Abstract
Background
Bisphosphonates are widely used in the clinical treatment of bone diseases with increased bone resorption. In terms of side effects, they are known to be associated with osteonecrosis of the jaw (BONJ).
The objective of this study was to evaluate the effect of bisphosphonates on osteoblast proliferation by cell count and gene expression analysis of cyclin D1 in vitro. Furthermore, the gene expression of the extracellular matrix protein collagen type I was evaluated. Nitrogen-containing and non-nitrogen-containing bisphosphonates have been compared on gene expression levels.
Methods
Human osteoblast obtained from hip bone were stimulated with zoledronate, ibandronate and clodronate at concentrations of 5 × 10-5M over the experimental periods of 1, 2, 5, 10 and 14 days. At each point in time, the cells were dissolved, the mRNA extracted, and the gene expression level of cyclin D1 and collagen type I were quantified by Real-Time RT-PCR. The gene expression was compared to an unstimulated osteoblast cell culture for control.
Results
The proliferation appeared to have been influenced only to a small degree by bisphosphonates. Zolendronate led to a lower cyclin D1 gene expression after 10 days. The collagen gene expression was enhanced by nitrogen containing bisphosphonates, decreased however after day 10. The non-nitrogen-containing bisphosphonate clodronate, however, did not significantly influence cyclin D1 and collagen gene expression.
Conclusions
The above data suggest a limited influence of bisphosphonates on osteoblast proliferation, except for zoledronate. The extracellular matrix production seems to be initially advanced and inhibited after 10 days. Interestingly, clodronate has little influence on osteoblast proliferation and extracellular matrix production in terms of cyclin D1 and collagen gene expression.
Similar content being viewed by others
Background
Bisphosphonates are widely used in the clinical treatment of bone diseases with increased bone resorption [1] such as Paget's disease, osteoporosis, and malignant diseases like multiple myeloma or metastasis to the bone. The increased bone mineral density has been attributed to a decreased bone turnover [2–5] by the inhibition of osteoclastic bone resorption.
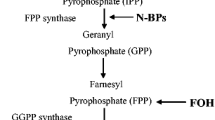
There is, however, increasing evidence, that bisphosphonates interact with osteoblasts. The bisphosphonates are a family of pyrophosphate analogs that can further be separated into nitrogen-containing and non-nitrogen-containing bisphosphonates. Non-nitrogen-containing bisphosphonates are build into ATP resulting in a non-hydrolysable adenine containing metabolite, whereas nitrogen-containing bisphosphonates interfere with the mevalonate pathway by inhibition of farnesyl pyrophosphate (FPP) synthase enzyme [6, 7]. This interference causes a reduction in geranyl geranyl diphosphate (GGPP), which is required for the prenylation of guanosin triphosphate (GTP)-binding proteins such as Rab, Rac, Ras, Rho and Cdc42 [8–12]. In contrast to older in vivo studies that attribute higher bone density to reduced bone turnover, newer studies have shown the potential of bisphosphonates to enhance osteoblast proliferation and differentiation in bone marrow-derived mesenchymal stem cells (MSC) and osteoblasts [13–15]. These actions could cause an altered cell metabolism, which is supposed to promote osteonecrosis that almost always occurs in the jaw as a serious side effect with exposed bone, fistulae and even pathological fractures [16, 17]. Especially after treatment by nitrogen containing bisphosphonates intravenously an incidence of 5%-19% has been reported [18–20]. In addition to a direct effect on osteoclasts and osteoblasts, some authors suggest that a bisphosphonate induced obliteration of the regional blood vessels could lead to an avascular osteonecrosis of the jaw [17, 21, 22].
The objective of this in vitro study was to illuminate the impact of bisphosphonates on osteoblast proliferation and extracellular matrix production over a period of 14 days. Therefore, the genes of cyclin D1 and collagen were quantified by Real Time RT-PCR. The nitrogen-containing bisphosphonates zoledronate and ibandronate were compared to the non-nitrogen-containing bisphosphonate clodronate.
Methods
Cell culture
Human hip bone osteoblasts (HOB-c, Promo Cell, Heidelberg, Germany) between passages 5-9 were cultured at a density of 200 000 cells per well using 6-well plates. They were allowed to attach for two days using an osteoblast specific medium (10% FCS/DMEM Dulbecco modified medium (Invitrogen, Carlsbad, Ca/US) containing 1% L-glutamin, 1% penicillin/streptomycin/neomycin, 1% ascorbic acid, and 20 μg/ml dexamethasone. The cells were stimulated by osteoblast specific medium containing zoledronate, ibandronate, or clodronate at a concentration of 5 × 10-5M. The osteoblast specific cell culture medium without bisphosphonate supplement was used for control. The media and bisphosphonates were renewed every 4 days for a period of 14 days to guarantee a constant stimulation und nutrition supply over the experimental period.
mRNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR)
On day 1, 2, 5, 10, and 14 of cultivation, the osteoblasts were detached with 0.05% trypsin-EDTA solution (Invitrogen, Carlsbad, Ca, US) and individually harvested. MRNA was extracted using a silicate gel technique that was provided by the Qiagen RNeasy extraction kit (Qiagen, Hilden, Germany). This included a DNAse digestion step. The amount of extracted mRNA was measured by extinction at 260nm; the contamination with proteins was determinated with the 260/280 ratio.
To detect the mRNA of cyclin D1 and collagen type I in osteoblasts, primers were designed using NCBI-nucleotide library and Primer3-design (Tab. 1). All primers had been matched to the mRNA sequences of the target genes (NCBI Blast software).
As housekeeping genes, human ribosomal protein (HuPO), actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ribosomal protein S18 (RPS18) were evaluated. We were able to show the most stable expression for the actin, GAPDH and RPS18 genes by comparing the bisphosphonate stimulated versus a non stimulated cell-culture using a specialized freeware, called GeNorm.
As a quantitative RT-PCR we used the SYBR Green Real Time PCR (oneStep RT-PCR, Bio-Rad, Hercules, CA/USA). This method enables reverse transcription using the individual primers immediately before PCR amplification and SYBR Green fluorescence measurement for quantification of gene expression. Samples were amplified in 96-well microplates in an IQ5-Cycler (Bio-Rad, Hercules, CA/USA) with an annealing temperature of 56°C and an elongation temperature of 71°C over 40 cycles. Background was to determine over 3-10 cycles and the threshold was set above this fluorescence, crossing the SYBR green fluorescence curve at the exponential part. This method was applied to calculate the cycle number and CT-value for quantitation. Furthermore, the CT-values of actin, GAPDH and RPS18 housekeeping genes and the individual primer efficacy were considered. Single product formation was confirmed by melting point analysis. For negative control, water instead of mRNA-samples was used.
CDNA from individual cell experiments was analyzed in triplicate PCR. The ΔΔCT method was applied [23, 24] for a statistical analysis of the CT-values. For each specific primer and Real-Time PCR, the efficiency was calculated on the basis of the SYBR Green fluorescence curves and the standard dilution series. The relative gene expression levels were standardized with those measured in the unstimulated control, which was set to 100%. Each point in time for relative mRNA is the mean +/- standard deviation. (See Table 1)
Statistical analysis
The mean values and standard deviations were calculated by the IQ5-software (BioRad, Hercules, CA/USA) to provide a descriptive data analysis.
Results
Effect of bisphosphonates on cyclin D1 gene expression
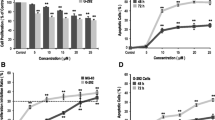
Time-course experiments were performed to determine the effects of zoledronate, ibandronate and clodronate on cyclin D1 gene expression. As shown in figure 1, treatment of human hip bone osteoblast [hOB] cells with ibandronate, zoledronate and clodronate did not significantly influence the gene expression of cyclin D1 during the first 6 days. Zoledronate, however, caused a decreasing cyclin D1 gene expression after the 6th day whereas ibandronate and clodronate did not significantly show enhanced or decreased gene expression levels.
Effect of bisphosphonates on collagen gene expression
The collagen gene expression was stimulated to the most extent by ibandronate, reaching a maximum of 400% at day 10 compared to the non-stimulated control. Zoledronate also caused osteoblasts to increase their gene expression to a maximum level of 330% on day 2. After 14 days of stimulation the gene expression of collagen type I has decreased to a level of 12% for zoledronate, respectively 30% for ibandronate compared to an unstimulated control.
The non-nitrogen-containing clodronate, however, did not cause a significant alteration of collagen gene expression (figure 2).
Discussion
Bisphosphonates are therapeutically applied to treat metabolic bone diseases, such as osteoporosis or metastasis to the bone. Clinical studies have shown their potency to increase bone density over an extended period of time [25–28]. This effect is not only caused by a positive bone turnover, but also by a direct stimulation of osteoblast and osteoblast precursor cells by applying nitrogen-containing bisphosphonates [15, 29]. An anabolic effect to the bone could be caused by proliferation and by extracellular matrix production, mainly of collagen type I. With respect to osteoblast proliferation, we examined cyclin D1, an important regulator of the cell cycle and a surrogate of cell proliferation. Our results did not show a significant impact on osteoblast proliferation during the first 6 days. However, after day 6 zoledronate led to a reduced Cyclin D1 gene expression. As shown in other in vitro studies, pamidronate, a nitrogen-containing bisphosphonate, decreased osteoblast proliferation in a dose dependent manner [29]. In contrast, bisphosphonates have been reported to induce proliferation of marrow osteoprogenitors [30]. These anabolic effects are evidenced by a positive bone turnover, evaluated in clinical studies of up to 10 years of bisphosphonate treatment [25, 31].
With respect to extracellular matrix production and early bone differentiation, type I collagen is the most important matrix protein. It is produced by osteoblasts and permits bone mineralization. The nitrogen-containing bisphosphonates appeared to induce collagen type I gene expression during the first 10 days. At day 14 the collagen gene expression level was lowered by the nitrogen containing bisphosphonates below 30%. These results are confirmed by Reinholz et al., who also found an enhanced collagen production [29]. The bisphosphonate as well effect bone marrow stromal cells by an enhanced collagen gene expression [15].
Our data suggest that nitrogen-containing bisphosphonate treatment enhances the differentiation of the osteoblasts from the proliferation stage into a nonproliferating matrix maturation stage. The lower proliferation but higher bone density through differentiation could explain the missing regeneration potential of BONJs.
In contrast, the non-nitrogen-containing bisphosphonate clodronate did not have any significant impact on osteoblasts cyclin D1 gene expression or type I collagen gene expression. These results support the assumption, that for the inhibition of farnesyl pyrophosphate (FPP) synthase enzyme [6, 7] non-nitrogen-containing bisphosphonates mainly effect the osteoclasts, but not the osteoblasts. This was also confirmed by the clinically higher potency of nitrogen-containing bisphosphonates for bone density evaluation and a lower incidence of BONJ.
Conclusions
Our data suggest that there is an antiproliferative effect of bisphosphonates on osteoblasts. Bisphosphonates, however, appear to enhance extracellular matrix production of collagen type I. The enhanced bone density mediated by bisphosphonates appears to be caused by the stimulation of osteoblast differentiation. Non-nitrogen-containing bisphosphonates do not appear to influence osteoblast proliferation and extracellular matrix production.
References
Russell RG, Rogers MJ: Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999, 25: 97-106. 10.1016/S8756-3282(99)00116-7.
Glatt M, Pataki A, Evans GP, Hornby SB, Green JR: Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int. 2004, 15: 707-715. 10.1007/s00198-004-1588-3.
Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR: Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int. 2003, 72: 519-527. 10.1007/s00223-002-2015-4.
Pataki A, Müller K, Green JR, Ma YF, Li QN, Jee WS: Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec. 1997, 249: 458-468. 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N.
Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D: The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest. 1993, 92: 2577-2586. 10.1172/JCI116872.
Fisher JE, Rodan GA, Reszka AA: In vivo effects of bisphosphonates on the osteoclast mevalonate pathway. Endocrinology. 2000, 141: 4793-4796. 10.1210/en.141.12.4793.
Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA: Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA. 1999, 96: 133-138. 10.1073/pnas.96.1.133.
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ: Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001, 296: 235-242.
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ: Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998, 13: 581-589. 10.1359/jbmr.1998.13.4.581.
Luckman SP, Hughes DE, Coxon FP, Russell GG, Rogers MJ: JBMR anniversary classic. Nitrogen-containing biophosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. Originally published in Volume 7, number 4, pp 581-9 (1998). J Bone Miner Res. 2005, 20: 1265-1274. 10.1359/jbmr.2005.20.7.1265.
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC: Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000, 88: 2961-2978. 10.1002/1097-0142(20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L.
van Beek ER, Cohen LH, Leroy IM, Ebetino FH, Löwik CW, Papapoulos SE: Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone. 2003, 33: 805-811. 10.1016/j.bone.2003.07.007.
Fromigue O, Body JJ: Bisphosphonates influence the proliferation and the maturation of normal human osteoblasts. J Endocrinol Invest. 2002, 25: 539-546.
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS: Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004, 25: 4105-4115. 10.1016/j.biomaterials.2003.11.024.
von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS: Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005, 26: 6941-6949. 10.1016/j.biomaterials.2005.04.059.
Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA: Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005, 23: 8580-8587. 10.1200/JCO.2005.02.8670.
Marx RE, Sawatari Y, Fortin M, Broumand V: Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005, 63: 1567-1575. 10.1016/j.joms.2005.07.010.
Walter C, Al-Nawas B, du Bois A, Buch L, Harter P, Grötz KA: Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer. 2009, 115: 1631-1637. 10.1002/cncr.24119.
Walter C, Al-Nawas B, Grötz KA, Thomas C, Thüroff JW, Zinser V, Gamm H, Beck J, Wagner W: Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol. 2008, 54: 1066-1072. 10.1016/j.eururo.2008.06.070.
Walter C, Grötz KA, Kunkel M, Al-Nawas B: Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer. 2007, 15: 197-202. 10.1007/s00520-006-0120-z.
Abu-Id MH, Açil Y, Gottschalk J, Kreusch T: [Bisphosphonate-associated osteonecrosis of the jaw]. Mund Kiefer Gesichtschir. 2006, 10: 73-81. 10.1007/s10006-005-0670-0.
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL: Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004, 62: 527-534. 10.1016/j.joms.2004.02.004.
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F: Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3: RESEARCH0034. 10.1186/gb-2002-3-7-research0034.
Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29: 45. 10.1093/nar/29.9.e45.
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA: Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004, 350: 1189-1199. 10.1056/NEJMoa030897.
Devogelaer JP, Broll H, Correa-Rotter R, Cumming DC, De Deuxchaisnes CN, Geusens P, Hosking D, Jaeger P, Kaufman JM, Leite M, Leon J, Liberman U, Menkes CJ, Meunier PJ, Reid I, Rodriguez J, Romanowicz A, Seeman E, Vermeulen A, Hirsch LJ, Lombardi A, Plezia K, Santora AC, Yates AJ, Yuan W: Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone. 1996, 18: 141-150. 10.1016/8756-3282(95)00436-X.
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Dequeker J, Favus M: Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995, 333: 1437-1443. 10.1056/NEJM199511303332201.
Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC: Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998, 83: 396-402. 10.1210/jc.83.2.396.
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC: Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60: 6001-6007.
Klein BY, Ben-Bassat H, Breuer E, Solomon V, Golomb G: Structurally different bisphosphonates exert opposing effects on alkaline phosphatase and mineralization in marrow osteoprogenitors. J Cell Biochem. 1998, 68: 186-194. 10.1002/(SICI)1097-4644(19980201)68:2<186::AID-JCB5>3.0.CO;2-R.
Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ: Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997, 100: 1475-1480. 10.1172/JCI119668.
Acknowledgements
Thanks to the laboratory staff.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
FK conceived of the study, organized and carried out the PCR studies, designed the primers and drafted the manuscript. CK carried out the PCR studies as well. TZ participated in the design of the study. RS and SSY participated in the study design, supported by scientific consulting and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Felix Peter Koch, Sareh Said Yekta contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Koch, F.P., Yekta, S.S., Merkel, C. et al. The impact of bisphosphonates on the osteoblast proliferation and Collagen gene expression in vitro . Head Face Med 6, 12 (2010). https://doi.org/10.1186/1746-160X-6-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1746-160X-6-12