan Gakkaishi 1981,47(1):95–104. 10.2331/suisan.47.95
Shimizu Y, Toyohara H, Lanier TC: Surimi production from fatty and dark-fleshed fish species. In Surimi technology. Edited by: Lanier TC, Lee CM. Marcel Dekker, New York; 1992:181–207.
Steel RGD, Torrie JH: Principle and procedure of statistics: a biometrical approach. 2nd edition. McGraw-Hill, New York; 1980.
Takagi J, Saito Y, Kikuchi T, Inada Y: Modification of transglutaminase assay: use of ammonium sulfate to stop the reaction. Anal Biochem 1986, 153: 295–298. 10.1016/0003-2697(86)90095-3
Takeda H, Seki N: Enzyme-catalyzed cross-linking and degradation of myosin heavy chain in walleye pollack surimi paste during setting. Fish Sci 1996, 62: 462–467.
Tanaka T: Gels. Sci Amer 1981, 244: 124–138. 10.1038/scientificamerican0181-124
Toyohara H, Nomata H, Makinodan Y, Shimizu Y: High molecular weight heat-stable alkaline proteinase from white croaker and chum salmon muscle: comparison of the activating effects by heating and urea. Comp Biochem Physiol B 1987, 86: 99–102. 10.1016/0305-0491(87)90181-7
Tsukamasa Y, Shimizu Y: Factors affecting the transglutaminase-associated setting phenomenon in fish meat sol. Nippon Suisan Gakkaishi 1991, 57: 535–540. 10.2331/suisan.57.535
Whistler RL, Daniel JR: Carbohydrates. In Food Chemistry. 2nd edition. Edited by: Fennema OR. Marcel Dekker, New York; 1985:69–137.
Wu J, Li CY, Ho ML, Jiang ST: Quality improvement of mackerel surimi with NADPH-sulfite reductase from Escherichia coli. J Food Sci 2000, 68: 1400–1403.
Yasueda H, Kumazawa Y, Motoki M: Purification and characterization of a tissue-type transglutaminase from red sea bream (Pagrus major). Biosci Biotechnol Biochem 1994, 58: 2041–2045. 10.1271/bbb.58.2041
Yoon WB, Park JW, Kim BY: Linear programming in blending various components of surimi seafood. J Food Sci 1997, 62: 561–567. 10.1111/j.1365-2621.1997.tb04430.x
Acknowledgement
The authors would like to express their sincere thanks to the Graduate School of Prince of Songkla University and the TRF Senior Research Scholar programme for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contribution
SB formulated the hypothesis and designed the studies. YAA performed the experiments and analyses. YAA and SB wrote the paper. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Arfat, Y.A., Benjakul, S. Gelling characteristics of surimi from yellow stripe trevally (Selaroides leptolepis). Int Aquat Res 4, 5 (2012). https://doi.org/10.1186/2008-6970-4-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2008-6970-4-5